Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Isothermal Differential Scanning Photocalorimetry (Photo-DSC)
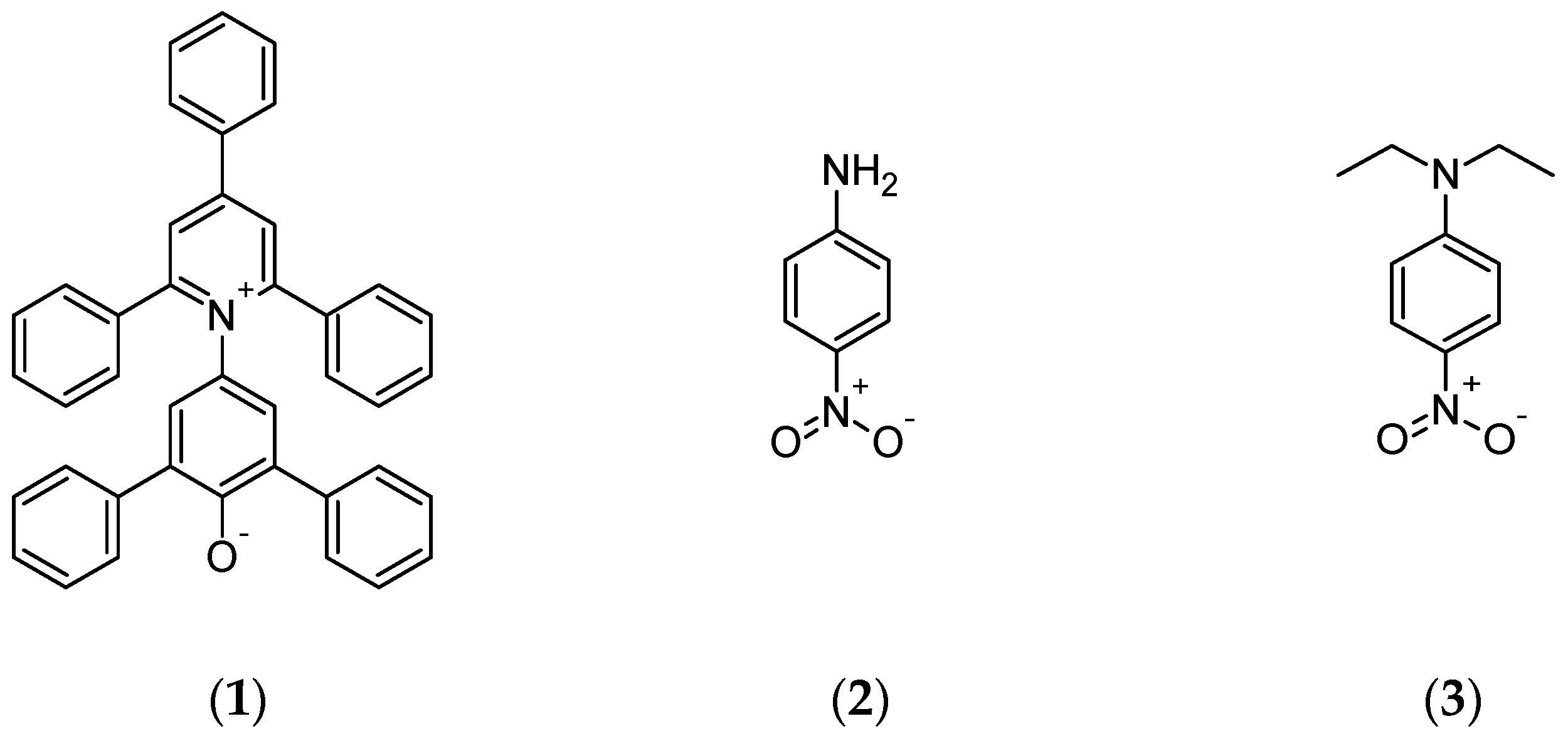
2.2.2. Solvatochromic Solvent Parameters
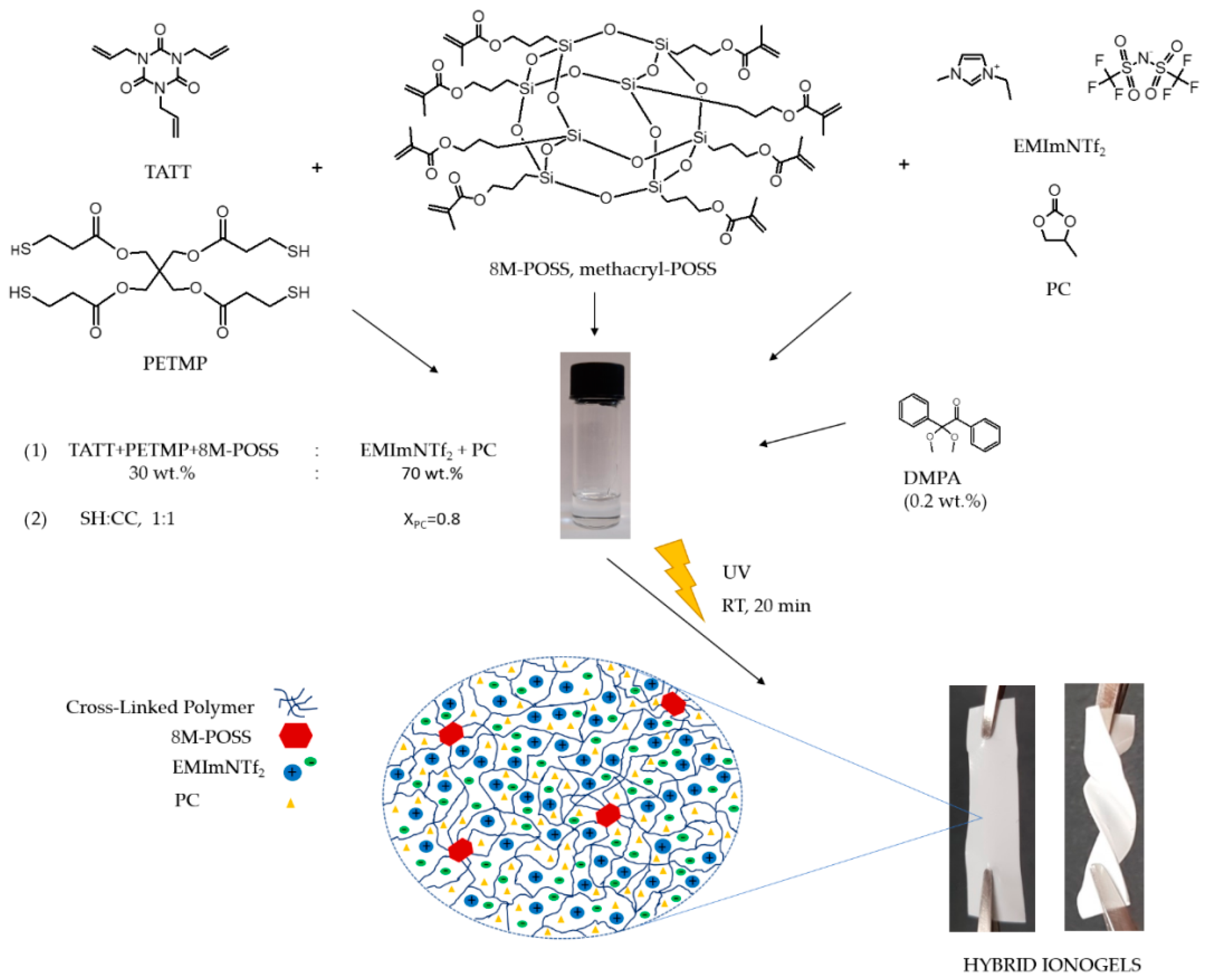
2.2.3. Ionogels Samples Preparation
2.2.4. Scanning Electron Microscope (SEM)
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. TGA
2.2.7. Puncture Resistance
2.2.8. Ionic Conductivity
2.2.9. Electrochemical Measurements of Capacitors
Preparation of Electrodes
Electrochemical Investigations
3. Results and Discussion
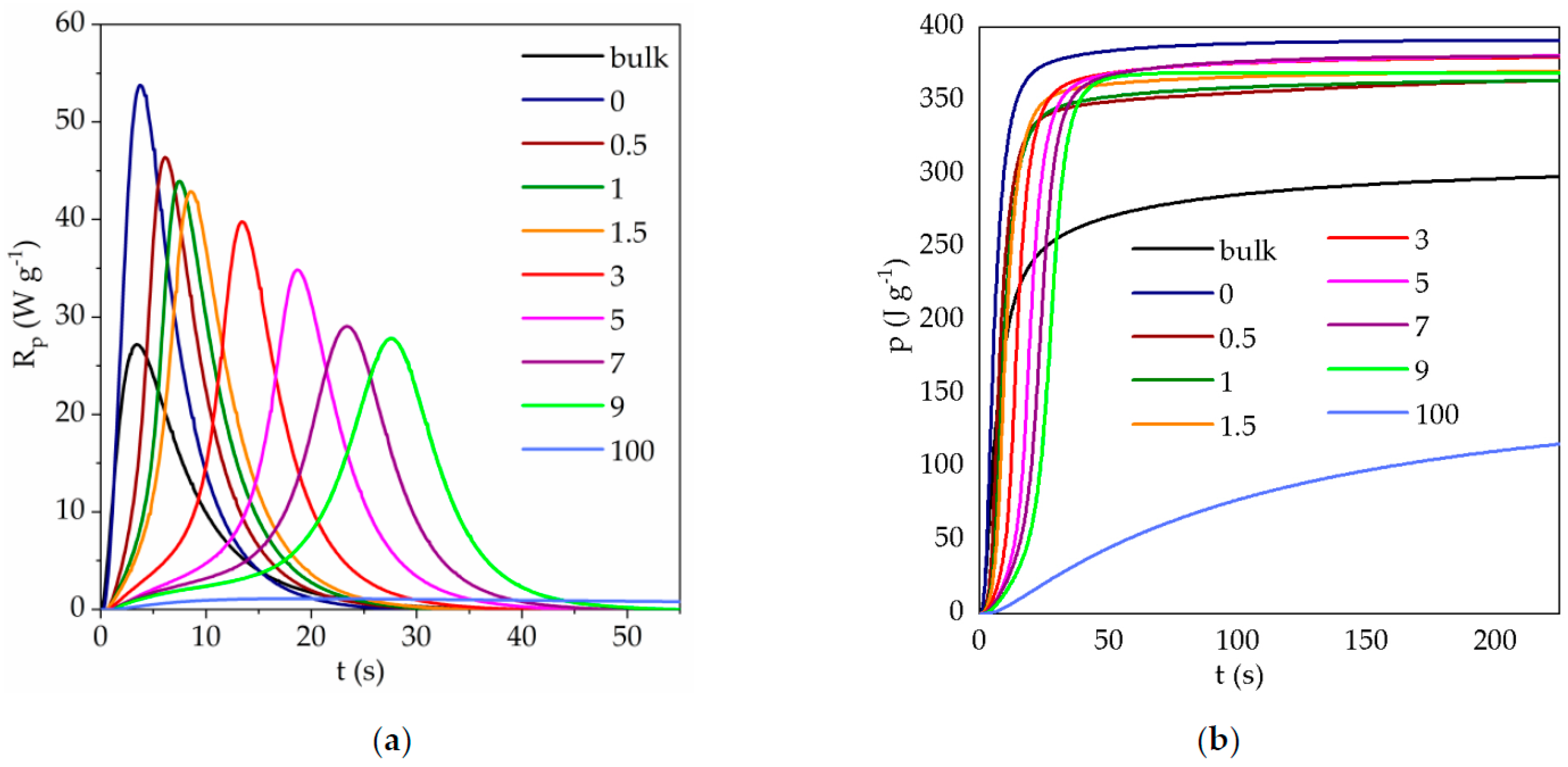
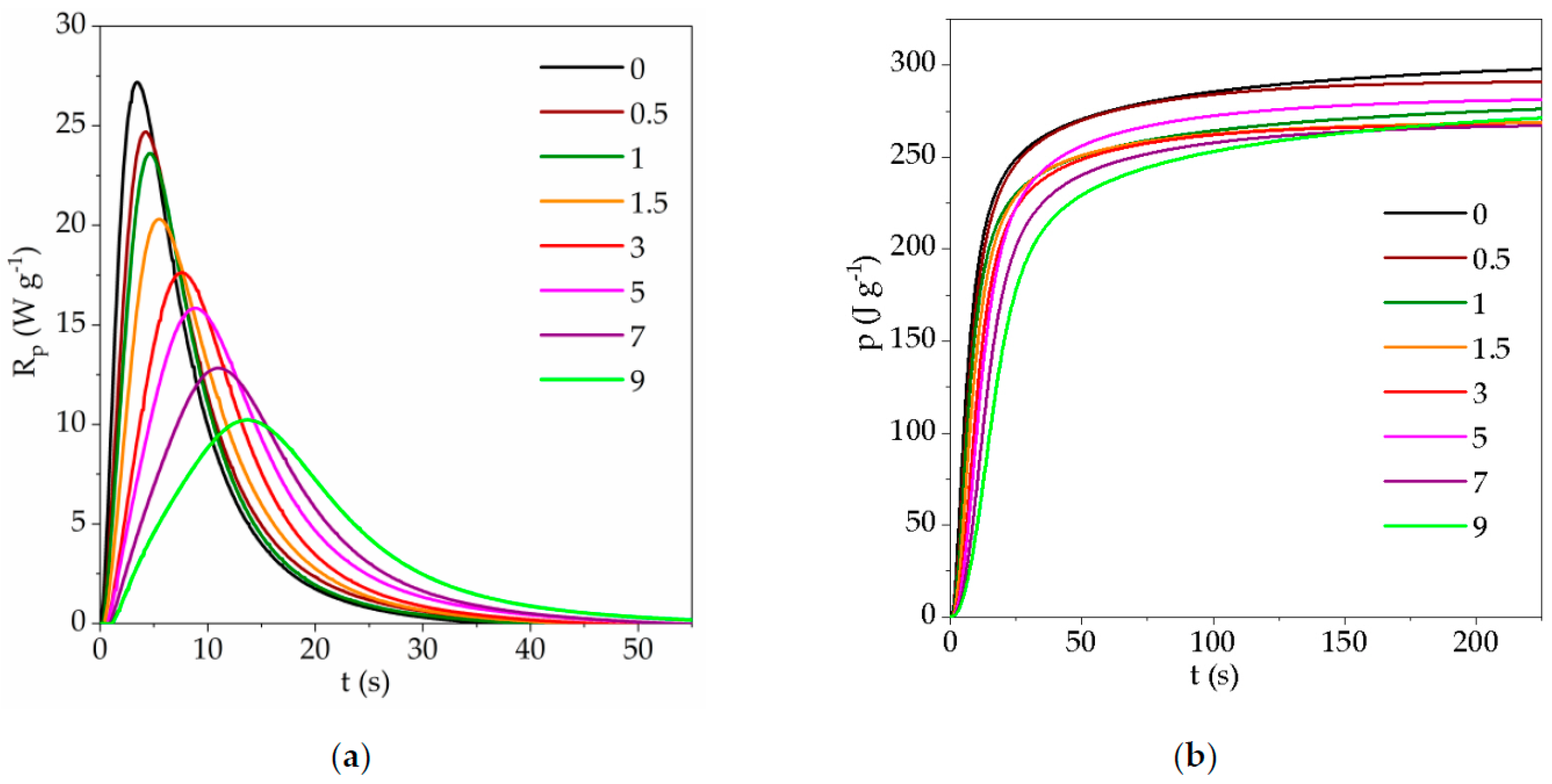
3.1. Photopolymerization Kinetics
3.2. Ionogels Morphology
3.3. Thermal and Mechanical Properties
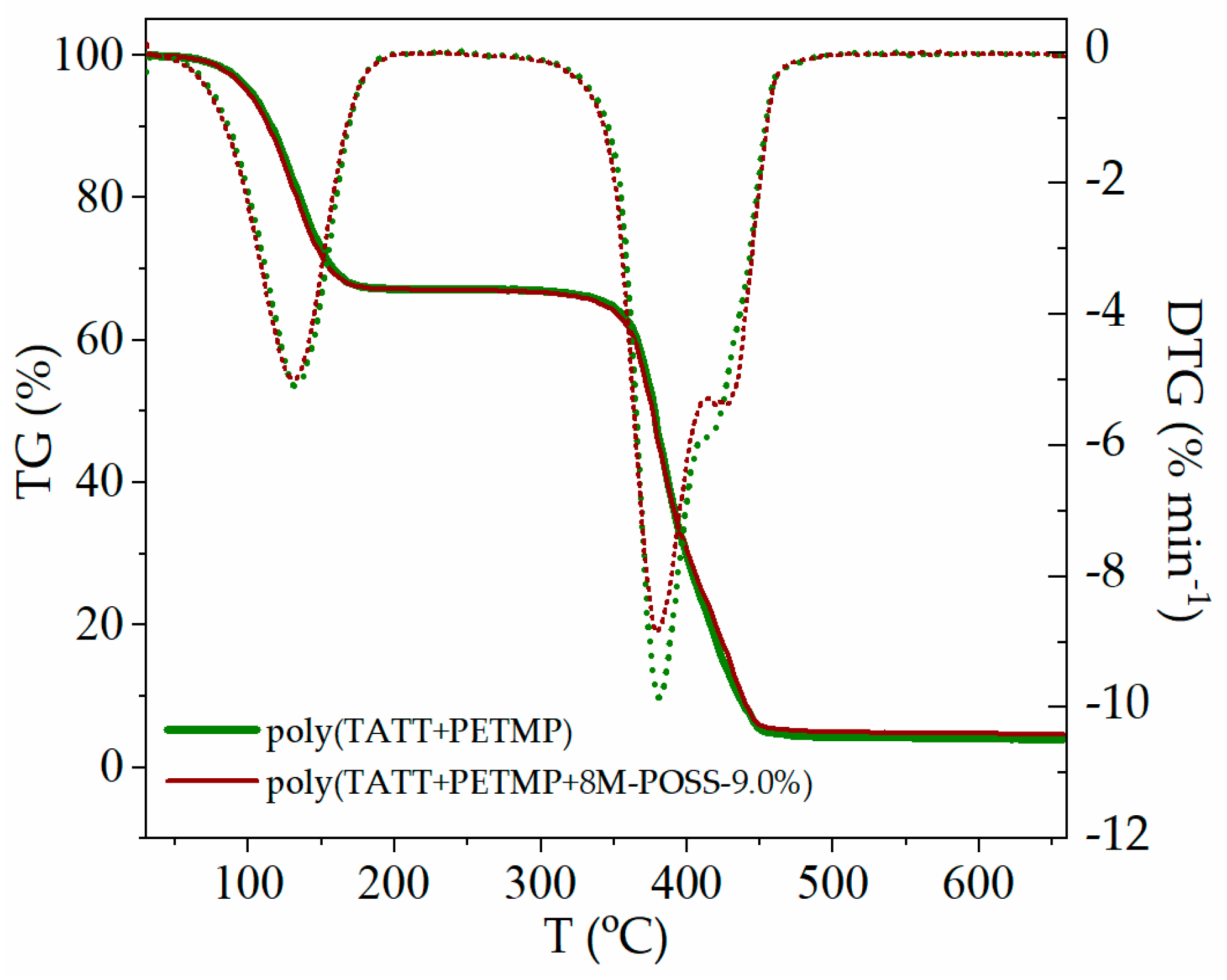
3.3.1. Thermal Properties
3.3.2. Mechanical Properties
3.4. Electrochemical Investigation
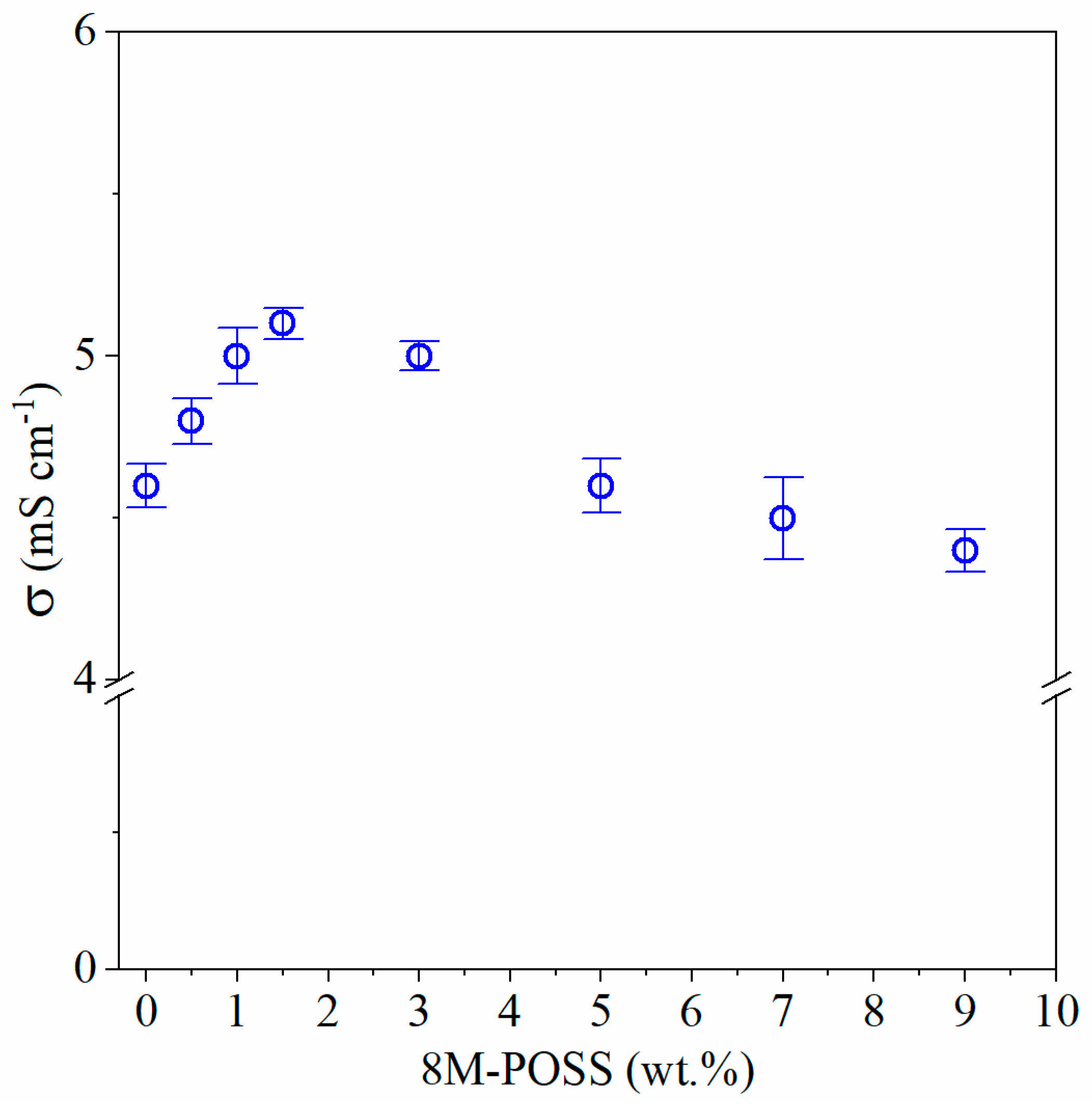
3.4.1. Ionic Conductivity
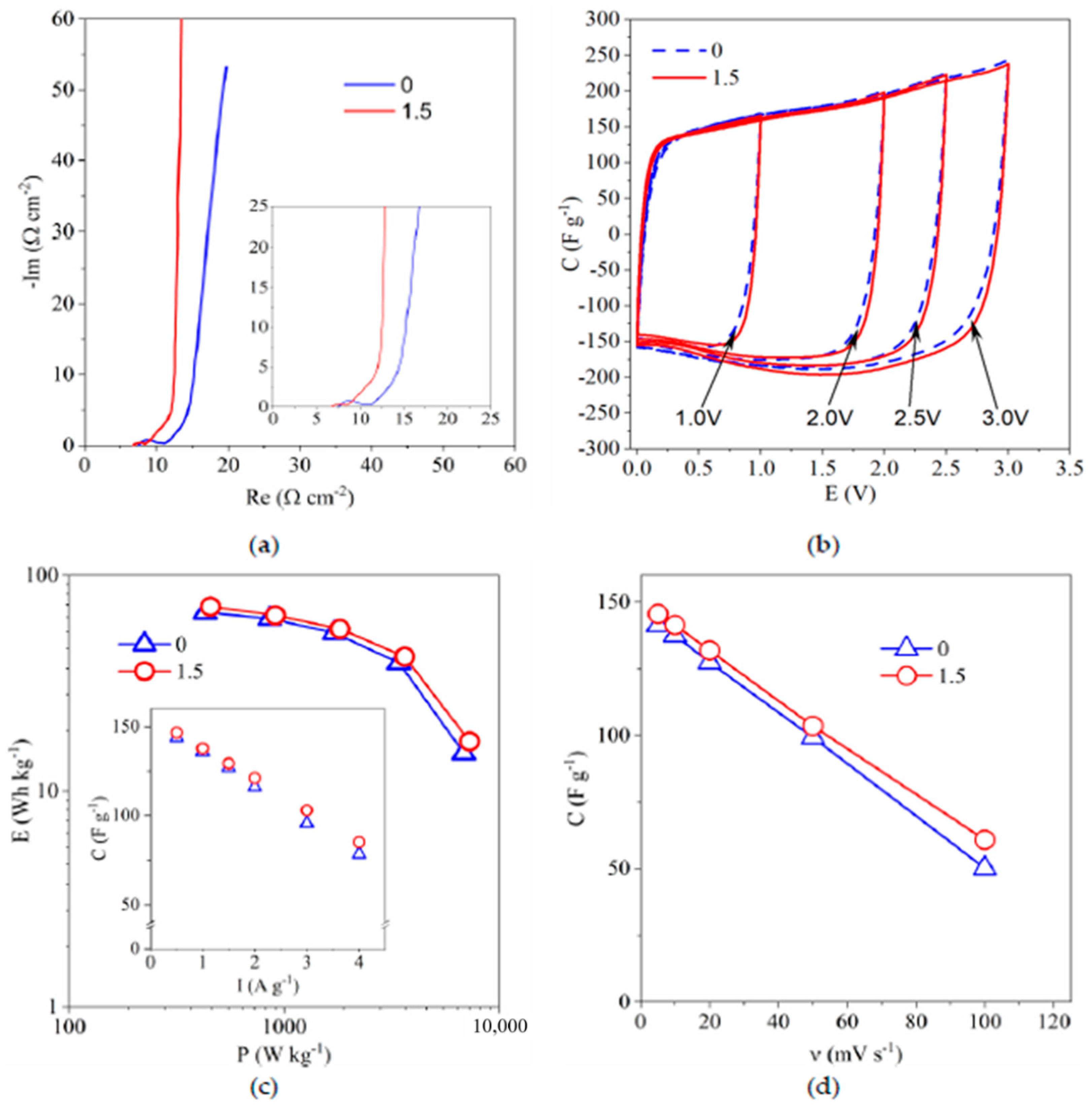
3.4.2. Electrochemical Capacitor Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. Eng. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Sadej, M.; Andrzejewska, E. Silica/aluminum oxide hybrid as a filler for photocurable composites. Prog. Org. Coat. 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Prządka, D.; Marcinkowska, A.; Andrzejewska, E. POSS-modified UV-curable coatings with improved scratch hardness and hydrophobicity. Prog. Org. Coat. 2016, 100, 165–172. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Peng, H.; Guo, M.; Feng, Y.; Xue, Z.; Ye, Y.; Xie, X. Flexible Organic–Inorganic Hybrid Solid Electrolytes Formed via Thiol–Acrylate Photopolymerization. Macromolecules 2017, 50, 1970–1980. [Google Scholar] [CrossRef]
- Sadej, M.; Andrzejewska, E.; Kurc, B.; Gojzewski, H.; Jesionowski, T. Surface-dependent effect of functional silica fillers on photocuring kinetics of hydrogel materials. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3472–3487. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Marcinkowska, A.; Zgrzeba, A. Ionogels—Materials containing immobilized ionic liquids. Polimery 2017, 62, 344–352. [Google Scholar] [CrossRef]
- Cramer, N.B.; Bowman, C.N. CHAPTER 1. Thiol-ene and Thiol-yne Chemistry in Ideal Network Synthesis. In Thiol-X Chemistries in Polymer and Materials Science; Lowe, A., Bowman, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 1–27. [Google Scholar]
- Cramer, N.B.; Couch, C.L.; Schreck, K.M.; Boulden, J.E.; Wydra, R.; Stansbury, J.W.; Bowman, C.N. Properties of methacrylate-thiol-ene formulations as dental restorative materials. Dent. Mater. 2010, 26, 799–806. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Wang, X.; Song, L.; Hu, Y.; Lo, S. Enhanced thermal and mechanical properties of functionalized graphene/thiol-ene systems by photopolymerization technology. Chem. Eng. J. 2013, 228, 318–326. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Zgrzeba, A.; Lota, G.; Kopczyński, K.; Andrzejewska, E. Ionogels by thiol-enephotopolymerization in ionic liquids: Formation, morphology and properties. Polymer 2019, 160, 272–281. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photoinitiated polymerization in ionic liquids and its application. Polym. Int. 2017, 66, 366–381. [Google Scholar] [CrossRef]
- Markovic, E.; Constantopolous, K.; Matisons, J.G. Polyhedral oligomericsilsesquioxanes: From early and strategic development through to materials application. In Adcance in Silicone Science—Application of Polyhedral OligomericSilsesquioxanes; Matisons, J.G., Hartmann-Thompson, C., Eds.; Springer Science + Buisnes Media B.V.: New York, NY, USA, 2011; pp. 1–4. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Rico, M.; Torres, A.; Barral, L.; López, J.; Montero, B. Epoxy/POSS organic–inorganic hybrids: ATR-FTIR and DSC studies. Eur. Polym. J. 2008, 44, 3035–3045. [Google Scholar] [CrossRef]
- Kim, D.-G.; Sohn, H.-S.; Kim, S.-K.; Lee, A.; Lee, J.-C. Star-shaped polymers having side chain poss groups for solid polymer electrolytes; synthesis, thermal behavior, dimensional stability, and ionic conductivity. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3618–3627. [Google Scholar] [CrossRef]
- Lin, H.-M.; Wu, S.-Y.; Chang, F.-C.; Yen, Y.-C. Photo-polymerization of photocurable resins containing polyhedral oligomericsilsesquioxane methacrylate. Mater. Chem. Phys. 2011, 131, 393–399. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Prządka, D.; Andrzejewska, E.; Marcinkowska, A. Multimethacryloxy-POSS as a crosslinker for hydrogel materials. Eur. Polym. J. 2015, 72, 34–49. [Google Scholar] [CrossRef]
- Luo, A.; Jiang, X.; Lin, H.; Yin, J. “Thiol-ene” photo-cured hybrid materials based on POSS and renewable vegetable oil. J. Mater. Chem. 2011, 21, 12753–12760. [Google Scholar] [CrossRef]
- Fang, Y.; Ha, H.; Shanmuganathan, K.; Ellison, C.J. Polyhedral OligomericSilsesquioxane-Containing Thiol-ene Fibers with Tunable Thermal and Mechanical Properties. ACS Appl. Mater. Interfaces 2016, 8, 11050–11059. [Google Scholar] [CrossRef]
- Lin, H.-M.; Wu, S.-Y.; Huang, P.-Y.; Huang, C.-F.; Kuo, S.-W.; Chang, F.-C. Polyhedral OligomericSilsesquioxane Containing Copolymers for Negative-Type Photoresists. Macromol. Rapid Commun. 2006, 27, 1550–1555. [Google Scholar] [CrossRef]
- Farooq, S.; Razzaq, H.; Razzaque, S.; Khan, B.A.; Qaisar, S. Structural and physical impacts of nanofillers in ionogels: A comprehensive overview. Polym. Compos. 2018, 12, E11–E23. [Google Scholar] [CrossRef]
- Gayet, F.; Viau, L.; Leroux, F.; Monge, S.; Robin, J.-J.; Vioux, A. Polymer nanocompositeionogels, high-performance electrolyte membranes. J. Mater. Chem. 2010, 20, 9456–9462. [Google Scholar] [CrossRef]
- Pan, Q.; Smith, D.M.; Qi, H.; Wang, S.; Li, C.Y. Hybrid Electrolytes with Controlled Network Structures for Lithium Metal Batteries. Adv. Mater. 2015, 27, 5995–6001. [Google Scholar] [CrossRef]
- Kim, D.-G.; Shim, J.; Lee, J.H.; Kwon, S.-J.; Baik, J.-H.; Lee, J.-C. Preparation of solid-state composite electrolytes based on organic/inorganic hybrid star-shaped polymer and PEG-functionalized POSS for all-solid-state lithium battery applications. Polymer 2013, 54, 5812–5820. [Google Scholar] [CrossRef]
- Balducci, A. Electrolytes for high voltage electrochemical double layer capacitors: A perspective article. J. Power Sources 2016, 326, 534–540. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The Solvatochromic Comparison Method. I. The β-Scale of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The α-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The .π* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Reichardt, C. Polarity of ionic liquids determined empirically by means of solvatochromicpyridinium N-phenolatebetaine dyes. Green Chem. 2005, 7, 339–351. [Google Scholar] [CrossRef]
- Lee, T.Y.; Bowman, C.N. The effect of functionalized nanoparticles on thiol-ene polymerization kinetics. Polymer 2006, 47, 6057–6065. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Guo, R.; Zhou, H.; Li, Z.; Chen, G.; Zhou, Z.; Li, Q. Preparation of novel UV-cured methacrylate hybrid materials with high thermal stability via thiol–enephotopolymerization. J. Mater. Sci. 2019, 54, 5877–5897. [Google Scholar] [CrossRef]
- Vogl, T.; Menne, S.; Balducci, A. Mixtures of protic ionic liquids and propylene carbonate as advanced electrolytes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 25014–25023. [Google Scholar] [CrossRef]
- Levy, N.R.; Lifshits, S.; Yohanan, E.; Ein-Eli, Y. Hybrid Ionic Liquid Propylene Carbonate-Based Electrolytes for Aluminum–Air Batteries. ACS Appl. Energy Mater. 2020, 3, 2585–2592. [Google Scholar] [CrossRef]
- Cramer, N.B.; Bowman, C.N. Kinetics of thiol-ene and thiol-acrylate photopolymerizations with real-time fourier transform infrared. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3311–3319. [Google Scholar] [CrossRef]
- Wei, H.; Senyurt, A.F.; Jönsson, S.; Hoyle, C.E. Photopolymerization of ternary thiol–ene/acrylate systems: Film and network properties. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 822–829. [Google Scholar] [CrossRef]
- Cramer, N.B.; Scott, A.J.P.; Bowman, C.N. Photopolymerizations of Thiol-Ene Polymers without Photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Zgrzeba, A.; Andrzejewska, E.; Marcinkowska, A. Ionic liquid—Containing ionogels by thiol-enephotopolymerization. Kinetics and solvent effect. RSC Adv. 2015, 5, 100354–100361. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Swift, L.; Hallett, J.P.; Welton, T.; Coutinho, J.A.P.; Freire, M.G. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 6593–6601. [Google Scholar] [CrossRef]
- Tronche, C.; Martinez, F.N.; Horner, J.H.; Newcomb, M.; Senn, M.; Giese, B. Polar substituent and solvent effects on the kinetics of radical reactions with thiols. Tetrahedron Lett. 1996, 37, 5845–5848. [Google Scholar] [CrossRef]
- Ye, S.; Cramer, N.B.; Smith, I.R.; Voigt, K.R.; Bowman, C.N. Reaction Kinetics and Reduced Shrinkage Stress of Thiol-Yne-Methacrylate and Thiol-Yne-Acrylate Ternary Systems. Macromolecules 2011, 44, 9084–9090. [Google Scholar] [CrossRef] [PubMed]
- Sodul, E.D. Dispersion polymerization. In Polymeric Dispersions: Principles and Applications; Asua, J.M., Ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 141–153. [Google Scholar]
- Wang, C.; Podgórski, M.; Bowman, C.N. Monodisperse functional microspheres from step-growth “click” polymerizations: Preparation, functionalization and implementation. Mater. Horiz. 2014, 1, 535–539. [Google Scholar] [CrossRef]



| Formulation | PETMP | TATT | 8M-POSS |
|---|---|---|---|
| wt.% | |||
| 8M-POSS-0% | 59.5 | 40.5 | 0 |
| 8M-POSS-0.5% | 59.3 | 40.2 | 0.5 |
| 8M-POSS-1.0% | 59.1 | 39.9 | 1.0 |
| 8M-POSS-1.5% | 58.9 | 39.6 | 1.5 |
| 8M-POSS-3.0% | 58.4 | 38.6 | 3.0 |
| 8M-POSS-5.0% | 57.6 | 37.4 | 5.0 |
| 8M-POSS-7.0% | 56.8 | 36.2 | 7.0 |
| 8M-POSS-9.0% | 56.0 | 35.0 | 9.0 |
| Formulation | Particle Size, nm |
|---|---|
| 8M-POSS-0% | 165 ± 15 |
| 8M-POSS-0.5% | 141 ± 13 |
| 8M-POSS-1.5% | 128 ± 12 |
| 8M-POSS-5.0% | 60 ± 6.1 |
| 8M-POSS-7.0% | 46 ± 4.5 |
| Investigated Materials | Tg, °C | T5%, °C | T10%, °C | |
|---|---|---|---|---|
| matrix | poly(TATT+PETMP) | 35.1 ± 0.77 | 360.2 | 368.3 |
| ionogels poly(TATT + PETMP + 8M-POSS) with 70 wt.% of EMImNTf2 + PC | 8M-POSS-0% | 13.4 ± 1.1 | 100.1 | 115.1 |
| 8M-POSS-0.5% | 14.2 ± 1.2 | 103.5 | 117.6 | |
| 8M-POSS-1.0% | 15.3 ± 0.75 | 103.1 | 117.1 | |
| 8M-POSS-1.5% | 16.3 ± 0.60 | 101.0 | 116.2 | |
| 8M-POSS-3.0% | 16.7 ± 0.79 | 98.2 | 112.8 | |
| 8M-POSS-5.0% | 16.0 ± 0.74 | 100.2 | 114.6 | |
| 8M-POSS-7.0% | 16.1 ± 0.50 | 100.1 | 114.7 | |
| 8M-POSS-9.0% | 16.5 ± 0.42 | 99.4 | 113.9 | |
| Formulation | Fmax, g | εmax, mm |
|---|---|---|
| 8M-POSS-0% | 247 ± 9.1 | 4.1 ± 0.21 |
| 8M-POSS-0.5% | 304 ± 12 | 4.3 ± 0.08 |
| 8M-POSS-1.0% | 308 ± 3.9 | 4.1 ± 0.13 |
| 8M-POSS-1.5% | 322 ± 6.4 | 4.2 ± 0.15 |
| 8M-POSS-3.0% | 331 ± 7.6 | 4.3 ± 0.15 |
| 8M-POSS-5.0% | 336 ± 14 | 4.4 ± 0.14 |
| 8M-POSS-7.0% | 305 ± 6.4 | 4.0 ± 0.07 |
| 8M-POSS-9.0% | 264 ± 8.0 | 3.9 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Sadej, M.; Patelski, P.; Marcinkowska, A. Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane. Polymers 2021, 13, 385. https://doi.org/10.3390/polym13030385
Lewandowska A, Gajewski P, Szcześniak K, Sadej M, Patelski P, Marcinkowska A. Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane. Polymers. 2021; 13(3):385. https://doi.org/10.3390/polym13030385
Chicago/Turabian StyleLewandowska, Aneta, Piotr Gajewski, Katarzyna Szcześniak, Mariola Sadej, Piotr Patelski, and Agnieszka Marcinkowska. 2021. "Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane" Polymers 13, no. 3: 385. https://doi.org/10.3390/polym13030385
APA StyleLewandowska, A., Gajewski, P., Szcześniak, K., Sadej, M., Patelski, P., & Marcinkowska, A. (2021). Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane. Polymers, 13(3), 385. https://doi.org/10.3390/polym13030385










