Fabrication and Characterization of Human Serum Albumin Particles Loaded with Non-Sericin Extract Obtained from Silk Cocoon as a Carrier System for Hydrophobic Substances
Abstract
1. Introduction
2. Materials and Methods
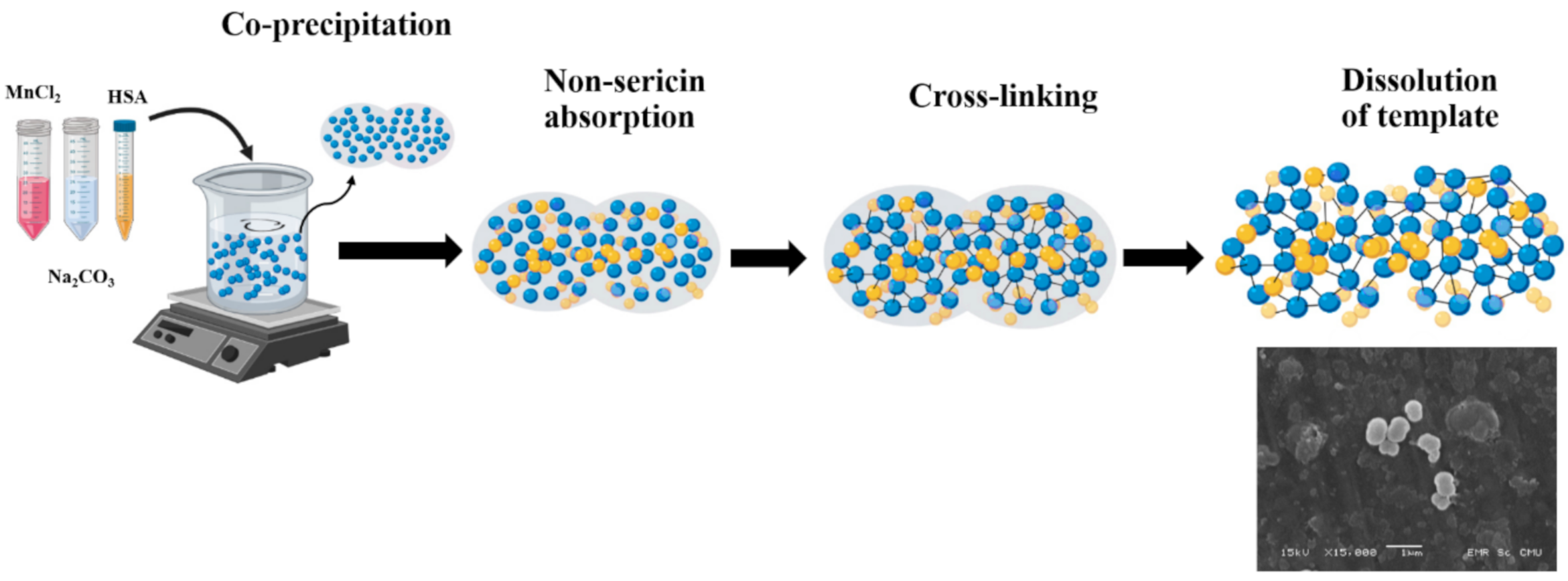
2.1. Preparation of Non-Sericin Loaded Human Serum Albumin Micro Particles (NS-HSA MPs)
2.2. Characterization of Non-Sericin Loaded Human Serum Albumin Micro Particles (NS-HSA MPs)
2.2.1. Size, Polydispersity Index (PDI), and Zeta Potential (ZP) of NS-HSA MPs
2.2.2. Human Serum Albumin Micro Particles (HSA-MPs) and Non-Sericin Loaded Human Serum Albumin Micro Particle (NS-HSA MPs) Morphology
2.3. Entrapment Efficiency of Non-Sericin Loaded Human Serum Albumin Micro Particles (NS-HSA MPs)
2.3.1. Entrapment efficiency of NS-HSA MPs
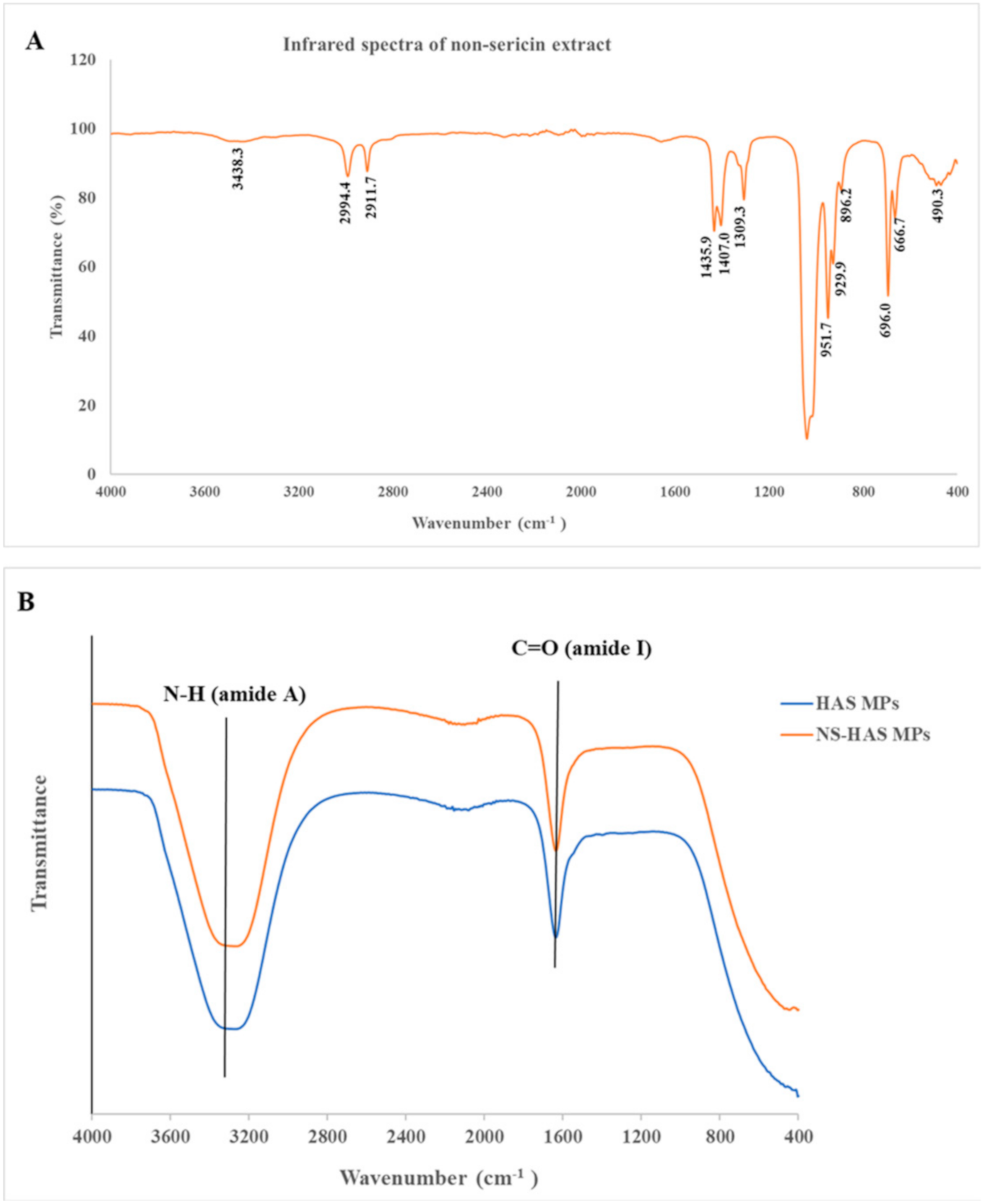
2.3.2. Fourier Trnsformed Infrared Spectroscopy (FTIR) Study
2.4. Uptake of Non-Sericin Loaded Human Serum Albumin Micro Particle (NS-HSA MPs) in A549 Cell Line
2.4.1. Cultivation of Cell Line
2.4.2. Analysis of Cellular Uptake by Flow Cytometric Analysis
2.4.3. Determination of Cellular Uptake by Confocal Laser Scanning Microscopy (CLSM)
2.4.4. Analysis of Three-Dimensional Localization of Particles by 3D Holotomography (HT) Microscopy
2.5. Effect of Particles on Macrophage Stimulation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Particle Preparation
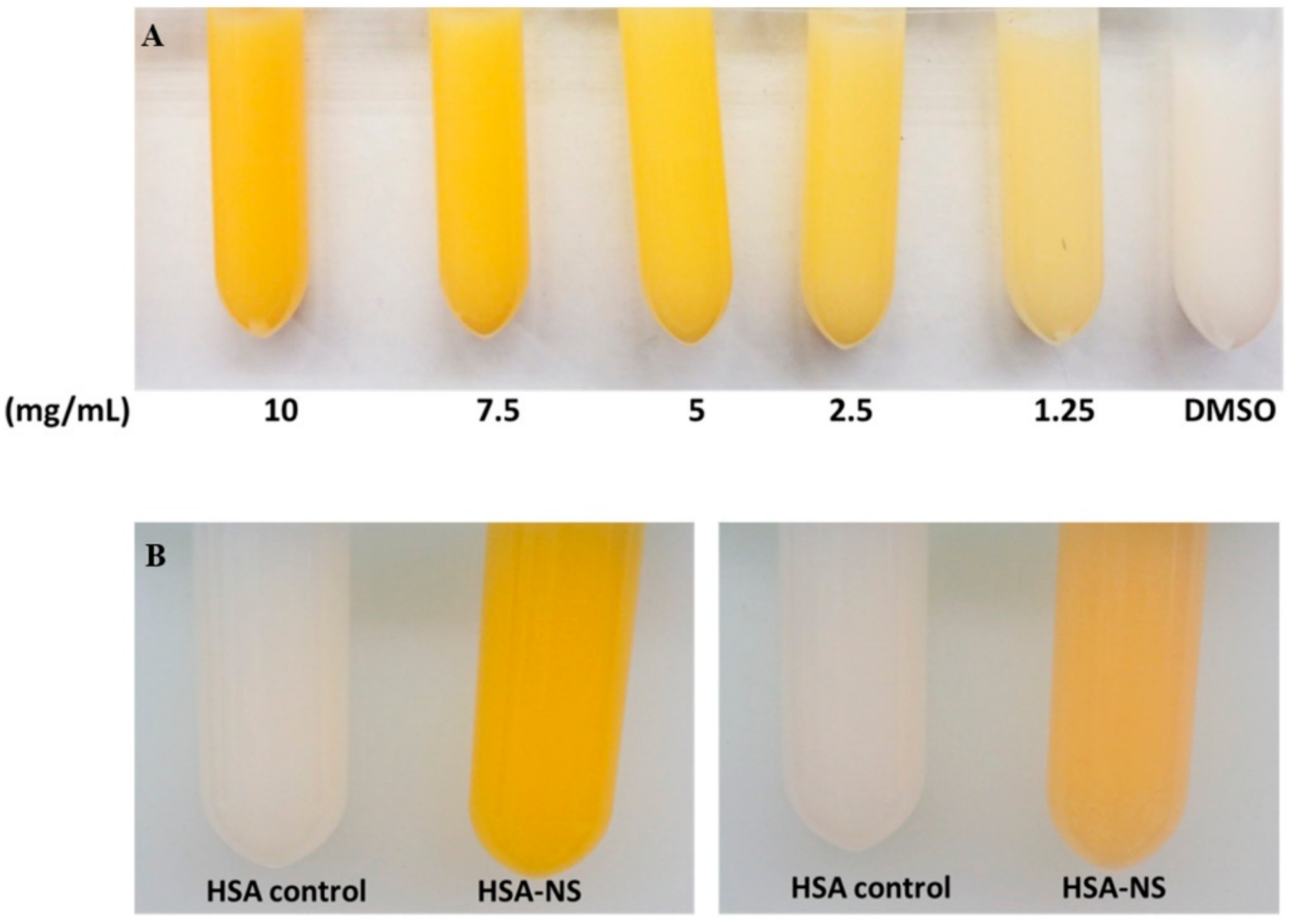
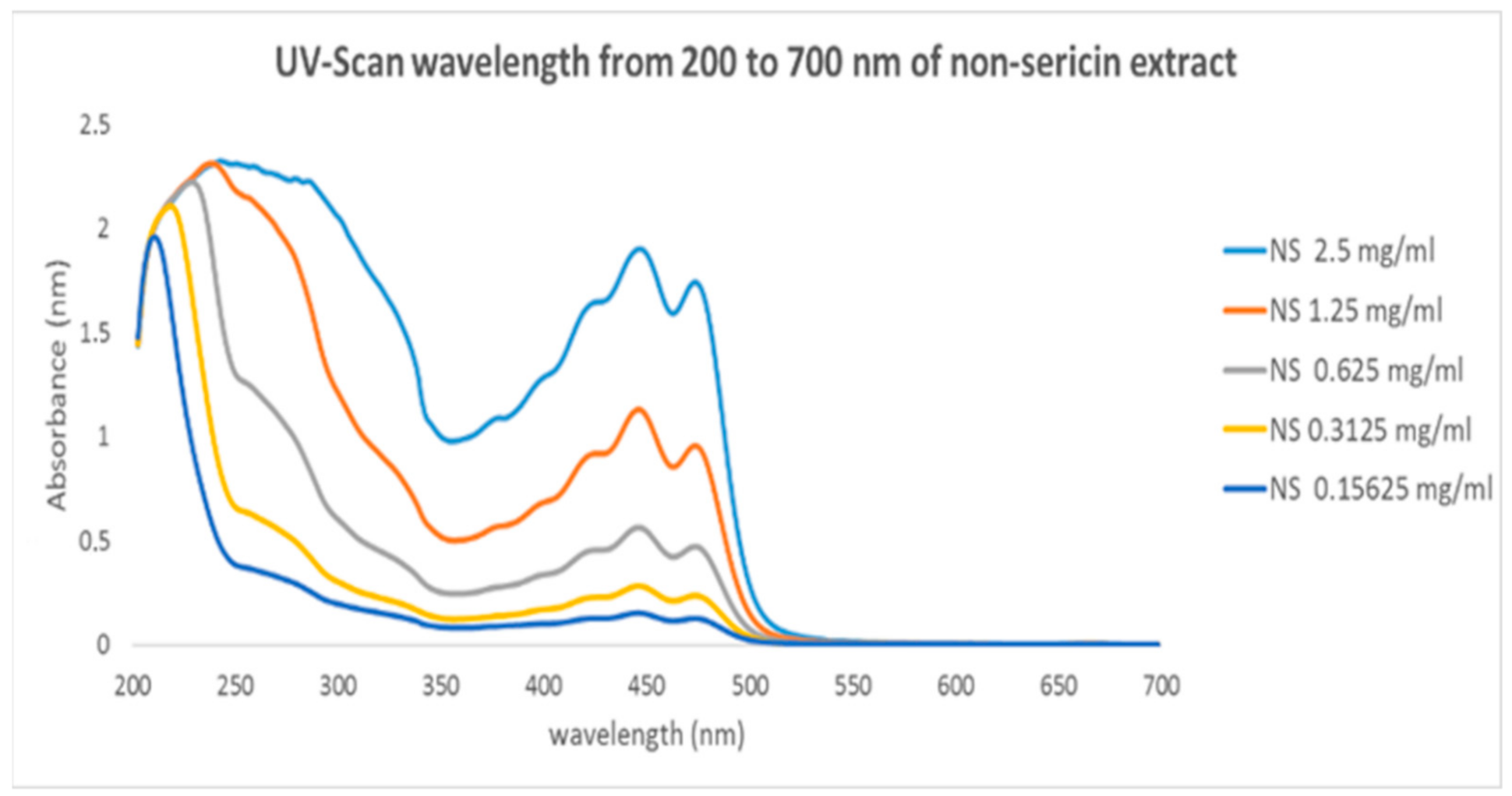
3.2. Characterization of Non-Sericin Loaded Human Serum Albumin Micro Particles (NS-HSA MPs)
3.3. Determination of Cellular Uptake of NS-HSA MPs and HSA MPs
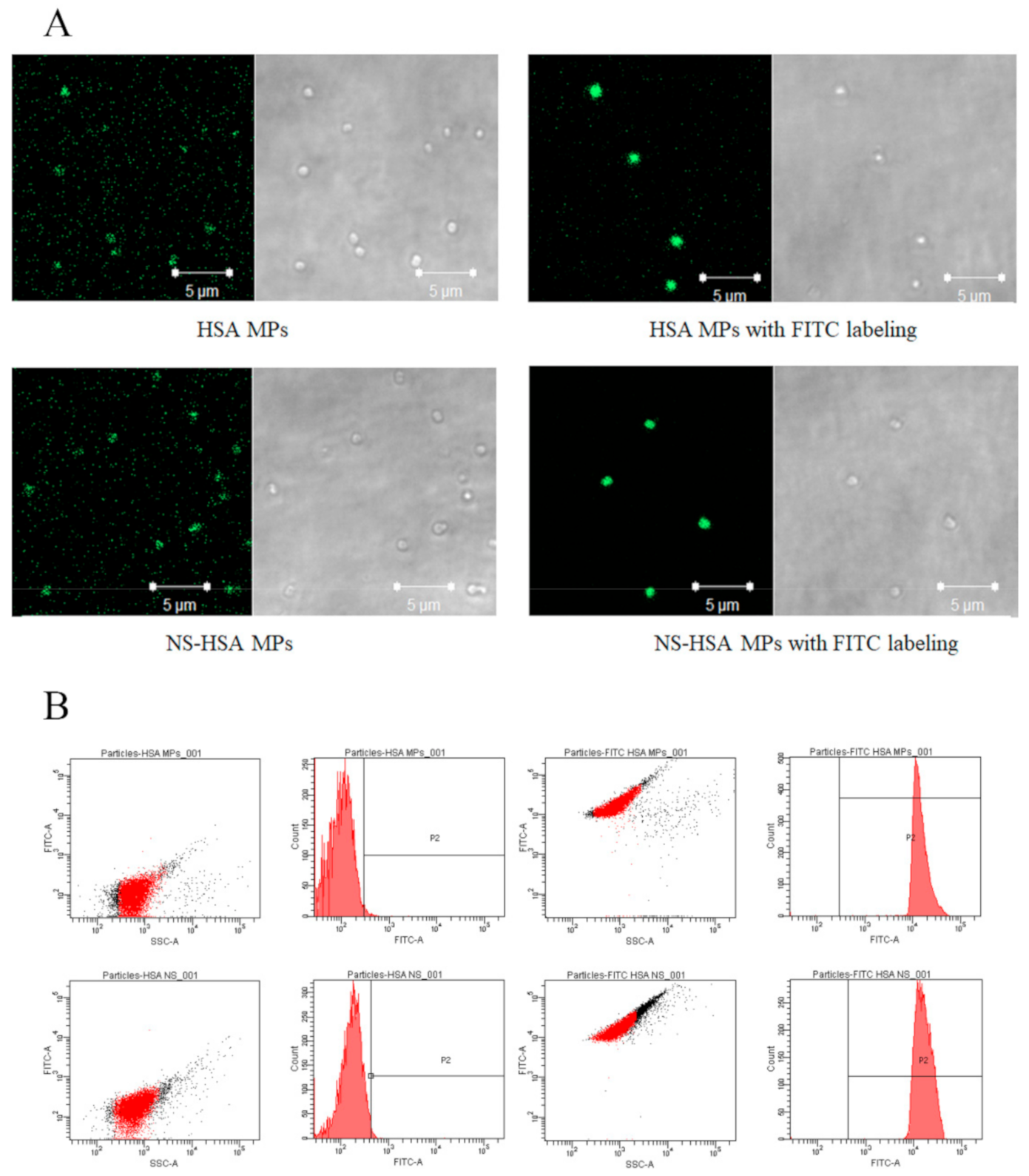
3.3.1. FITC-Labeling HSA MPs and NS-HSA MPs
3.3.2. Determination of Cellular Uptake of NS-HSA MPs by Flow Cytometry
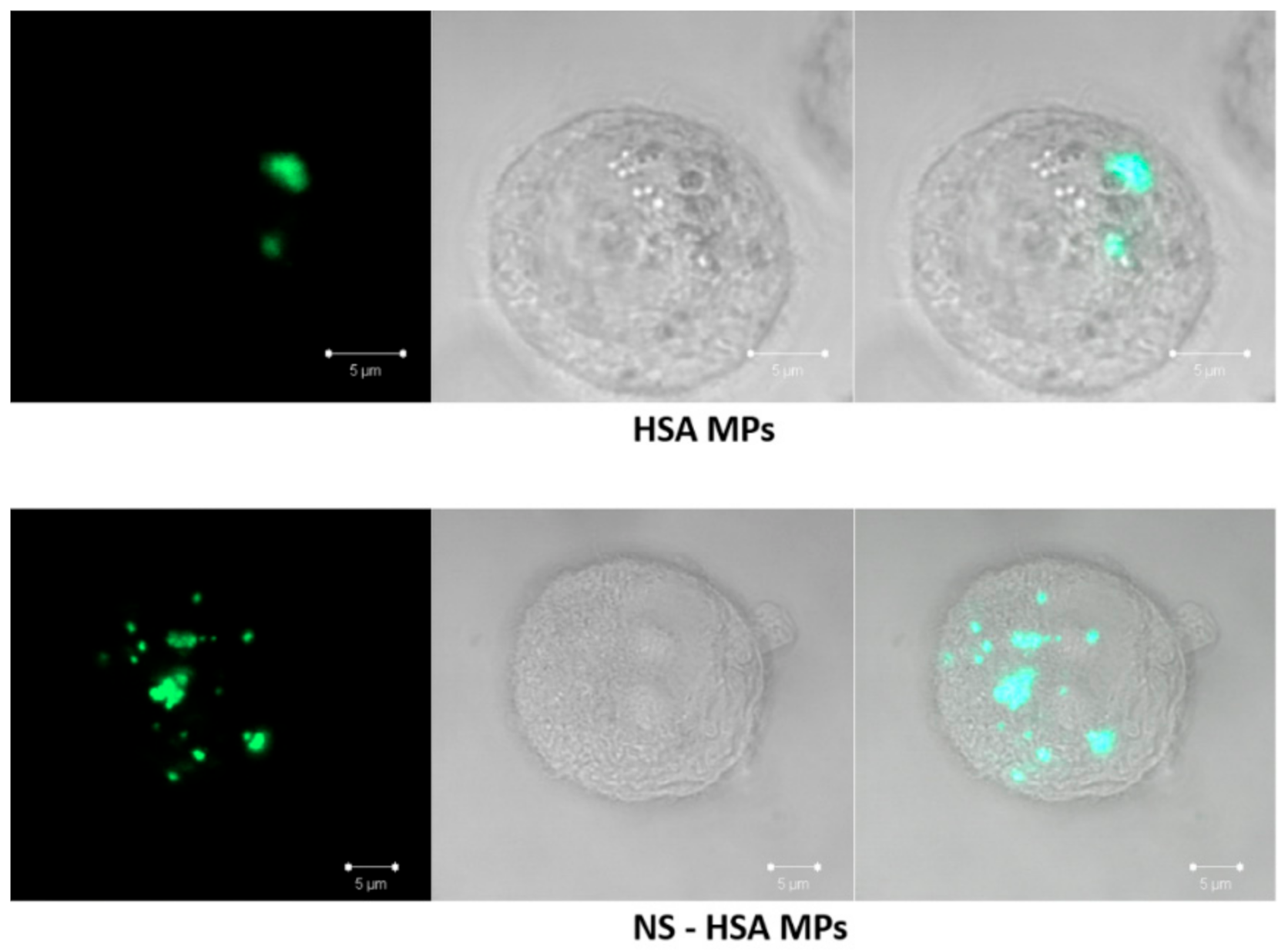
3.3.3. Determination of Cellular Uptake of NS-HSA MPs by Confocal Laser Scanning microscope (CLSM)
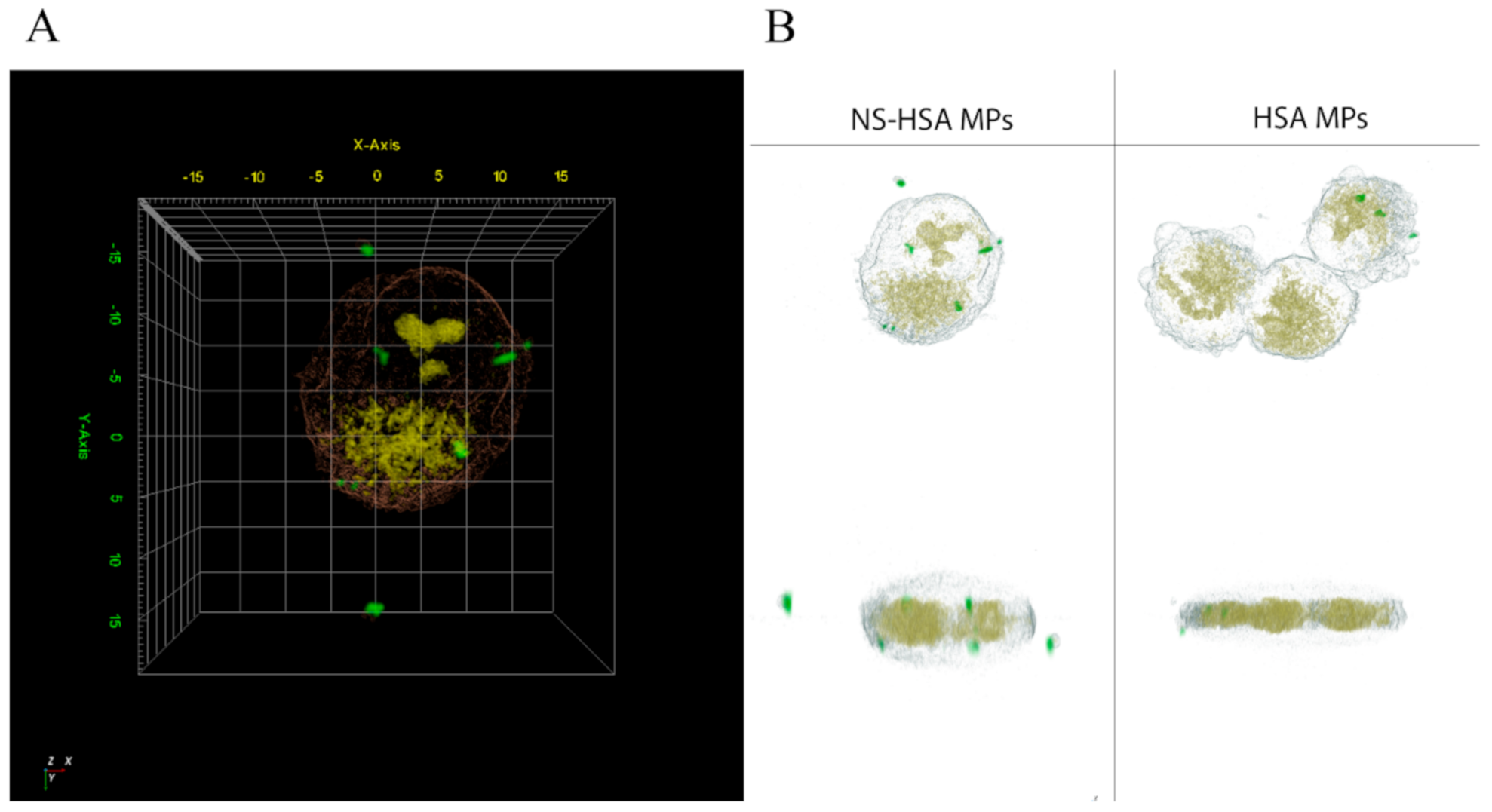
3.3.4. Cellular Uptake of NS-HSA MPs by 3D Holotomography (HT) Microscopy
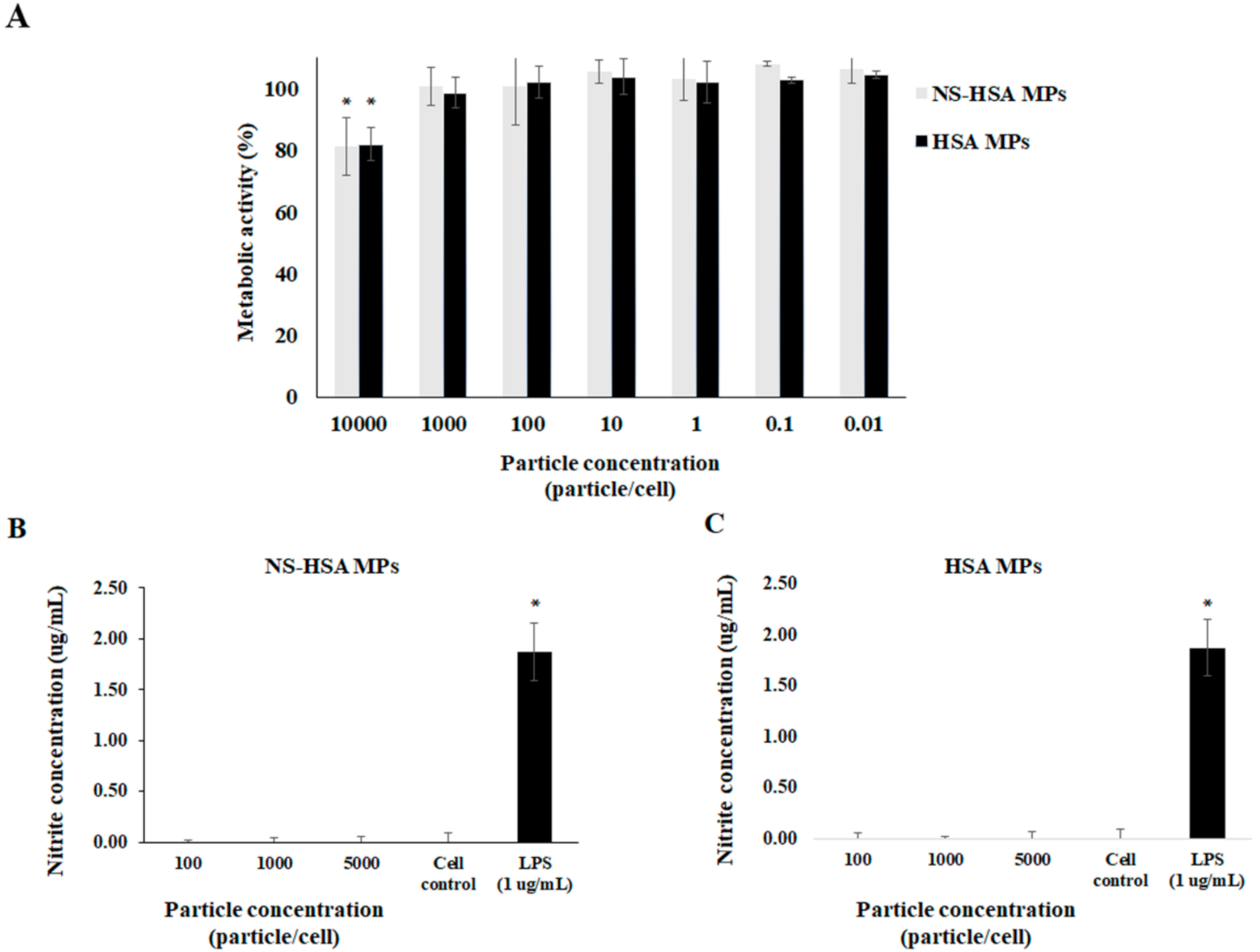
3.4. Effect of Particles on Macrophage Stimulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Sangwong, G.; Sumida, M.; Sutthikhum, V. Antioxidant activity of chemically and enzymatically modified sericin extracted from cocoons of Bombyx mori. Biocatal. Agric. Biotechnol. 2016, 5, 155–161. [Google Scholar] [CrossRef]
- Ma, M.; Hussain, M.; Dong, S.; Zhou, W. Characterization of the pigment in naturally yellow-colored domestic silk. Dyes. Pigm. 2016, 124, 6–11. [Google Scholar] [CrossRef]
- Tabunoki, H.; Higurashi, S.; Ninagi, O.; Fujii, H.; Banno, Y.; Nozaki, M.; Kitajima, M.; Miura, N.; Atsumi, S.; Tsuchida, K.; et al. A carotenoid-binding protein (CBP) plays a crucial role in cocoon pigmentation of silkworm (Bombyx mori) larvae. FEBS. Lett. 2004, 567, 175–178. [Google Scholar] [CrossRef]
- Tamura, Y.; Nakajima, K.; Nagayasu, K.; Takabayashi, C. Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochemistry 2002, 59, 275–278. [Google Scholar] [CrossRef]
- Kurioka, A.; Yamazaki, M. Purification and Identification of Flavonoids from the Yellow Green Cocoon Shell (Sasamayu) of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2003, 66, 1396–1399. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.J.; Zhou, L.X.; Zhu, L.; Zhang, Y.Q. Isolation and bioactivities of a non-sericin component from cocoon shell silk sericin of the silkworm Bombyx mori. Food Funct. 2012, 3, 150–158. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Kalay, S.; Yilmaz, Z.; Sen, O.; Emanet, M.; Kazanc, E.; Çulha, M. Synthesis of boron nitride nanotubes and their applications. Beilstein J. Nanotechnol. 2015, 6, 84–102. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Z.Y.; Cheng, X.W.; Tang, R.C.; Qiao, Y.F. Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber. Polymers 2019, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery-New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mu, J.; Xing, B. Recent Advances on the Development of Pharmacotherapeutic Agents on the Basis of Human Serum Albumin. Curr. Pharm. Des. 2015, 21, 1866–1888. [Google Scholar] [CrossRef]
- Xu, R.; Fisher, M.; Juliano, R.L. Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconjug. Chem. 2011, 22, 870–878. [Google Scholar] [CrossRef]
- Chaiwaree, S.; Prapan, A.; Suwannasom, N.; Laporte, T.; Neumann, T.; Pruß, A.; Georgieva, R.; Bäumler, H. Doxorubicin-loaded human serum albumin submicron particles: Preparation, characterization and in vitro cellular uptake. Pharmaceutics 2020, 12, 224. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Z.Z.; Georgieva, R.; Smuda, K.; Steffen, A.; Sendeski, M.; Voigt, A.; Patzak, A.; Bäumler, H. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano. 2013, 7, 7454–7461. [Google Scholar] [CrossRef]
- Suwannasom, N.; Smuda, K.; Kloypan, C.; Kaewprayoon, W.; Baisaeng, N.; Prapan, A.; Chaiwaree, S.; Georgieva, R.; Bäumler, H. Albumin submicron particles with entrapped riboflavin-fabrication and characterization. Nanomaterials 2019, 9, 482. [Google Scholar] [CrossRef]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Bäumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef]
- Pinkaew, R. Exhaustive Separation of Fibroin, Sericin and Pigments from Yellow Thai Silk Cocoon. Master’s Thesis, Chiang Mai University, Chiangmai, Thailand, October 2011. [Google Scholar]
- Ghosh, C.; Hong, B.; Batabyal, S.; Jeon, T.; Yang, S.H.; Hwang, S.G. Anti-inflammatory activity of the ethanol extract of Dictamnus dasycarpus leaf in lipopolysaccharide-activated macrophages. BMC Complement. Altern. Med. 2014, 14, 330–336. [Google Scholar] [CrossRef]
- Rehman, A.Q.T.; Jafari, S.M.; Assadpour, E.Q.S.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Sajid, B.; Mushtaq, W.A. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2019, 275, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Chinnaswamy, A. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and Adsorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.Q. Identification and analysis of the pigment composition and sources in the colored cocoon of the silkworm, Bombyx mori, by HPLC-DAD. J. Insect Sci. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Redox responsive curcumin-loaded human serum albumin nanoparticles: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 114, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.S.M.; Sousa, F.; Gübitz, G.; Cavaco-Paulo, A. Chemical Modifications on Proteins Using Glutaraldehyde. Food Technol. Biotech. 2004, 42, 51–56. [Google Scholar]
- Xiong, Y.; Steffen, A.; Andreas, K.; Müller, S.; Sternberg, N.; Georgieva, R.; Baumler, H. Hemoglobin-based oxygen carrier microparticles: Synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules 2012, 13, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Tazhbayev, Y.; Mukashev, O.; Burkeyev, M.; Lozinsky, V.I. Synthesis and Comparative Study of Nanoparticles Derived from Bovine and Human Serum Albumins. Polymers 2020, 12, 1301. [Google Scholar] [CrossRef]
- Ramappa, V.K.; Dev, P.; Shankar, S.; Shanker, U. Analysis of chemical compounds in different mulberry and non mulberry silkworm pupae powder by FTIR and EDX. Int. J. Sci. Innov. Res. 2016, 4, 120–135. [Google Scholar]
- Abrosimova, K.V.; Shulenina, O.V.; Paston, S.V. FTIR study of secondary structure of bovine serum albumin and ovalbumin. J. Phys. Conf. Ser. 2016, 769, 1–6. [Google Scholar] [CrossRef]
- Ghosh, P.; Roy, A.S.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of albumin based nanoparticles for delivery of fisetin and evaluation of its cytotoxic activity. Int. J. Biol. Macromol. 2016, 86, 408–417. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.S.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol. Sci. Appl. 2017, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baidukova, O.; Wang, Q.; Chaiwaree, S.; Freyer, D.; Prapan, A.; Georgieva, R.; Zhao, L.; Bäumler, H. Antioxidative protection of haemoglobin microparticles (HbMPs) by PolyDopamine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.W.; Ko, W.H.; Yeh, M.K.; Chiang, C.H.; Chen, J.L. The mechanism of high transfection efficiency of human serum albumin conjugated polyethylenimine in A549 cells. J. Med. Sci. 2015, 35, 57–61. [Google Scholar]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]
- Ueno, T.; Nagano, T. Fluorescent Probes for Sensing and Imaging. Nat. Methods 2011, 8, 642–645. [Google Scholar] [CrossRef]
- Yumoto, R.; Suzuka, S.; Oda, K.; Nagai, J.; Takano, M. Endocytic uptake of FITC-albumin by human alveolar epithelial cell line A549. Drug Metab. Pharmacokinet. 2012, 27, 336–443. [Google Scholar] [CrossRef]
- Das, S.K.; Dey, T.; Kundu, S.C. Fabrication of sericin nanoparticles for controlled gene delivery. RSC Adv. 2014, 4, 2137–2142. [Google Scholar] [CrossRef]
- Kim, D.; Oh, N.; Kim, K.; Lee, S.Y.; Pack, C.G.; Park, J.H.; Park, Y. Label-free high-resolution 3-D imaging of gold nanoparticles inside live cells using optical diffraction tomography. Methods 2017, 136, 160–167. [Google Scholar] [CrossRef]
- Simon, B.; Debailleul, M.; Beghin, A.; Tourneur, Y.; Haeberle, O. High-resolution tomographic diffractive microscopy of biological samples. J. Biophotonics 2010, 3, 462–467. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.E.; Yoon, J.; Heo, J.H.; Choi, C.; Park, Y.K. Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes. Sci. Rep. 2016, 6, 1–8. [Google Scholar]


| Absorbance | Entrapment Efficiency * (%) | ||
|---|---|---|---|
| 436 nm | 460 nm | 488 nm | |
| Concentration of | |||
| NS (mg/mL) | 57.77 ± 6.25 | 57.60 ± 7.56 | |
| 1.25 | 54.47 ± 5.59 | 44.59 ± 1.71 | 44.44 ± 2.21 |
| 2.5 | 43.48 ± 3.03 | 42.28 ± 7.14 | 43.65 ± 6.17 |
| 5.0 | 42.28 ± 7.14 | 38.68 ± 4.81 | 39.34 ± 5.27 |
| 7.5 | 33.87 ± 4.86 | 32.35 ± 1.38 | 32.19 ± 1.60 |
| 10.0 | 30.30 ± 0.34 | ||
| NS 5 mg/mL incubation step | 42.97 ± 6.28 | 43.65 ± 6.17 | |
| before cross-linking | 42.28 ± 7.14 | 7.60 ± 1.43 | 7.31 ± 1.12 |
| Particle | Size * | Zeta Potential * | |||
|---|---|---|---|---|---|
| Z-Average Size (nm) | PDI | ZP (mV) | Mob (µmCm/Vs) | Cond (mS/cm) | |
| NS-HSA MPs | 918.50 ± 55 | 0.21 ± 0.04 | −14.81 ± 0.51 | −1.14 ± 0.04 | 17.38 ± 0.58 |
| HSA MPs | 893.57 ± 48 | 0.19 ± 0.06 | −14.36 ± 0.38 | −1.10 ± 0.03 | 17.38 ± 0.60 |
| Particle FITC Labeling | Dilution Solution | Size * | Zeta Potential * | |||
|---|---|---|---|---|---|---|
| Z-Average Size (nm) | PDI | ZP (mV) | Mob (µmCm/Vs) | Cond (mS/cm) | ||
| NS-HSA MPs | PBS pH7.4 | 813 ± 70 | 0.39 ± 0.07 | −14.73 ± 0.24 | −1.13 ± 0.02 | 17.33 ± 0.58 |
| HSA MPs | PBS pH7.4 | 901 ± 60 | 0.18 ± 0.01 | −14.89 ± 0.97 | −1.15 ± 0.08 | 17.41 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantakee, K.; Prapan, A.; Chaiwaree, S.; Suwannasom, N.; Kaewprayoon, W.; Georgieva, R.; Tragoolpua, Y.; Bäumler, H. Fabrication and Characterization of Human Serum Albumin Particles Loaded with Non-Sericin Extract Obtained from Silk Cocoon as a Carrier System for Hydrophobic Substances. Polymers 2021, 13, 334. https://doi.org/10.3390/polym13030334
Jantakee K, Prapan A, Chaiwaree S, Suwannasom N, Kaewprayoon W, Georgieva R, Tragoolpua Y, Bäumler H. Fabrication and Characterization of Human Serum Albumin Particles Loaded with Non-Sericin Extract Obtained from Silk Cocoon as a Carrier System for Hydrophobic Substances. Polymers. 2021; 13(3):334. https://doi.org/10.3390/polym13030334
Chicago/Turabian StyleJantakee, Kanyaluck, Ausanai Prapan, Saranya Chaiwaree, Nittiya Suwannasom, Waraporn Kaewprayoon, Radostina Georgieva, Yingmanee Tragoolpua, and Hans Bäumler. 2021. "Fabrication and Characterization of Human Serum Albumin Particles Loaded with Non-Sericin Extract Obtained from Silk Cocoon as a Carrier System for Hydrophobic Substances" Polymers 13, no. 3: 334. https://doi.org/10.3390/polym13030334
APA StyleJantakee, K., Prapan, A., Chaiwaree, S., Suwannasom, N., Kaewprayoon, W., Georgieva, R., Tragoolpua, Y., & Bäumler, H. (2021). Fabrication and Characterization of Human Serum Albumin Particles Loaded with Non-Sericin Extract Obtained from Silk Cocoon as a Carrier System for Hydrophobic Substances. Polymers, 13(3), 334. https://doi.org/10.3390/polym13030334









