Alginate as Dispersing Agent for Compounding Natural Rubber with High Loading Microfibrillated Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
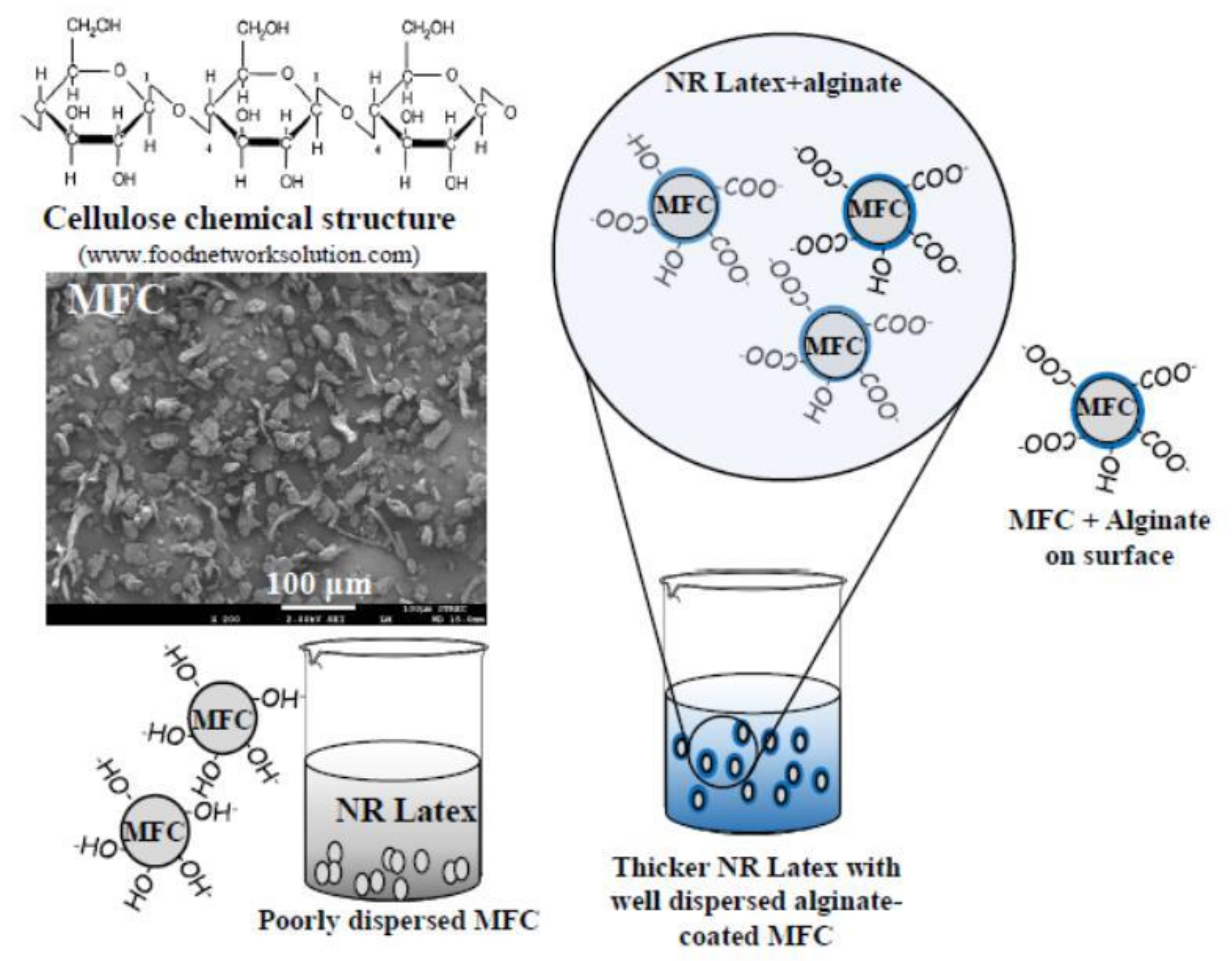
2.2. Preparation of MFC–NR Composite Films
2.3. Characterization of MFC–NR Composite Films
3. Results and Discussion
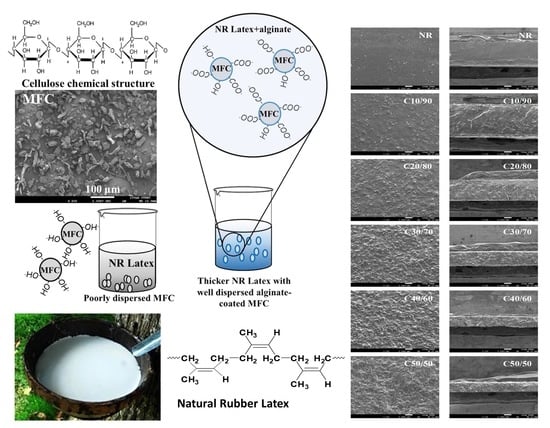
3.1. Morphology of the MFC–NR Composite Films
3.2. Mechanical Properties
3.3. FTIR
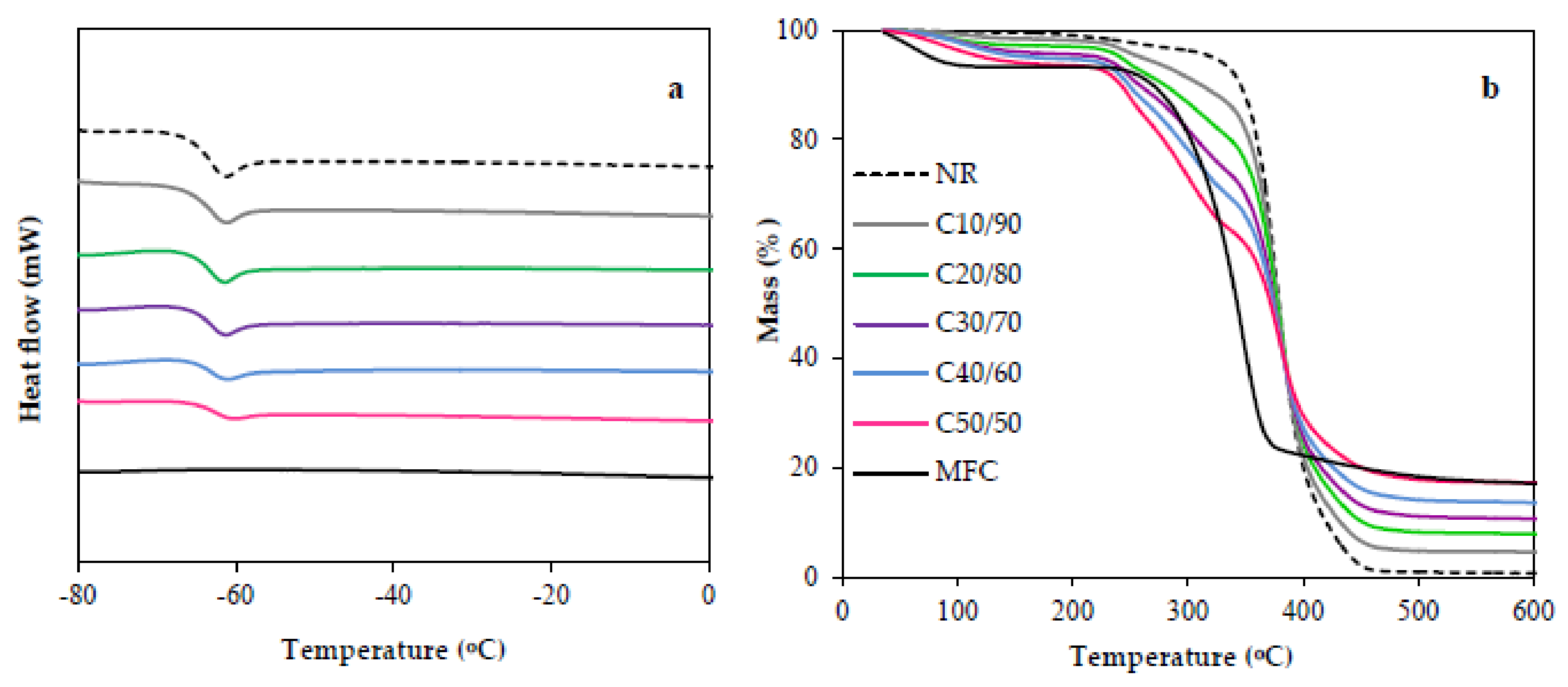
3.4. Thermal Properties
3.5. Water Absorption and Toluene Uptake
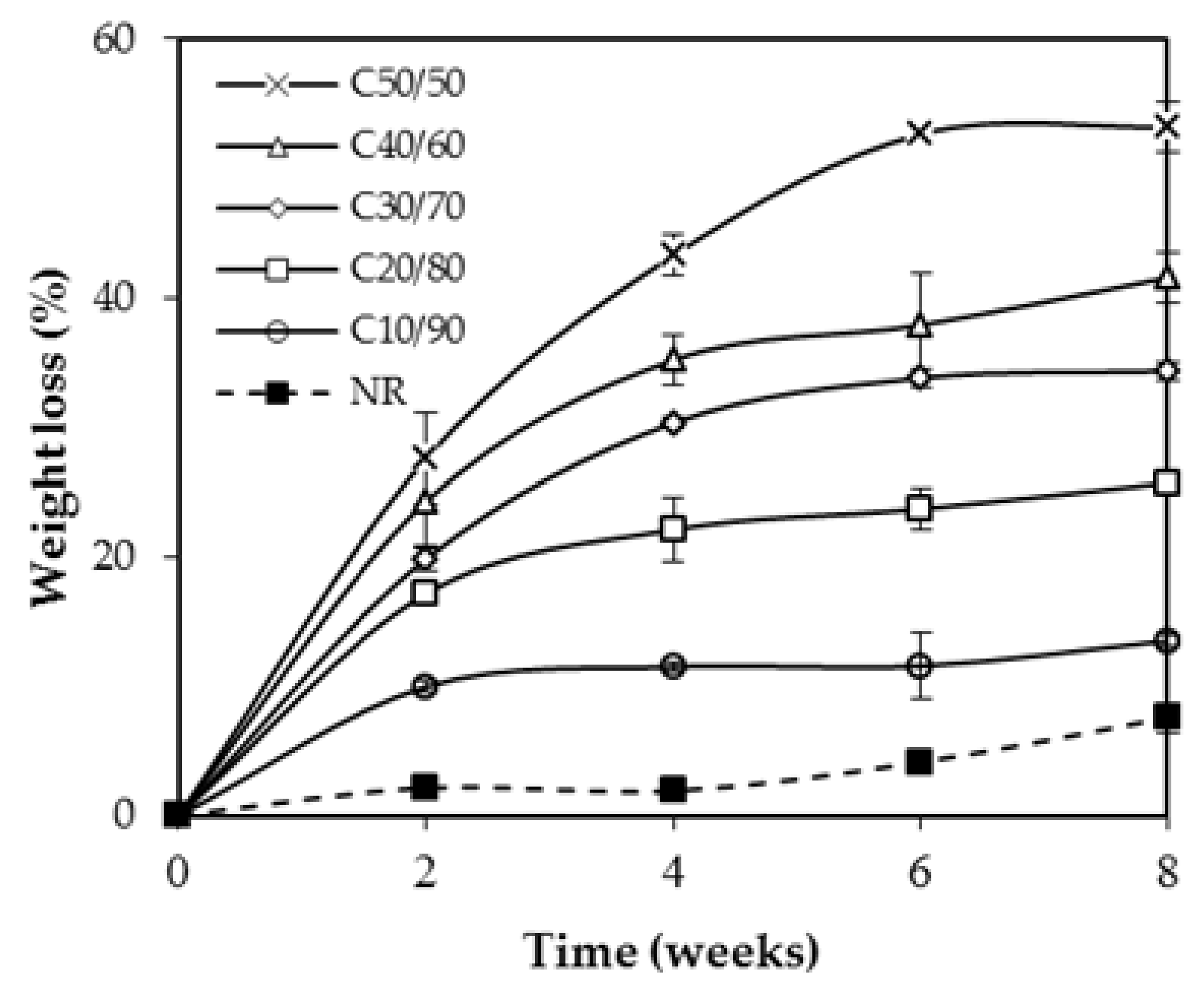
3.6. Biodegradation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Visakh, P.M.; Thomas, S.; Oksman, K.; Mathew, A.P. Crosslinked natural rubber nanocomposites reinforced with cellulose whiskers isolated from bamboo waste: Processing and mechanical/thermal properties. Compos. Part. A Appl. Sci. 2012, 43, 735–741. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Poirier, Y. Establishment of new crops for the production of natural rubber. Trends Biotechnol. 2007, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Phiriyawirut, M.; Chotirat, N.; Phromsiri, S.; Lohapaisarn, I. Preparation and Properties of Natural Rubber—Cellulose Microfibril Nanocomposite Films. Adv. Mat. Res. 2010, 93–94, 328–331. [Google Scholar] [CrossRef]
- Rajisha, K.R.; Maria, H.J.; Pothan, L.A.; Ahmad, Z.; Thomas, S. Preparation and characterization of potato nanocrystal reinforced natural rubber nanocomposites. Int. J. Biol. Macromol. 2014, 67, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, A.; Kaddami, H.; Dufresne, A. Investigation on the effect of cellulosic nanoparticles’ morphology on properties of natural rubber based nanocomposites. Eur. Polym. J. 2010, 46, 609–620. [Google Scholar] [CrossRef]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef]
- Grasland, F.; Chazeau, L.; Chenal, J.-M.; schach, R. About thermo-oxidative ageing at moderate temperature of conventionally vulcanized natural rubber. Polym. Degrad. Stabil. 2019, 161, 74–84. [Google Scholar] [CrossRef]
- Trovatti, E.; Carvalho, A.J.F.; Ribeiro, S.J.L.; Gandini, A. Simple Green Approach to Reinforce Natural Rubber with Bacterial Cellulose Nanofibers. Biomacromolecules 2013, 14, 2667–2674. [Google Scholar] [CrossRef]
- Bai, W.; Li, K. Partial replacement of silica with microcrystalline cellulose in rubber composites. Compos. Part. A Appl. Sci. 2009, 40, 1597–1605. [Google Scholar] [CrossRef]
- Kohjiya, S.; Kato, A.; Ikeda, Y. Visualization of nanostructure of soft matter by 3D-TEM: Nanoparticles in a natural rubber matrix. Prog. Polym. Sci. 2008, 33, 979–997. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. A review on reinforcement of natural rubber by silica fillers for use in low-rolling resistance tyres. J. Rubber Res. 2015, 18, 203–233. [Google Scholar]
- Abraham, E.; Elbi, P.A.; Deepa, B.; Jyotishkumar, P.; Pothen, L.A.; Narine, S.S.; Thomas, S. X-ray diffraction and biodegradation analysis of green composites of natural rubber/nanocellulose. Polym. Degrad. Stabil. 2012, 97, 2378–2387. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Soheilmoghaddam, M.; Uzir Wahit, M.; Ibrahim Akos, N. Regenerated cellulose/epoxidized natural rubber blend film. Mater. Lett. 2013, 111, 221–224. [Google Scholar] [CrossRef]
- Bonini, C.; Heux, L.; Cavaillé, J.-Y.; Lindner, P.; Dewhurst, C.; Terech, P. Rodlike Cellulose Whiskers Coated with Surfactant: A Small-Angle Neutron Scattering Characterization. Langmuir 2002, 18, 3311–3314. [Google Scholar] [CrossRef]
- Andresen, M.; Johansson, L.-S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Taokaew, S.; Phisalaphong, M.; Newby, B.M.Z. Modification of bacterial cellulose with organosilanes to improve attachment and spreading of human fibroblasts. Cellulose 2015, 22, 2311–2324. [Google Scholar] [CrossRef]
- Martins, M.A.; Forato, L.A.; Mattoso, L.H.C.; Colnago, L.A. A solid state 13C high resolution NMR study of raw and chemically treated sisal fibers. Carbohydr. Polym. 2006, 64, 127–133. [Google Scholar] [CrossRef]
- Phomrak, S.; Phisalaphong, M. Reinforcement of natural rubber with bacterial cellulose via a latex aqueous microdispersion Process. J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Agrebi, F.; Ghorbel, N.; Bresson, S.; Abbas, O.; Kallel, A. Study of nanocomposites based on cellulose nanoparticles and natural rubber latex by ATR/FTIR spectroscopy: The impact of reinforcement. Polym. Compos. 2019, 40, 2076–2087. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Suratago, T.; Taokaew, S.; Kanjanamosit, N.; Kanjanaprapakul, K.; Burapatana, V.; Phisalaphong, M. Development of bacterial cellulose/alginate nanocomposite membrane for separation of ethanol–water mixtures. J. Ind. Eng. Chem. 2015, 32, 305–312. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Wikranvanich, J.; Phisalaphong, M. Synthesis and characterization of natural rubber/coal fly ash composites via latex aqueous microdispersion. J. Mater. Cycles Waste Manag. 2019, 21, 134–144. [Google Scholar] [CrossRef]
- Phomrak, S.; Phisalaphong, M. Lactic acid modified natural rubber–bacterial cellulose composites. Appl. Sci. 2020, 10, 3583. [Google Scholar] [CrossRef]
- Gleadall, A. 9—Mechanical properties of biodegradable polymers for medical applications. In Modelling Degradation of Bioresorbable Polymeric Medical Devices; Pan, J., Ed.; Woodhead Publishing: Sawston, UK; University of Leicester: Leicester, UK, 2015; pp. 163–199. [Google Scholar]
- Shamsuri, A.A.; Azid, K.M.; Ariff, H.A.; Sudari, K.A. Influence of Surface Treatment on Tensile Properties of Low-Density Polyethylene/Cellulose Woven Biocomposites: A Preliminary Study. Polymers 2014, 6, 2345–2356. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.; John, M.; Narine, S.; Thomas, S.; Anandjiwala, R. Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Celluloses 2013, 20, 417–427. [Google Scholar] [CrossRef]
- Bras, J.; Hassan, M.L.; Bruzesse, C.; Hassan, E.A.; El-Wakil, N.A.; Dufresne, A. Mechanical, barrier, and biodegradability properties of bagasse cellulose whiskers reinforced natural rubber nanocomposites. Ind. Crop. Prod. 2010, 32, 627–633. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohyd. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Bettas Ardisson, G.; Tosin, M.; Barbale, M.; Degli-Innocenti, F. Biodegradation of plastics in soil and effects on nitrification activity. A laboratory approach. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Béguin, P. Molecular biology of cellulose degradation. Annu. Rev. Microbiol. 1990, 44, 219–248. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. UK 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sander, M. Biodegradation of Polymeric Mulch Films in Agricultural Soils: Concepts, Knowledge Gaps, and Future Research Directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef] [PubMed]




| Samples | MFC (g) | NR (g) |
|---|---|---|
| NR | 0 | 100 |
| C10/90 | 10 | 90 |
| C20/80 | 20 | 80 |
| C30/70 | 30 | 70 |
| C40/60 | 40 | 60 |
| C50/50 | 50 | 50 |
| Samples | σm (MPa) | E (MPa) | εb (%) | Cr (%) | Tg (°C) | Td (°C) |
|---|---|---|---|---|---|---|
| NR | 1.2 ± 0.1 | 3.3 ± 0.4 | 72.9 ± 4.0 | 0 | −64.7 | 321.4 |
| C10/90 | 1.8 ± 0.0 | 4.4 ± 0.1 | 147.4 ± 11.7 | 1.0 | −64.7 | 259.6 |
| C20/80 | 6.5 ± 0.2 | 9.9 ± 0.5 | 302.7 ± 1.2 | 3.4 | −64.4 | 241.7 |
| C30/70 | 10.4 ± 0.7 | 87.5 ± 4.9 | 313.3 ± 11.7 | 7.1 | −64.2 | 223.1 |
| C40/60 | 10.6 ± 0.3 | 528.7 ± 18.9 | 12.0 ± 1.0 | 10.6 | −64.3 | 167.1 |
| C50/50 | 13.6 ± 0.3 | 1085.7 ± 45.2 | 1.9 ± 0.1 | 14.6 | −63.4 | 123.5 |
| MFC | - | - | - | 23.2 | - | 77.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supanakorn, G.; Varatkowpairote, N.; Taokaew, S.; Phisalaphong, M. Alginate as Dispersing Agent for Compounding Natural Rubber with High Loading Microfibrillated Cellulose. Polymers 2021, 13, 468. https://doi.org/10.3390/polym13030468
Supanakorn G, Varatkowpairote N, Taokaew S, Phisalaphong M. Alginate as Dispersing Agent for Compounding Natural Rubber with High Loading Microfibrillated Cellulose. Polymers. 2021; 13(3):468. https://doi.org/10.3390/polym13030468
Chicago/Turabian StyleSupanakorn, Goragot, Nanthaphak Varatkowpairote, Siriporn Taokaew, and Muenduen Phisalaphong. 2021. "Alginate as Dispersing Agent for Compounding Natural Rubber with High Loading Microfibrillated Cellulose" Polymers 13, no. 3: 468. https://doi.org/10.3390/polym13030468
APA StyleSupanakorn, G., Varatkowpairote, N., Taokaew, S., & Phisalaphong, M. (2021). Alginate as Dispersing Agent for Compounding Natural Rubber with High Loading Microfibrillated Cellulose. Polymers, 13(3), 468. https://doi.org/10.3390/polym13030468