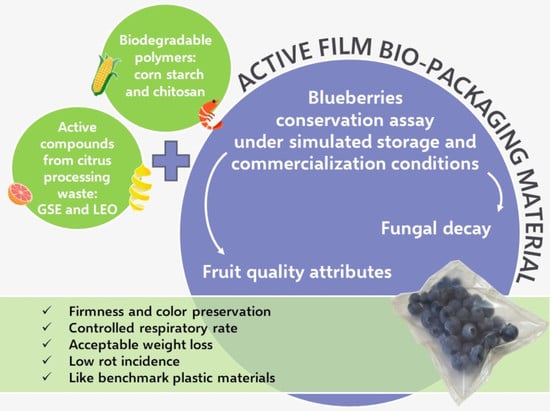
Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
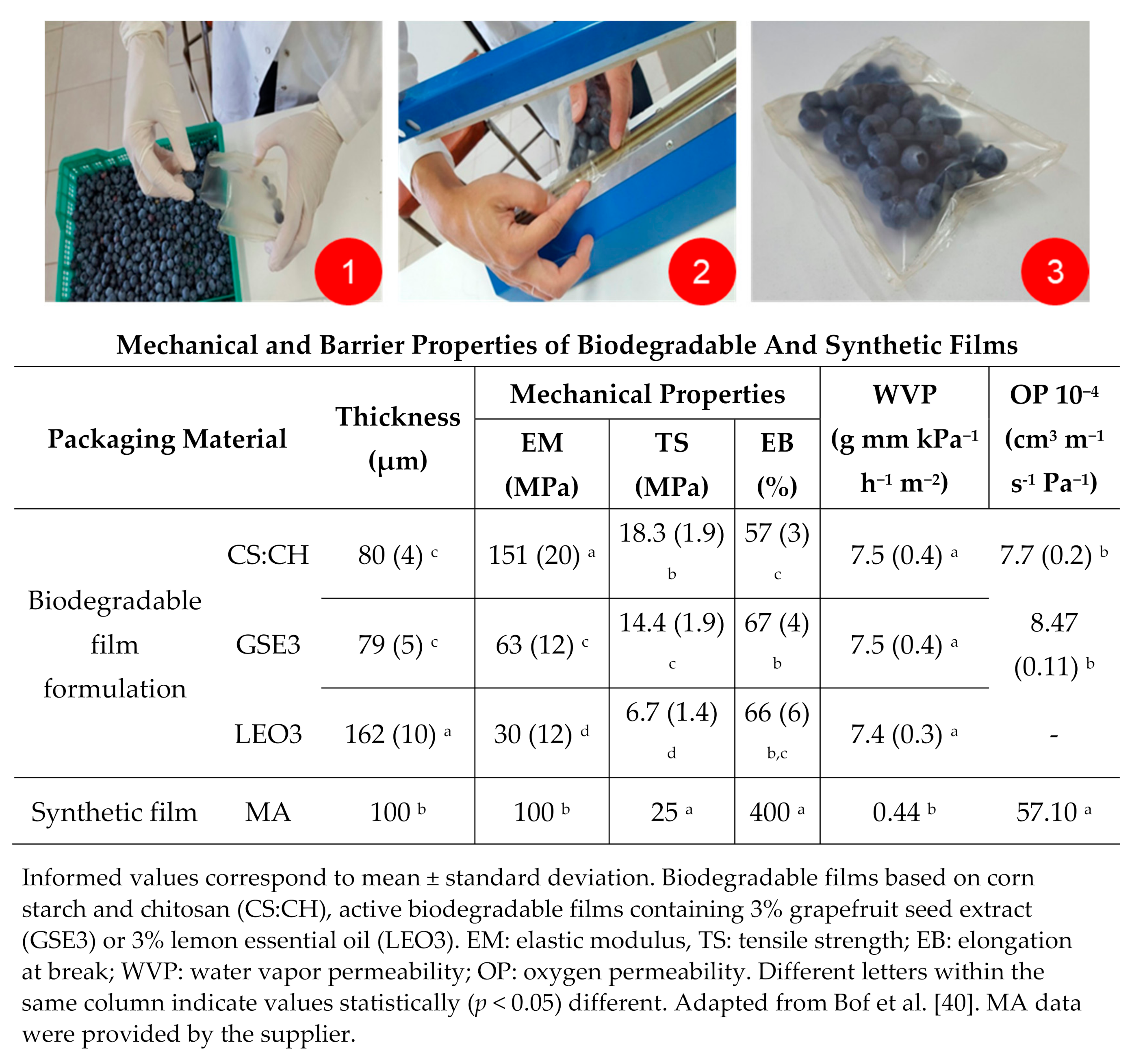
2.2. Biodegradable Films Preparation
2.3. Blueberries Preservation Assays under Transport and Market Conditions
2.4. Quality Attributes of Packed Fruit
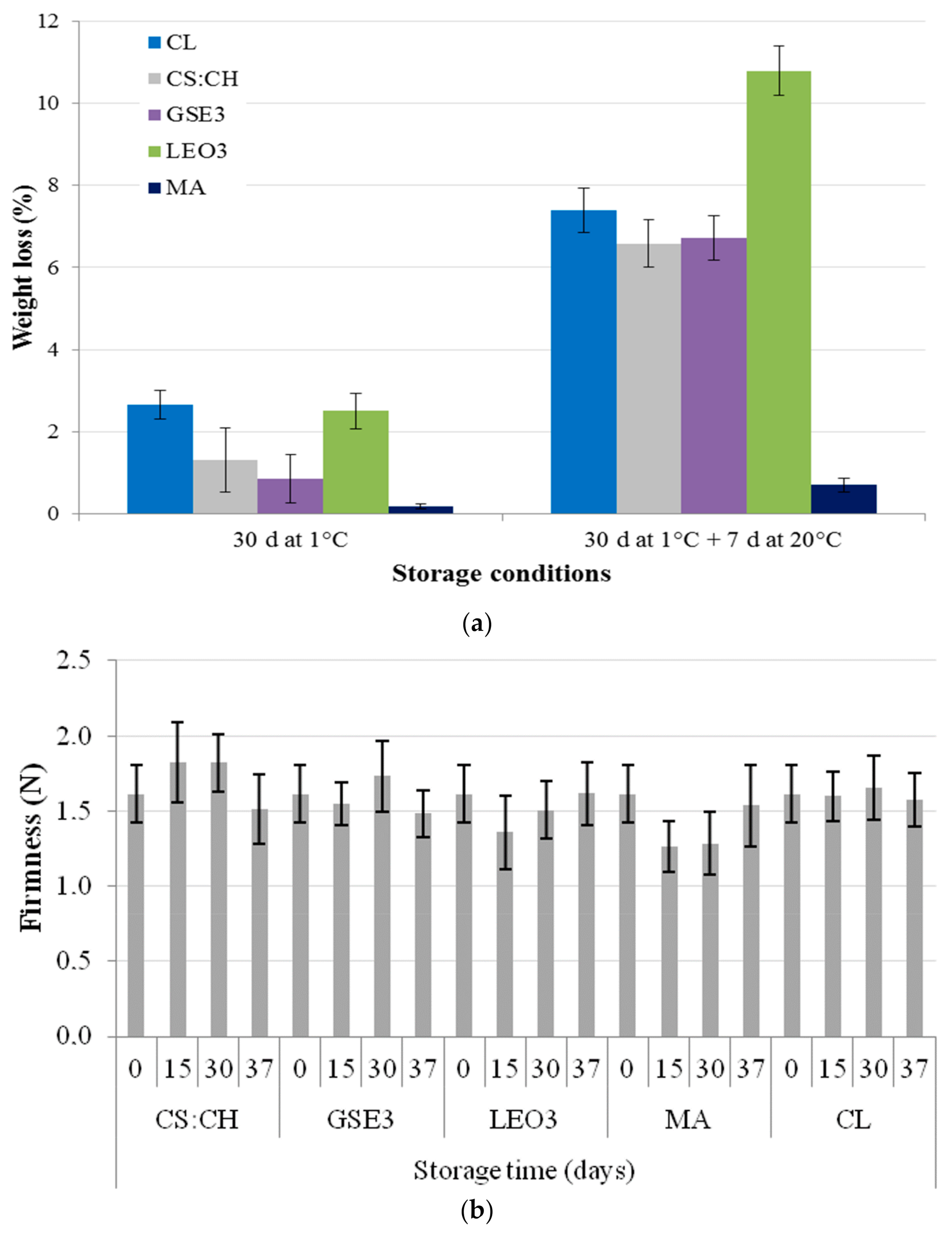
2.4.1. Weight Loss
2.4.2. Firmness
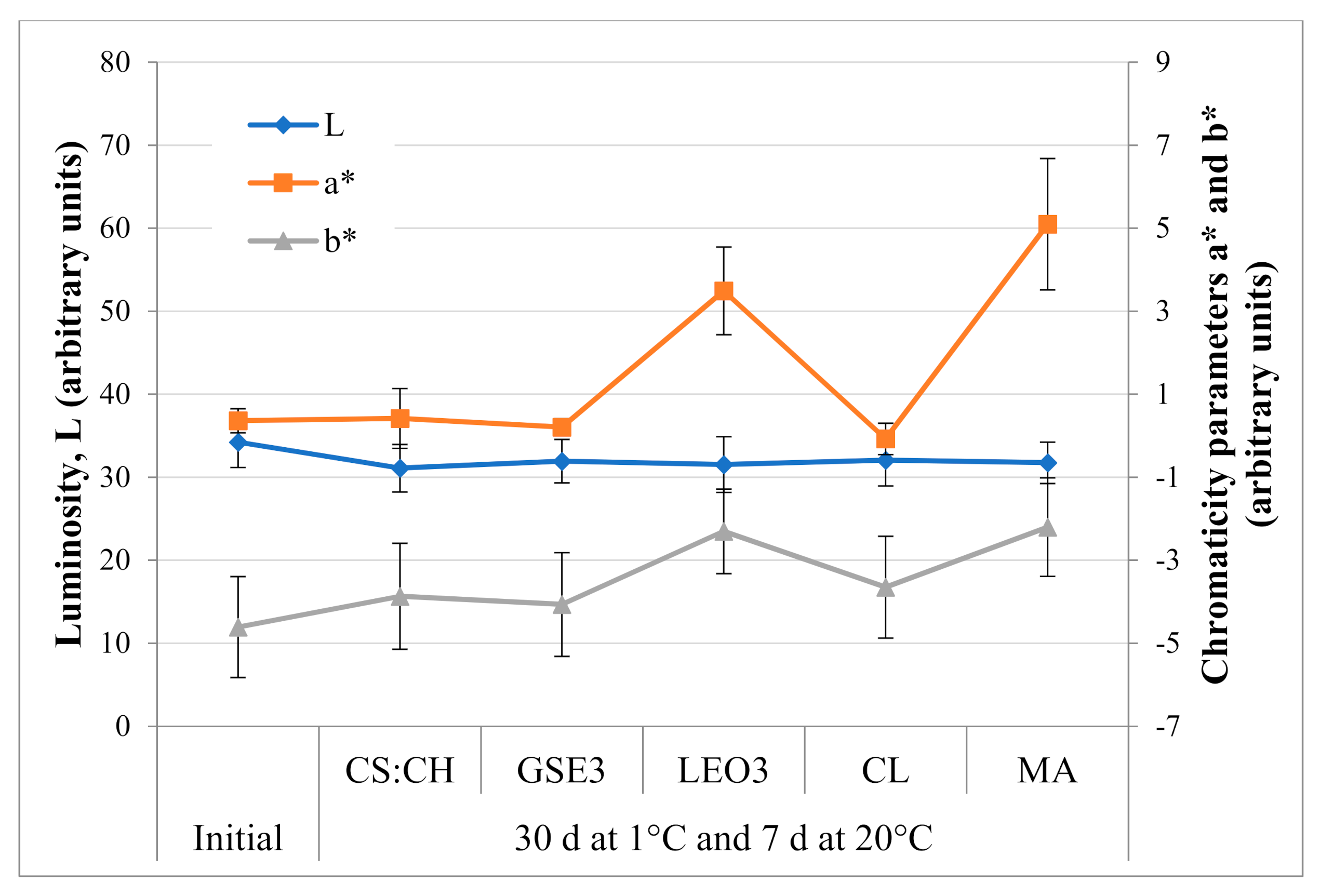
2.4.3. Color
2.4.4. Titratable Acidity and Total Soluble Solids
2.4.5. Respiration Rate
2.4.6. Antioxidant Activity
2.4.7. Fungal Decay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Blueberries Quality Attributes
3.2. Fruit Internal Quality
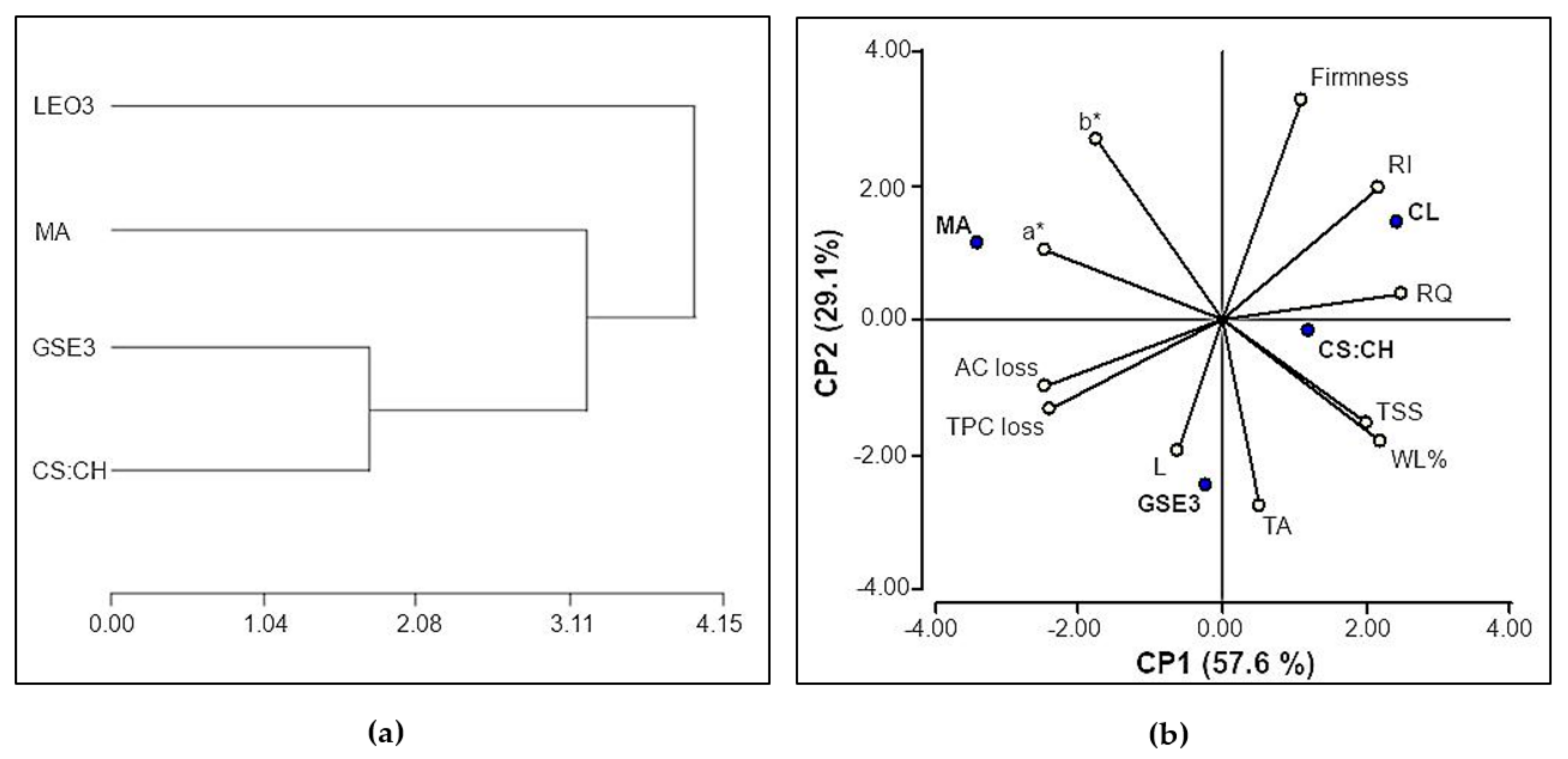
3.3. Materials Properties Effect on Fruit Quality Parameters
4. Practical Applications and Future Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affair. The Sustainable Development Goals Report 2020; Department of Economic and Social Affair: New York, NY, USA, 2020. [Google Scholar]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential oils as antimicrobial agents in biopolymer-based food packaging—A comprehensive review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Plastics Europe; EPRO. Plastics—The Facts 2019; Plastics Europe: Brussels, Belgium, 2019. [Google Scholar]
- Yadav, A.; Mangaraj, S.; Singh, R.; Das, K.; Kumar, N.; Arora, S. Biopolymers as packaging material in food and allied industry. Int. J. Chem. Stud. 2018, 6, 2411–2418. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers—A Review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Jabeen, N.; Majid, I.; Nayik, G.A.; Yildiz, F. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan composites in packaging industry-current trends and future challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.J.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with D-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Sinelli, N.; Spinardi, A.; Di Egidio, V.; Mignani, I.; Casiraghi, E. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharvest Biol. Technol. 2008, 50, 31–36. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.S.; Casto, B.C. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Yin, J.J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Medina, R.B.; Cantuarias-Avilés, T.E.; Angolini, S.F.; Da Silva, S.R. Performance of ‘emerald’ and ‘jewel’ blueberry cultivars under no-chill incidence. Pesqui. Agropecu. Trop. 2018, 48, 147–152. [Google Scholar] [CrossRef]
- Giacalone, G.; Chiabrando, V. Problems and methods to improve the market-life of berry fruit. In Berries: Properties, Consumption and Nutrition; Tuberoso, C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 179–196. ISBN 9781614702573. [Google Scholar]
- Zheng, Y.; Yang, Z.; Chen, X. Effect of high oxygen atmospheres on fruit decay and quality in Chinese bayberries, strawberries and blueberries. Food Control 2008, 19, 470–474. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Shelf-life extension of highbush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chem. 2011, 126, 1812–1816. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Cantín, C.M.; Minas, I.S.; Goulas, V.; Jiménez, M.; Manganaris, G.A.; Michailides, T.J.; Crisosto, C.H. Sulfur dioxide fumigation alone or in combination with CO 2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 2012, 67, 84–91. [Google Scholar] [CrossRef]
- Cantín, C.M.; Palou, L.; Bremer, V.; Michailides, T.J.; Crisosto, C.H. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 2011, 59, 150–158. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Li, S.; Xu, L.; Wang, R.; Chen, R.; Sun, Y. The size-controllable preparation of chitosan/silver nanoparticle composite microsphere and its antimicrobial performance. Carbohydr. Polym. 2019, 220, 22–29. [Google Scholar] [CrossRef]
- Passaretti, M.G.; Ninago, M.D.; Di Anibal, C.; Pacheco, C.; Vega, D.A.; Villar, M.A.; López, O.V. Composite films with UV barrier capacity to minimize flavored waters degradation. Food Packag. Shelf Life 2019, 21, 100334. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from Marine Crustaceans: Production, Characterization and Applications. Biol. Act. Appl. Mar. Polysaccharides 2017. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Do Evangelho, J.A.; Da Silva Dannenberg, G.; Biduski, B.; El Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; Da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222. [Google Scholar] [CrossRef]
- Bof, M.J.; Jiménez, A.; Locaso, D.E.; García, M.A.; Chiralt, A. Grapefruit Seed Extract and Lemon Essential Oil as Active Agents in Corn Starch–Chitosan Blend Films. Food Bioprocess Technol. 2016, 9, 2033–2045. [Google Scholar] [CrossRef]
- Silva, J.L.; Marroquin, E.; Matta, F.B.; Garner, J.O.; Stojanovic, J. Physicochemical, carbohydrate and sensory characteristics of highbush and rabbiteye blueberry cultivars. J. Sci. Food Agric. 2005, 85, 1815–1821. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf life determination of fresh blueberries (vaccinium corymbosum) stored under controlled atmosphere and ozone. Int. J. Food Sci. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Swift, J.E. Effects of Frozen Storage and Harvest Time on the Textural and Sensory Properties of Rabbiteye Blueberries (Vaccinium virgatum Aiton); North Carolina State University: Raleigh, NC, USA, 2010. [Google Scholar]
- Bof, M.J.; Bordagaray, V.C.; Locaso, D.E.; García, M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Biomed. Sci. 2013, 126–139. [Google Scholar] [CrossRef]
- Ogbo, E.M.; Oyibo, A.E. Effects of three plant extracts (Ocimum gratissimum, Acalypha wilkesiana and Acalypha macrostachya) on post harvest pathogen of Persea americana. J. Med. Plants Res. 2008, 2, 311–314. [Google Scholar]
- Essghaier, B.; Fardeau, M.L.; Cayol, J.L.; Hajlaoui, M.R.; Boudabous, A.; Jijakli, H.; Sadfi-Zouaoui, N. Biological control of grey mould in strawberry fruits by halophilic bacteria. J. Appl. Microbiol. 2009, 106, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Infostat Software; FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2011. [Google Scholar]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- Jackson, E.D.; Sanford, K.A.; Lawrence, R.A.; McRae, K.B.; Stark, R. Lowbush blueberry quality changes in response to prepacking delays and holding temperatures. Postharvest Biol. Technol. 1999, 15, 117–126. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Influence of initial gas modification on physicochemical quality attributes and molecular changes in fresh and fresh-cut fruit during modified atmosphere packaging. Food Packag. Shelf Life 2019, 21. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.P.; Brecht, J.K. Quality curves for highbush blueberries as a function of the storage temperature. Small Fruits Rev. 2004, 3, 423–440. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. Postharvest shelf life extension of blueberries using a biodegradable package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G.; Rolle, L. Mechanical behaviour and quality traits of highbush blueberry during postharvest storage. J. Sci. Food Agric. 2009, 89, 989–992. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Methods of testing strawberry fruit firmness. Acta Agric. Scand. Sect. B Plant Soil Sci. 2002, 52, 43–51. [Google Scholar] [CrossRef]
- Khazaei, J.; Mann, D.D. Effects of Temperature and Loading Characteristics on Mechanical and Stress Relaxation Properties of Sea Buckthorn Berries. Part 2. Puncture Tests; International Commission of Agricultural Engineering: Liège, Belgium, 2004; pp. 1–16. [Google Scholar]
- Fava, J.; Alzamora, S.M.; Castro, M.A. Structure and nanostructure of the outer tangential epidermal cell wall in vaccinium corymbosum L. (Blueberry) fruits by blanching, freezing-thawing and ultrasound. Food Sci. Technol. Int. 2006, 12, 241–251. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Goswami, T.K. Extended storage life of litchi fruit using controlled atmosphere and low temperature. J. Food Process. Preserv. 2004, 28, 388–403. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Rodriguez, J.; Zoffoli, J.P. Effect of sulfur dioxide and modified atmosphere packaging on blueberry postharvest quality. Postharvest Biol. Technol. 2016, 117, 230–238. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Harb, J.Y.; Streif, J. Controlled atmosphere storage of highbush blueberries cv. “Duke”. Eur. J. Hortic. Sci. 2004, 69, 66–72. [Google Scholar]
- Eichholz, I.; Huyskens-Keil, S.; Rohn, S. Blueberry Phenolic Compounds: Fruit Maturation, Ripening and Post-Harvest Effects. Fruit Maturation, Ripening and Post-Harvest Effects.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124047099. [Google Scholar]
- Giacalone, G.; Peano, C.; Guarinoni, A.; Beccaro, G.; Bounous, G. Ripening Curve of Early, Midseason and Late Maturing Highbush Blueberry Cultivars. Acta Hortic. 2002, 119–121. [Google Scholar] [CrossRef]
- Iqbal, T.; Rodrigues, F.A.S.; Mahajan, P.V.; Kerry, J.P. Mathematical modeling of the influence of temperature and gas composition on the respiration rate of shredded carrots. J. Food Eng. 2009, 91, 325–332. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Cameron, A.C.; Shirazi, A.; Dostal-Lange, D.L. Modified-atmosphere Packaging of Blueberry Fruit: Effect of Temperature on Package O2 and CO2. J. Am. Soc. Hortic. Sci. 2019, 117, 436–441. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Wood, B. Use of edible coating to preserve pecans at room temperature. HortScience 2006, 41, 188–192. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Carmona, A.; Rodríguez, F.J.; Soliva-Fortuny, R.; Martin-Belloso, O. Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008, 22, 1493–1503. [Google Scholar] [CrossRef]
- Suman, S.P.; Mancini, R.A.; Joseph, P.; Ramanathan, R.; Konda, M.K.R.; Dady, G.; Yin, S. Packaging-specific influence of chitosan on color stability and lipid oxidation in refrigerated ground beef. Meat Sci. 2010, 86, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Ravishankar, C.N. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Tapia, M.S. Edible coatings as carriers of food additives on fresh-cut fruits and vegetables. Stewart Postharvest Rev. 2010, 6, 1–7. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, J. Consumer acceptance of fresh blueberries in bio-based packages. J. Sci. Food Agric. 2010, 90, 1121–1128. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Girgenti, V.; Peano, C. Qualitative Performance and Consumer Acceptability of Starch Films for the Blueberry Modified Atmosphere Packaging Storage. Polish J. Food Nutr. Sci. 2017, 67, 129–136. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Bustamante, L.; Vergara, C.; Von Baer, D.; Hermosín-Gutiérrez, I.; Obando, L.; Mardones, C. Hydroxycinnamic acids and flavonols in native edible berries of South Patagonia. Food Chem. 2015, 167, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Castilla, S.; Guillén-Bejarano, R.; Jaramillo-Carmona, S.; Jiménez-Araujo, A.; Rodríguez-Arcos, R. Funcionalidad de distintas variedades de arándanos. In Proceedings of the CESIA 2012—Universidad de Castilla, VII Congreso Español de Ingeniería de Alimentos, Ciudad Real, Spain, 7–9 November 2012; p. 11. [Google Scholar]
- Guiné, R.P.F.; Matos, S.; Gonçalves, F.J.; Costa, D.; Mendes, M. Evaluation of phenolic compounds and antioxidant activity of blueberries and modelization by artificial neural networks. Int. J. Fruit Sci. 2018, 18, 199–214. [Google Scholar] [CrossRef]
- Rodrigues, E.; Poerner, N.; Rockenbach, I.I.; Gonzaga, L.V.; Mendes, C.R.; Fett, R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Food Sci. Technol. 2011, 31, 911–917. [Google Scholar] [CrossRef]
- Remberg, S.F.; Haffner, K.; Blomhoff, R. Total antioxidant capacity and other quality criteria in blueberries cvs “Bluecrop”, “Hardyblue”, “Patriot”, “Putte” and “Aron” after storage in cold store and controlled atmosphere. Acta Hortic. 2003, 600, 595–598. [Google Scholar] [CrossRef]
- Martins da Costa, J.C.; Lima Miki, K.S.; Da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Ares, A.; Abad, M.J.; Barral, L.; García-Garabal, S. Barrier and physical properties of polypropylene/high-barrier ethylene vinyl alcohol copolymer blends compatibilized with a sodium ionomer. e-Polymers 2009, 9, 1–13. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Abejón, R.; Bala, A.; Vázquez-Rowe, I.; Aldaco, R.; Fullana-i-Palmer, P. When plastic packaging should be preferred: Life cycle analysis of packages for fruit and vegetable distribution in the Spanish peninsular market. Resour. Conserv. Recycl. 2020, 155, 104666. [Google Scholar] [CrossRef]
- Glutal, S.A. Available online: www.glutal.com.ar (accessed on 18 January 2021).
- Droguería Saporiti SACIFIA. Available online: www.drogueriasaporiti.com.ar (accessed on 18 January 2021).
- Alibaba. Available online: www.alibaba.com/showroom/price-of-glycerol.html (accessed on 18 January 2021).
- EUMA SAICIYF. Available online: http://www.euma.com.ar/catalog/index.php/manufacturers_id/2/filter_id/33/page/1/sort/2d (accessed on 18 January 2021).

| Sample | RCO2 (mL kg−1 h−1) | RO2 (mL kg−1 h−1) | RQ | RI |
|---|---|---|---|---|
| Initial | 1.31 ± 0.07 d | 1.05 ± 0.05 d | 1.25 | - |
| CS:CH | 6.30 ± 0.32 b | 6.06 ± 0.30 b | 1.04 | 12 |
| GSE3 | 12.87 ± 0.65 a | 12.98 ± 0.65 a | 0.99 | 0 |
| LEO3 | 5.84 ± 0.29 b | 4.96 ± 0.25 b,c | 1.18 | 28 |
| CL | 2.82 ± 0.14 c | 2.09 ± 0.10 d | 1.35 | 24 |
| MA | 3.19 ± 0.16 c | 4.66 ± 0.23 c | 0.68 | 0 |
| Sample | TPC (mg CA kg−1) | TPC Loss (%) | AC (mg TEAC kg−1) | AC Loss (%) |
|---|---|---|---|---|
| Initial | 5765 ± 4.32 a | - | 4042 ± 68.82 a | - |
| CS:CH | 4802 ± 73.71 b | 16.7 | 3428 ± 156.54 b | 15.2 |
| GSE3 | 2703 ± 102.65 c | 53.1 | 1827 ± 99.01 c | 54.8 |
| CL | 5606 ± 122.70 a | 2.8 | 3816 ± 233.73 a | 5.6 |
| MA | 2091 ± 25.49 d | 63.7 | 1115 ± 29.24 d | 72.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bof, M.J.; Laurent, F.E.; Massolo, F.; Locaso, D.E.; Versino, F.; García, M.A. Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers 2021, 13, 481. https://doi.org/10.3390/polym13040481
Bof MJ, Laurent FE, Massolo F, Locaso DE, Versino F, García MA. Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers. 2021; 13(4):481. https://doi.org/10.3390/polym13040481
Chicago/Turabian StyleBof, María Julieta, Franco Emanuel Laurent, Facundo Massolo, Delia Elisa Locaso, Florencia Versino, and María Alejandra García. 2021. "Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions" Polymers 13, no. 4: 481. https://doi.org/10.3390/polym13040481
APA StyleBof, M. J., Laurent, F. E., Massolo, F., Locaso, D. E., Versino, F., & García, M. A. (2021). Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers, 13(4), 481. https://doi.org/10.3390/polym13040481