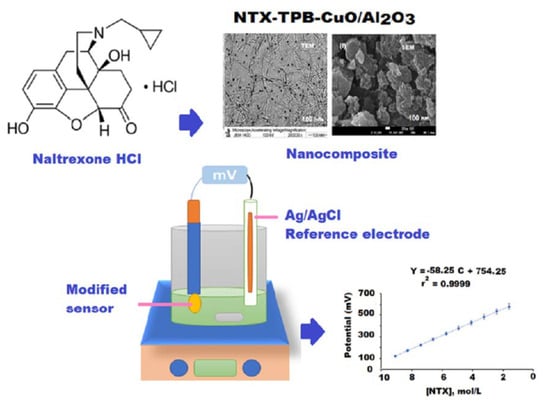
New Construction of Functionalized CuO/Al2O3 Nanocomposite-Based Polymeric Sensor for Potentiometric Estimation of Naltrexone Hydrochloride in Commercial Formulations
Abstract
:1. Introduction
2. Materials and methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of NTX-TPB Electroactive Complex
2.4. Synthesis of CuO and Al2O3 Nanoparticles
2.5. Synthesis of Polymeric NTX-TPB-CuO/Al2O3 Nanocomposite
2.6. Characterization of Synthesized Nanomaterials
2.7. Preparation of Standard NTX Solution
2.8. Sensor Construction and Membrane Composition
2.9. Calibration Graphs
2.10. Optimization of Analytical Conditions
2.11. Estimation of Naltrexone Hydrochloride® Tablets
3. Results and Discussion
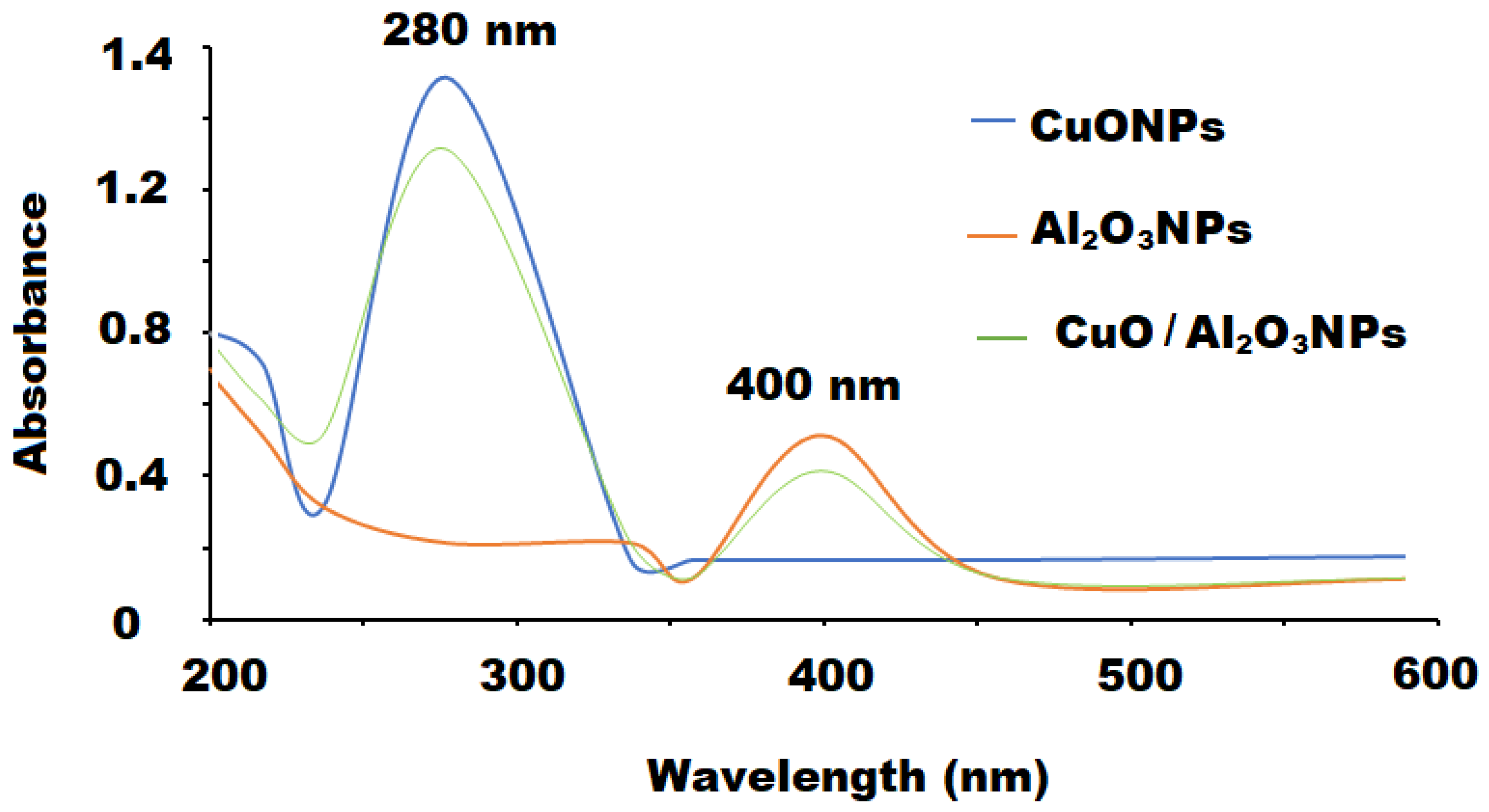
3.1. Characterization of CuO/Al2O3 Nanocomposite
3.2. Characteristics of the Constructed Sensors
3.3. Estimation of NTX in Bulk Form
3.4. Validation Study
3.5. Analysis of NTX in Naltrexone Hydrochloride® Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional nanocomposites and their potential applications: A review. J. Polym. Res. 2021, 28, 36. [Google Scholar] [CrossRef]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Mi, T.; Zhao, Y.; Wang, X.; Zhang, X. Preparation of superhydrophobic fabric based on the SiO2@ PDFMA nanocomposites by an emulsion graft polymerization and a hot-Pressing process. ChemistrySelect 2021, 6, 5646–5654. [Google Scholar] [CrossRef]
- Joseph, J.E.; Mary, P.R.; Haritha, K.V.; Panwar, D.; Kapoor, M. Soluble and cross-linked aggregated forms of α-galactosidase from Vigna mungo immobilized on magnetic nanocomposites: Improved stability and reusability. Appl. Biochem. Biotechnol. 2021, 193, 238–256. [Google Scholar] [CrossRef]
- Noor, M.; Al Mamun, M.A.; Ullah, A.A.; Matsuda, A.; Kawamura, G.; Hakim, M.A.; Islam, M.F.; Matin, M.A. Physics of Ce3+ ↔ Ce4+ electronic transition in phytosynthesized CeO2/CePO4 nanocomposites and its antibacterial activities. J. Phys. Chem. Solids 2021, 148, 109751. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for electrochemical sensors and their applications on the detection of trace metals in environmental water samples. Sensors 2021, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Al-Hossainy, A.F.; Abdelaal, R.M.; El Sayed, W.N. Novel synthesis, structure characterization, DFT and investigation of the optical properties of cyanine dye/zinc oxide [4-CHMQI/ZnO] C nanocomposite thin film. J. Mol. Struct. 2021, 1224, 128989. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhang, Y. A comprehensive review on metal-oxide nanocomposites for high-performance lithium-ion battery anodes. Energy Fuels 2021, 35, 6420–6442. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Li, Z.; Govindan, R.; Jayakumar, R.; Wang, C.; Gu, F.L. Zinc oxide-quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin nanoformulations with metal oxide nanomaterials for biomedical applications. Nanomaterials 2021, 11, 460. [Google Scholar] [CrossRef]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A novel and highly sensitive sandwich-type immunosensor for prostate-specific antigen detection based on MWCNTs-Fe3O4 nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Ali, M.; Ijaz, M.; Ikram, M.; Ul-Hamid, A.; Avais, M.; Anjum, A.A. Biogenic synthesis, characterization and antibacterial potential evaluation of copper oxide nanoparticles against Escherichia coli. Nanoscale Res. Lett. 2021, 16, 148. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D.; Chelouche, S.; Mezroua, A. Catalytic effect of green CuO nanoparticles on the thermal decomposition kinetics of ammonium perchlorate. Z. Anorg. Allg. Chem. 2021, 647, 312–325. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Dong, Q.; Zhou, F.; Wang, Q.; Wang, Z.; Huang, K.; Yu, H.; Xiong, X. One-step synthesis of CuO nanoparticles based on flame synthesis: As a highly effective non-enzymatic sensor for glucose, hydrogen peroxide and formaldehyde. J. Electroanal. Chem. 2021, 881, 114965. [Google Scholar] [CrossRef]
- Fakhree, F.M.; Waheed, I.F.; Mahmoud, K.M. Synthesis and characterization of CuO nanoparticles stabilized by quercetin and its application for anti-breast cancer activity. Egypt. J. Chem. 2021, 64, 22–23. [Google Scholar] [CrossRef]
- Resan, S.A.; Essa, A.F. Preparation and study of the optical properties for polyaniline-Al2O3 nanocomposite. Mater. Today Proc. 2021, 45, 5819–5822. [Google Scholar] [CrossRef]
- Zulkifli, F.W.A.; Yazid, H.; Jani, A.M.M. Immobilization of carbon nanotubes decorated gold nanoparticles on anodized aluminium oxide (Au-CNTs-AAO) membrane for enhanced catalytic performance. Mater. Chem. Phys. 2021, 264, 124445. [Google Scholar] [CrossRef]
- Li, Z.; Wray, P.R.; Su, M.P.; Tu, Q.; Andaraarachchi, H.P.; Jeong, Y.J.; Atwater, H.A.; Kortshagen, U.R. Aluminum oxide nanoparticle films deposited from a nonthermal plasma: Synthesis, characterization, and crystallization. ACS Omega 2020, 5, 24754–24761. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Anjum, M.A.R.; Khan, M.R.; Janjua, N.K. Electro-oxidation of ammonia over copper oxide impregnated γ-Al2O3 nanocatalysts. Coatings 2021, 11, 313. [Google Scholar] [CrossRef]
- Sankar, S.; Parvathi, K.; Ramesan, M.T. Structural characterization, electrical properties and gas sensing applications of polypyrrole/Cu-Al2O3 hybrid nanocomposites. High Perform. Polym. 2020, 32, 719–728. [Google Scholar] [CrossRef]
- Baratto, C.; Kumar, R.; Faglia, G.; Vojisavljevic, K.; Malic, B. p-Type copper aluminum oxide thin films for gas-sensing applications. Sens. Actuators B Chem. 2015, 209, 287–296. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Aroh, K.; Ehrman, S.H. Copper oxide nanoparticle made by flame spray pyrolysis for photoelectrochemical water splitting–Part I. CuO nanoparticle preparation. Int. J. Hyd. Energy 2012, 37, 4871–4879. [Google Scholar] [CrossRef]
- Salem, S.; Salem, A.; Parni, M.H.; Jafarizad, A. Microwave-assisted pyrolysis of organometallic gel prepared through ternary combination of surfactants for fabrication of nano-porous gamma alumina: Adsorptive properties, characterization. J. Chem. Technol. Biotechnol. 2021, 96, 1187–1196. [Google Scholar] [CrossRef]
- Lillo-Ramiro, J.; Guerrero-Villalba, J.M.; Mota-Gonzalez, M.D.L.; Aguirre-Tostado, F.S.; Gutierrez-Heredia, G.; Mejia-Silva, I.; Carrillo-Castillo, A. Optical and microstructural characteristics of CuO thin films by sol gel process and introducing in non-enzymatic glucose biosensor applications. Optik 2021, 229, 166238. [Google Scholar] [CrossRef]
- Rutkowska, I.; Marchewka, J.; Jelen, P.; Odziomek, M.; Korpys, M.; Paczkowska, J.; Sitarz, M. Chemical and structural characterization of amorphous and crystalline alumina obtained by alternative sol–gel preparation routes. Materials 2021, 14, 1761. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, Y.; Li, J.; Liu, Y.; Zhang, J.; Xiang, G.; Zhu, X.; Li, R.; Wang, H.; Zhao, Y. Annealing temperature induced physical characteristics of CuO films grown by magnetron sputtering. Mater. Sci. Semicond. Process. 2021, 131, 105883. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, C.; Fan, X.; Liu, Z.; Liu, J. Preparation of non-stoichiometric Al2O3 film with broadband antireflective by magnetron sputtering. Chem. Phys. Lett. 2021, 764, 138299. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: Preparation, characterization, and photoactivated antibacterial activity. Nano-Struct Nano-Objects 2020, 22, 100464. [Google Scholar] [CrossRef]
- ElFaham, M.M.; Okil, M.; Mostafa, A.M. Effects of post-laser irradiation on the optical and structure properties of Al2O3 nanoparticles produced by laser ablation. J. Appl. Phys. 2020, 128, 153104. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper oxide nanoparticles: Synthesis, characterization and their antibacterial activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Merati, Z.; Parsa, J.B.; Babaei-Sati, R. Electrochemically synthesized polypyrrole/MWCNTs-Al2O3 ternary nanocomposites supported Pt nanoparticles toward methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 20993–21005. [Google Scholar] [CrossRef]
- Sodeifian, G.; Behnood, R. Application of microwave irradiation in preparation and characterization of CuO/Al2O3 nanocomposite for removing MB dye from aqueous solution. J. Photochem. Photobiol. A Chem. 2017, 342, 25–34. [Google Scholar] [CrossRef]
- Sudha, V.; Murugadoss, G.; Thangamuthu, R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Sci. Rep. 2021, 11, 3413. [Google Scholar] [CrossRef]
- Zhou, X.; Pu, H.; Sun, D.W. DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021, 61, 2277–2296. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Thulasi, G.; Al-Ansari, M.M.; Al-Humaid, L.A.; Saravanan, M. Experimental investigation and electrochemical characterization of titanium coated nanocomposite materials for biomedical applications. J. Mol. Struct. 2021, 1231, 129932. [Google Scholar] [CrossRef]
- Shawky, A.M.; El-Tohamy, M.F. Highly functionalized modified metal oxides polymeric sensors for potentiometric determination of letrozole in commercial oral tablets and biosamples. Polymers 2021, 13, 1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Yan, R.; Gao, Y.; Wang, P. Bimetallic AuCu nanoparticles coupled with multi-walled carbon nanotubes as ion-to-electron transducers in solid-contact potentiometric sensors. Electrochim. Acta 2020, 331, 135370. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Colozza, N.; Tazzioli, S.; Sassolini, A.; Agosta, L.; di Monte, M.G.; Hermansson, K.; Arduini, F. Multiparametric analysis by paper-assisted potentiometric sensors for diagnostic and monitoring of reinforced concrete structures. Sens. Actuators B Chem. 2021, 345, 130352. [Google Scholar] [CrossRef]
- Al-Tamimi, S.A. Biogenic green synthesis of metal oxide nanoparticles using oat biomass for ultrasensitive modified polymeric sensors. Green Chem. Lett. Rev. 2021, 14, 166–179. [Google Scholar] [CrossRef]
- Elashery, S.E.; Frag, E.Y.; Sleim, A.A. Novel and selective potentiometric sensors for Cinchocaine HCl determination in its pure and Co-formulated dosage form: A comparative study of in situ carbon sensors based on different ion pairing agents. Measurement 2021, 173, 108549. [Google Scholar] [CrossRef]
- Nunes, E.V., Jr.; Scodes, J.M.; Pavlicova, M.; Lee, J.D.; Novo, P.; Campbell, A.N.; Rotrosen, J. Sublingual buprenorphine-naloxone compared with injection naltrexone for opioid use disorder: Potential utility of patient characteristics in guiding choice of treatment. Am. J. Psych. 2021, 178, 660–671. [Google Scholar] [CrossRef] [PubMed]
- El-Didamony, A.M.; Hassan, W.S. Spectrophotometric and fluorimetric methods for determination of naltrexone in urine, serum and tablets by oxidation with cerium (IV). J. Chil. Chem. Soc. 2012, 57, 1404–1408. [Google Scholar] [CrossRef] [Green Version]
- Jafari-Nodoushan, M.; Barzin, J.; Mobedi, H. A stability-indicating HPLC method for simultaneous determination of morphine and naltrexone. J. Chromatogr. B 2016, 1011, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; El-Gamal, R.M. Simultaneous determination of naltrexone and bupropion in their co-formulated tablet utilizing green chromatographic approach with application to human urine. Saudi Pharm. J. 2018, 26, 169–176. [Google Scholar] [CrossRef]
- Ghorbani-Bidkorbeh, F.; Shahrokhian, S.; Mohammadi, A.; Dinarvand, R. Electrochemical determination of naltrexone on the surface of glassy carbon electrode modified with Nafion-doped carbon nanoparticles: Application to determinations in pharmaceutical and clinical preparations. J. Electroanal. Chem. 2010, 638, 212–217. [Google Scholar] [CrossRef]
- Draz, M.E.; Darwish, H.W.; Darwish, I.A.; Saad, A.S. Solid-state potentiometric sensor for the rapid assay of the biologically active biogenic amine (tyramine) as a marker of food spoilage. Food Chem. 2021, 346, 128911. [Google Scholar] [CrossRef] [PubMed]
- FDA. ICH-Q2 (R1) Validation and Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization Guidelines, Geneva, Switzerland, 17 November 2005. [Google Scholar]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z. Fabrication of CuO nanoparticles for structural, optical and dielectric analysis using chemical precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 12591–12597. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Zamani, R.M.; Beiygie, E.; Nekouei, H. Synthesis of micro-mesopores flowerlike γ-Al2O3 nano-architectures. J. Serbian Chem. Soc. 2014, 79, 1007–1017. [Google Scholar] [CrossRef]
- Rao, K.V.; Smakula, A. Dielectric properties of cobalt oxide, nickel oxide, and their mixed crystals. J. Appl. Phys. 1965, 36, 2031–2038. [Google Scholar] [CrossRef]
- Saleem, M.; Fang, L.; Ruan, H.B.; Wu, F.; Huang, Q.L.; Xu, C.L.; Kong, C.Y. Effect of zinc acetate concentration on the structural and optical properties of ZnO thin films deposited by Sol-Gel method. Int. J. Phys. Sci. 2012, 7, 2971–2979. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Koblova, E.; Ustinov, A.Y.; Shcheka, O. Theoretical study of copper (II) oxide clusters and their interaction with CO. Appl. Mech. Mater. 2015, 709, 358–363. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Apitz, S.E. Molecular cluster models of aluminum oxide and aluminum hydroxide surfaces. Am. Mineral. 1998, 83, 1054–1066. [Google Scholar] [CrossRef]
- Bitra, H.C.R.; Rao, A.V.; Babu, K.S.; Rao, G.N. Synthesis and enhanced dielectric properties of copper oxidenanoparticles. Mat. Chem. Phys. 2020, 254, 123379. [Google Scholar] [CrossRef]
- Yadav, N.; Dabrowski, R.; Dhar, R. Effect of alumina nanoparticles on dielectric permittivity, electrical conductivity, director relaxation frequency, threshold and switching voltages of a nematic liquid crystalline material. Liq. Cryst. 2014, 41, 1803–1810. [Google Scholar] [CrossRef]
- Kakhkiz, R.M. Application of nanoparticles in the potentiometric ion selective electrodes. Russ. J. Electrochem. 2013, 49, 458–465. [Google Scholar] [CrossRef]
- Isa, I.M.; Sohaimi, N.M.; Hashim, N.; Kamari, A.; Mohamed, A.; Ahmad, M.; Ghani, S.A. Determination of salicylate ion by potentiometric membrane electrode based on zinc aluminium layered double hydroxides-4(2,4-dichlorophenoxy) butyrate nanocomposites. Int. J. Electrochem. Sci. 2013, 8, 2112–2121. [Google Scholar]
- Ma, T.S.; Hassan, S.S. Organic Analysis Using Ion Selective Electrodes; Academic Press: London, UK, 1982; Volume 1–2. [Google Scholar]
- Ganjali, M.R.; Alipour, A.; Riahi, S.; Norouzi, P. Design and construction of a naltrexone selective sensor based on computational study for application in pharmaceutical analysis. Int. J. Electrochem. Sci. 2009, 4, 1153–1166. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry, 3rd ed.; Ellis Horwood PTR Prentice Hall: New York, NY, USA, 1993. [Google Scholar]
- Huang, X.; Jiang, P. Core-shell structured high-k polymer nanocomposites for energy storage and dielectric applications. Adv. Mater. 2015, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Wallace, R.M. High-K materials and metal gates for CMOS applications. Mater. Sci. Eng. R Rep. 2015, 88, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef]
- Prakash, S.; Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosen. Bioelectron. 2013, 41, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Bidkorbeh, F.; Shahrokhian, S.; Mohammadi, A.; Dinarvand, R. Preparation of a naltrexone HCl potentiometric sensor and its application to pharmaceutical analysis and drug determination in biological fluids. J. Food Drug Anal. 2011, 19, e51. [Google Scholar] [CrossRef]
- AlRabiah, H.; Abounassif, M.; Al-Majed, A.; Mostafa, G. Comparative investigation of β-and γ-cyclodextrin as ionophores in potentiometric based sensors for naltrexone. Int. J. Electrochem. Sci. 2016, 11, 4930–4942. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]








| Parameter | Conventional Coated Wire NTX-TPB Sensor | Modified NTX-TPB-CuO/Al2O3 Nanocomposite Sensor |
|---|---|---|
| Linear conc. (mol L−1) | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−11–1.0 × 10−2 |
| Regression equation | EmV = (52.1 ± 0.2) log [NTX] + 406.6 | EmV = (58.25 ± 0.3) log [NTX] + 754.25 |
| Correlation Coefficient (r) | 0.9995 | 0.9999 |
| Slope (mV. Decade−1) | 52.1 ± 0.2 | 58.25 ± 0.3 |
| Intercept | 406.6 | 754.25 |
| LOD | 5.0 × 10−6 | 5.0 × 10−10 |
| Suitable pH range | 2–5 | 2–5 |
| Temperature (°C) | 25 | 25 |
| Lifetime (day) | 30 | 60 |
| Accuracy (%) | 98.73 ± 1.09 | 99.72 ± 0.4 |
| Robustness | 98.45 ± 0.7 | 99.63 ± 0.3 |
| Ruggedness | 98.59 ± 0.4 | 99.68 ± 0.4 |
| Interferences | Conventional Coated Wire NTX-TPB Sensor (KPotNTX+) | Modified NTX-TPB-CuO/Al2O3 Nanocomposite Coated Wire Sensor (KPotNTX+) |
|---|---|---|
| Povidone | 5.6 × 10−3 | 1.5 × 10−5 |
| Hydroxypropyl methylcellulose | 1.9 × 10−3 | 5.6 × 10−4 |
| Lactose monohydrate | 4.2 × 10−3 | 2.8 × 10−5 |
| Magnesium stearate | 9.4 × 10−3 | 3.9 × 10−5 |
| Microcrystalline cellulose | 4.9 × 10−3 | 1.7 × 10−4 |
| Polyethylene glycol | 6.3 × 10−3 | 3.3 × 10−5 |
| Polysorbate 80 | 1.2 × 10−3 | 6.9 × 10−5 |
| Tryptophan | 5.6 × 10−3 | 2.4 × 10−4 |
| Lysine | 5.3 × 10−3 | 5.0 × 10−5 |
| Glycine | 3.3 × 10−3 | 3.6 × 10−5 |
| Conventional Coated Wire NTX-TPB Sensor | Modified NTX-TPB-CuO/Al2O3 Nanocomposite Coated Wire Sensor | |||
|---|---|---|---|---|
| Test Sample −log [NTX] mol L−1 | % Recovery | Test Sample −log [NTX] mol L−1 | % Recovery | |
| Statistical analysis | 6.0 | 99.5 | 9.0 | 99.9 |
| 5.3 | 98.9 | 8.0 | 99.6 | |
| 5.0 | 99.0 | 7.0 | 100.0 | |
| 4.0 | 97.3 | 6.0 | 99.7 | |
| 3.0 | 97.3 | 5.0 | 99.6 | |
| 2.0 | 98.0 | 4.0 | 100.0 | |
| 3.0 | 99.7 | |||
| 2.0 | 100.0 | |||
| Mean ± SD | 98.33 ± 0.9 | 99.81 ± 0.2 | ||
| n | 6 | 8 | ||
| Variance | 0.81 | 0.04 | ||
| %SE * | 0.37 | 0.07 | ||
| %RSD | 0.92 | 0.20 | ||
| Conventional Coated Wire NTX-TPB Sensor | Modified NTX-TPB-CuO/Al2O3 Nanocomposite Coated Wire Sensor | |||
|---|---|---|---|---|
| Test Sample −log [NTX] mol L−1 | % Recovery | Test Sample −log [NTX] mol L−1 | % Recovery | |
| Statistical Analysis | 6.0 | 99.9 | 9.0 | 99.9 |
| 5.3 | 97.9 | 8.3 | 99.7 | |
| 5.0 | 99.5 | 8.0 | 99.9 | |
| 4.3 | 98.3 | 7.0 | 99.6 | |
| 4.0 | 97.3 | 6.0 | 100.0 | |
| 3.3 | 98.9 | 5.0 | 99.7 | |
| 3.0 | 99.7 | 4.0 | 98.8 | |
| 2.3 | 97.2 | 3.0 | 100.0 | |
| 2.0 | 99.9 | 2.0 | 99.9 | |
| Mean ± SD | 98.73 ± 1.09 | 99.72 ± 0.4 | ||
| n | 9 | 9 | ||
| Variance | 1.18 | 0.16 | ||
| %SE * | 0.36 | 0.13 | ||
| %RSD | 1.10 | 0.40 | ||
| Modified NTX-TPB-CuO/Al2O3 Nanocomposite Coated Wire Sensor | ||||||
|---|---|---|---|---|---|---|
| Intra-Day Assay | Inter-Day Assay | |||||
| Sample −log [NTX] mol L−1 | Found −log [NTX] mol L−1 | % Recovery | Sample −log [NTX] mol L−1 | Found −log [NTX] mol L−1 | % Recovery | |
| Statistical Analysis | 11.0 | 10.99 | 99.9 | 11.0 | 11.0 | 100.0 |
| 7.0 | 7.0 | 100.0 | 7.0 | 6.98 | 99.7 | |
| 2.0 | 1.99 | 99.5 | 2.0 | 2.01 | 100.2 | |
| Mean ± SD | 99.8 ± 0.3 | 99.9 ± 0.2 | ||||
| n | 3 | 3 | ||||
| Variance | 0.09 | 0.04 | ||||
| %SE * | 0.17 | 0.11 | ||||
| %RSD | 0.30 | 0.20 | ||||
| Conventional Coated Wire NTX-TPB Sensor | Modified NTX-TPB-CuO/Al2O3 Nanocomposite Coated Wire Sensor | Reported Method [63] | |||
|---|---|---|---|---|---|
| Test Sample −log [NTX] mol L−1 | % Recovery | Test Sample −log [NTX] mol L−1 | % Recovery | ||
| Statistical analysis | 6.0 | 99.8 | 11.0 | 100.00 | |
| 5.3 | 99.6 | 9.0 | 99.9 | ||
| 5.0 | 98.8 | 7.0 | 99.8 | 99.52 ± 0.3 | |
| 4.0 | 98.5 | 5.0 | 99.6 | 6 | |
| 3.0 | 98.7 | 3.0 | 99.4 | 0.09 | |
| 2.0 | 98.9 | 2.0 | 99.5 | 0.12 | |
| 0.30 | |||||
| Mean ± SD | 99.05 ± 0.5 | 99.70 ± 0.2 | |||
| n | 6 | 6 | |||
| Variance | 0.25 | 0.04 | |||
| %SE * | 0.20 | 0.08 | |||
| %RSD | 0.50 | 0.20 | |||
| t-student test | 2.015 (2.228) * | 1.248(2.228) * | |||
| F-test | 2.77 (5.05) * | 2.25(5.05) * | |||
| No. | Ion-Pair Complex | Concentration Range (mol L−1) | LOD (mol L−1) | Reference |
|---|---|---|---|---|
| 1. | NTX-tetraphenylborate | 1.0 × 10−5–1.0 × 10−2 | 8.0 × 10−6 | [63] |
| 2. | NTX-tetraphenylborate | 1.0 × 10−5–1.0 × 10−3 | 5.0 × 10−6 | [69] |
| 3. | NTX-tetrakis-4 chlorophenyl borate | 5.8 × 10−6–1.0 × 10−2 | 5.0 × 10−6 | [70] |
| 4. | NTX-tetraphenylbotate-CuO/Al2O3 nanocomposite | 1.0 × 10−9–1.0 × 10−2 | 5.0 × 10−10 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mohaimeed, A.M.; Mostafa, G.A.E.; El-Tohamy, M.F. New Construction of Functionalized CuO/Al2O3 Nanocomposite-Based Polymeric Sensor for Potentiometric Estimation of Naltrexone Hydrochloride in Commercial Formulations. Polymers 2021, 13, 4459. https://doi.org/10.3390/polym13244459
Al-Mohaimeed AM, Mostafa GAE, El-Tohamy MF. New Construction of Functionalized CuO/Al2O3 Nanocomposite-Based Polymeric Sensor for Potentiometric Estimation of Naltrexone Hydrochloride in Commercial Formulations. Polymers. 2021; 13(24):4459. https://doi.org/10.3390/polym13244459
Chicago/Turabian StyleAl-Mohaimeed, Amal M., Gamal A. E. Mostafa, and Maha F. El-Tohamy. 2021. "New Construction of Functionalized CuO/Al2O3 Nanocomposite-Based Polymeric Sensor for Potentiometric Estimation of Naltrexone Hydrochloride in Commercial Formulations" Polymers 13, no. 24: 4459. https://doi.org/10.3390/polym13244459
APA StyleAl-Mohaimeed, A. M., Mostafa, G. A. E., & El-Tohamy, M. F. (2021). New Construction of Functionalized CuO/Al2O3 Nanocomposite-Based Polymeric Sensor for Potentiometric Estimation of Naltrexone Hydrochloride in Commercial Formulations. Polymers, 13(24), 4459. https://doi.org/10.3390/polym13244459