Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model
Abstract
1. Introduction
2. Materials and Methods
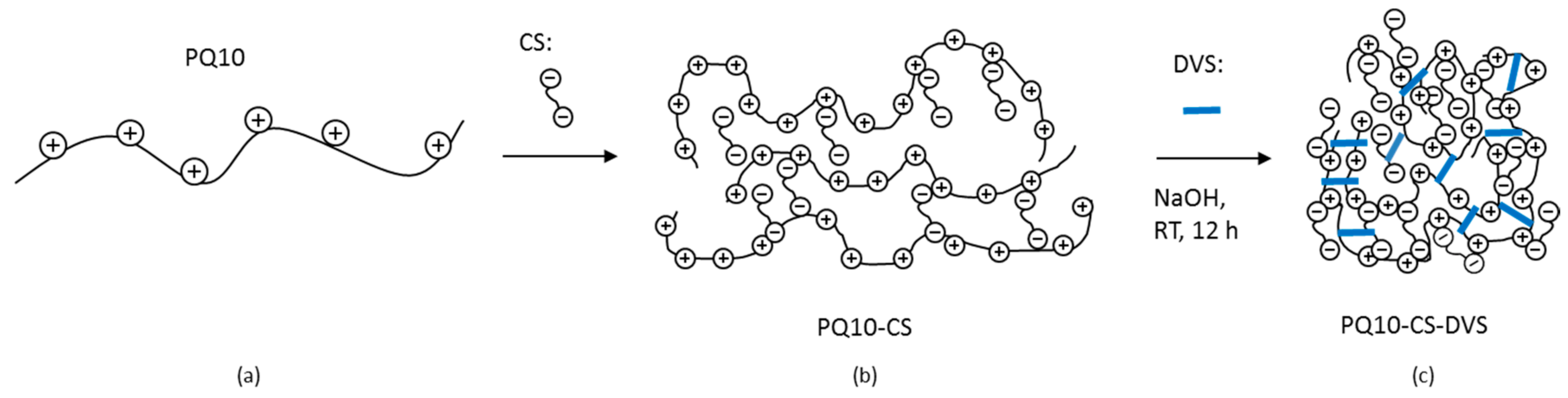
2.1. Preparation of the Crosslinked PQ10-CS-DVS
2.2. Hydrogel Characterization
2.2.1. FTIR Measurements
2.2.2. Differential Scanning Calorimetric (DSC) Analysis
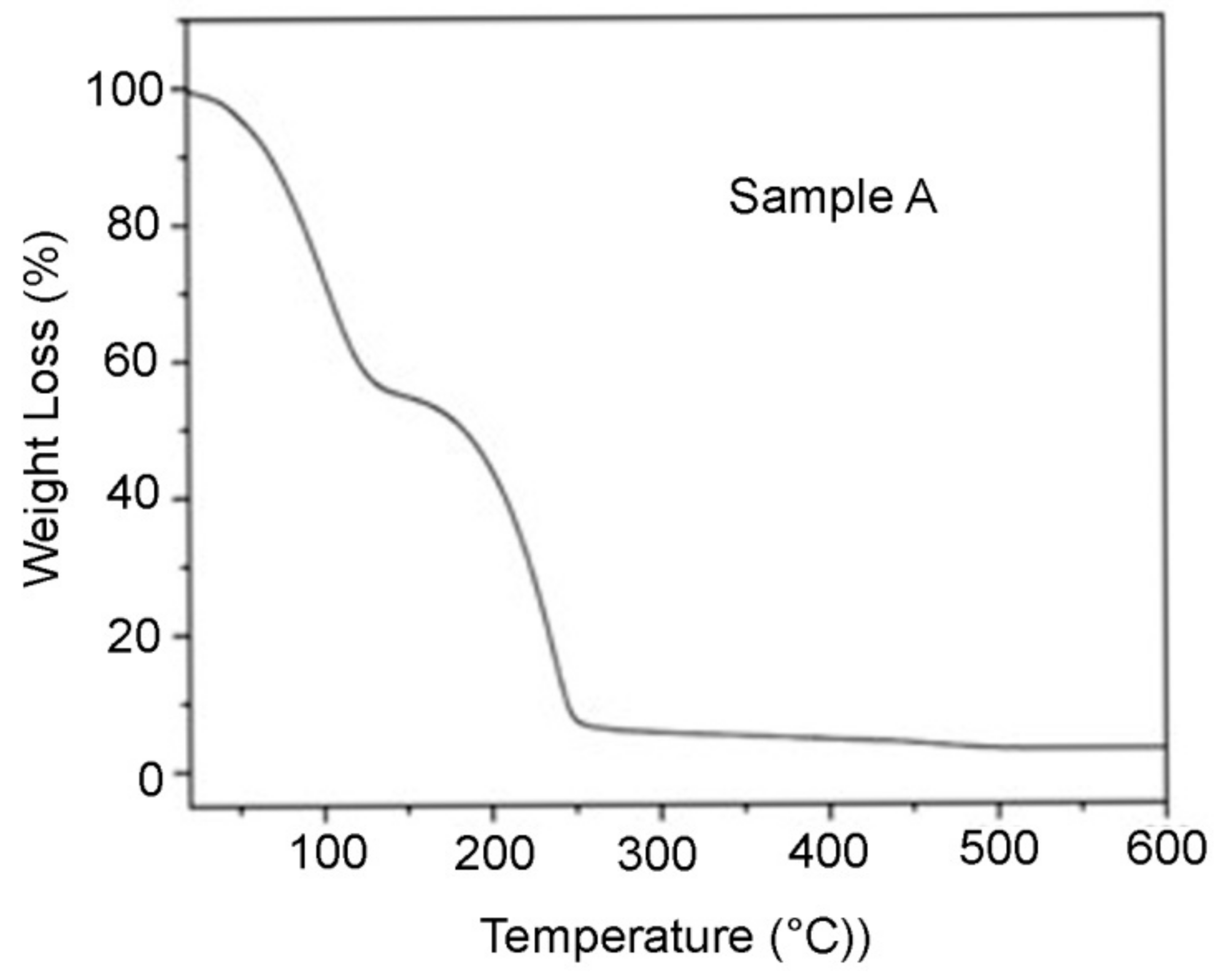
2.2.3. Thermogravimetric (TGA) Analysis
2.2.4. Texture Analysis
2.2.5. Dynamic Viscosity and Storage Module Measurement
2.3. ISO Guinea Pig Maximization Sensitization Test
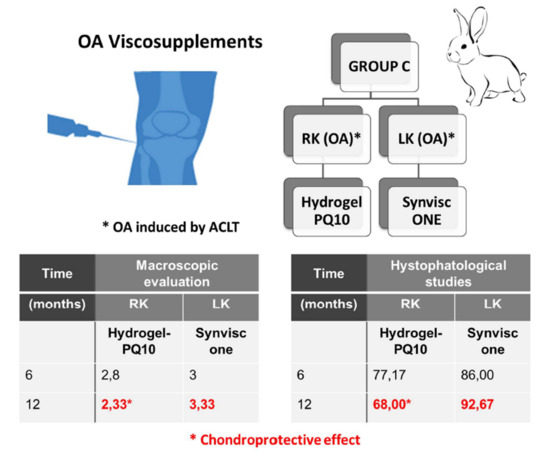
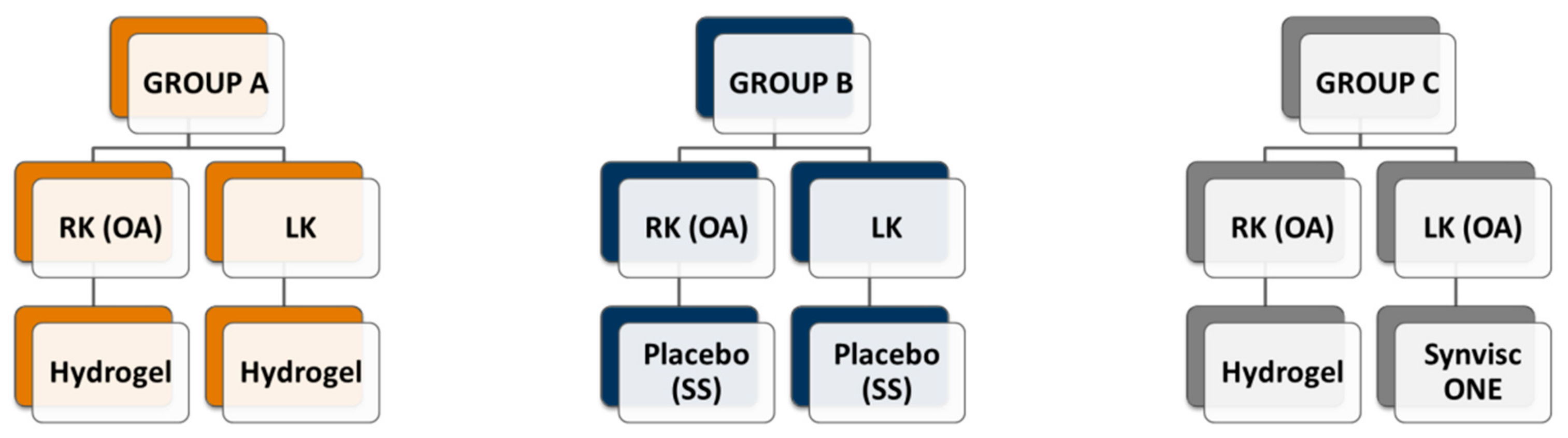
2.4. Rabbit’s Experimental Design
2.4.1. Surgical Procedure
2.4.2. Radiological Studies
2.4.3. Euthanasia and Necropsy
2.4.4. Macroscopic Scoring
2.4.5. Microscopic Scoring
2.4.6. Hematology and Serum Chemistry
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of PQ10-CS-DVS Hydrogel
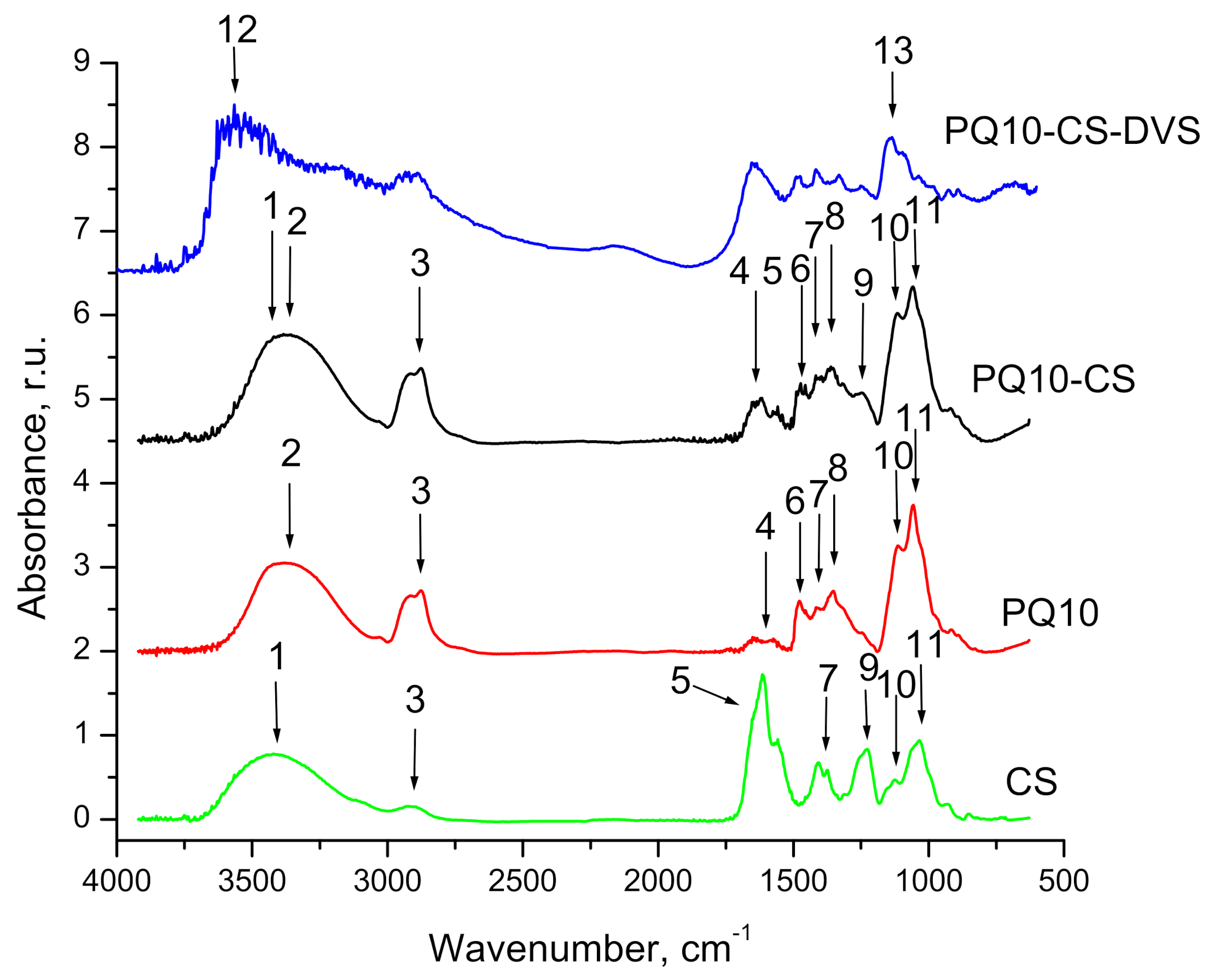
3.1.1. FTIR Analysis
3.1.2. Differential Scanning Calorimetry (DSC)
3.1.3. Thermogravimetric Analysis (TGA)
3.1.4. Rheology
3.1.5. Thermal Stability of Covalently Crosslinked Hydrogel
3.2. In Vivo Studies
3.2.1. Sensitization Test
3.2.2. Implantation of PQ10-CS-DVS in Rabbits’ Knees
Radiological Studies
Macroscopic Evaluation
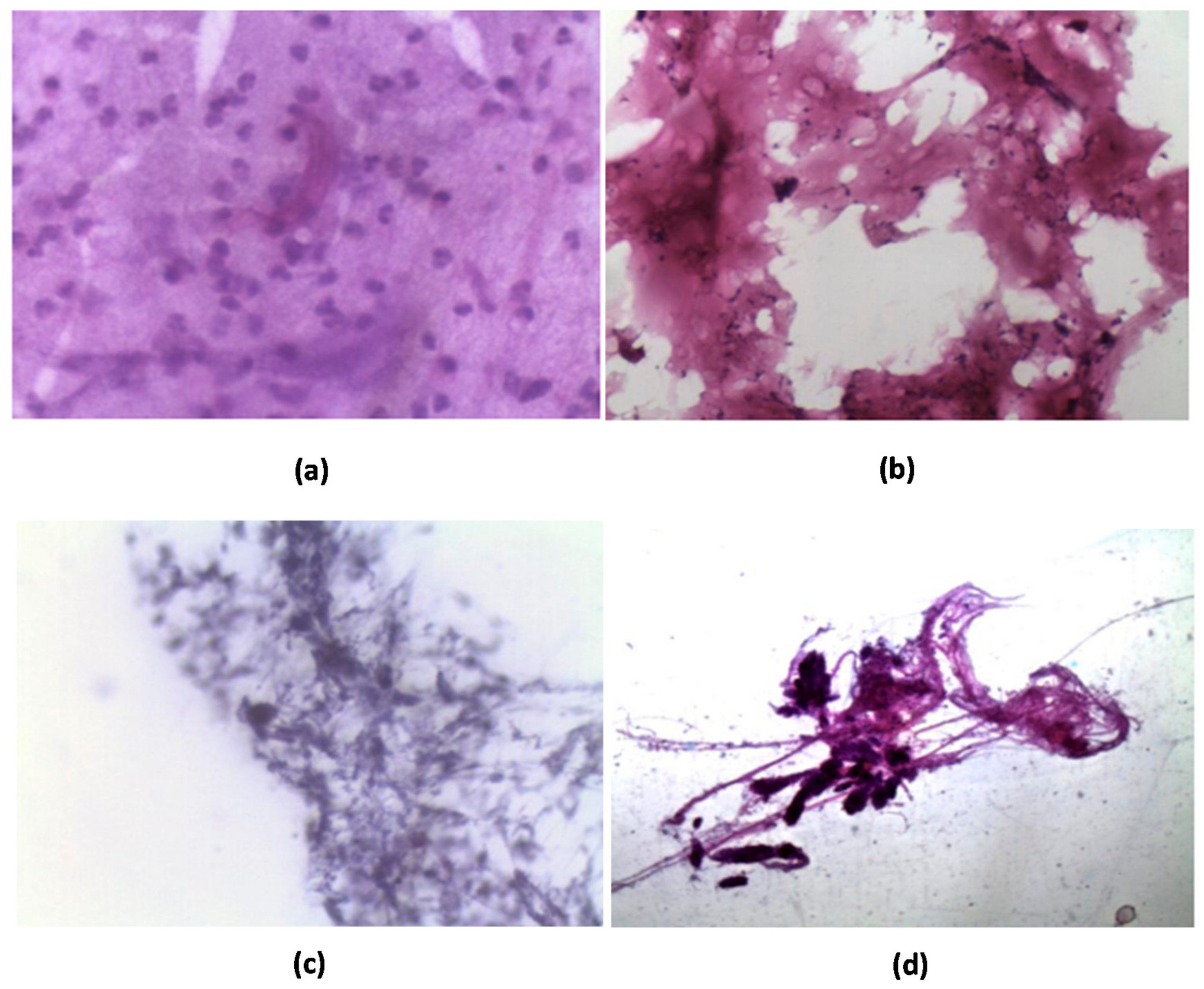
Histopathological Study
Hematology and Serum Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, J.W.P.; Schlüter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd Edition. J. Am. Acad. Orthop. Surg. 2013, 21, 571–576. [Google Scholar] [CrossRef]
- Lotz, B.; Bothe, F.; Deubel, A.K.; Hesse, E.; Renz, Y.; Werner, C.; Schäfer, S.; Böck, T.; Groll, J.; von Rechenberg, B.; et al. Preclinical testing of new hydrogel materials for cartilage repair: Overcoming fixation issues in a large animal model. Int. J. Biomater. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J. Viscosupplementation for osteoarthritis of the knee. N. Engl. J. Med. 2015, 372, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Renier, D. Nuevos Derivados Entrecruzados de Ácido Hialurónico. European Patent 2,227,297, 1 April 2005. [Google Scholar]
- Borrás-Verdera, A.; Calcedo-Bernal, V.; Ojeda-Levenfeld, J.; Clavel-Sainzc, C. Efficacy and safety of a single intra-articular injection of 2% hyaluronic acid plus manitol in knee osteoarthritis over a 6-month period. J. Orthop. Surg. Traum. 2012, 56, 274–280. [Google Scholar] [CrossRef]
- Iannitti, T.; Elhensheri, M.; Bingöl, A.Ö.; Palmieri, B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J. Mol. Hist. 2013, 44, 191–201. [Google Scholar] [CrossRef]
- Raman, R.; Dutta, A.; Day, N.; Sharma, H.K.; Shaw, C.J.; Johnson, G.V. The efficacy of Hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—A prospective randomized clinical trial. Knee 2008, 15, 318–324. [Google Scholar] [CrossRef]
- Ong, K.L.; Farr, J.; Gudeman, A.S.; Murray, I.R.; McIntyre, L.F.; Hummer, C.D.; Ngai, W.; Lau, E.; Altman, R.D.; Sherman, S.L. Risk of severe acute localized reactions for different intra-articular hyaluronic acid knee injections in a real-world setting. Cartilage 2021, 1–11. [Google Scholar] [CrossRef]
- Brode, G.L. Glycosaminoglycan and Cationic Polymer Combinations. U.S. Patent 4,767,463, 30 August 1988. [Google Scholar]
- Brode, G.L. Processes for Managing Keratinous Material Using Glycosaminoglycan and Cationic Polymer Combinations. U.S. Patent 4,913,743, 3 April 1990. [Google Scholar]
- Baeurle, S.A.; Kiselev, M.G.; Makarova, E.S.; Nogovitsin, E.A. Effect of the counterion behavior on the frictional-compressive properties of chondroitin sulfate solutions. Polymer 2009, 50, 1805–1813. [Google Scholar] [CrossRef]
- What You Need to Know about Cartilage Damage. Available online: https://www.medicalnewstoday.com/articles/171780 (accessed on 15 December 2017).
- When You Have No Cartilage in Your Knee. Available online: https://www.verywellhealth.com/treatments-for-loss-of-cartilage-in-knee-5096944 (accessed on 6 May 2021).
- Alasino, R.V.; Garcia, L.G.; Gramajo, A.L.; Pusterla, J.P.; Beltramo, D.M.; Luna, J.D. Ocular biocompatibility of polyquaternium 10 gel: Functional and morphological results. J. Mat. Sci. Mat. Med. 2015, 26, 64. [Google Scholar] [CrossRef]
- Malmsten, M.; Ljusegren, I.; Carlstedt, I. Ellipsometry studies of the mucoadhesion of cellulose derivatives. Coll.Surf. B Bioint. 1994, 2, 463–470. [Google Scholar] [CrossRef]
- Salamone, J.C. Polymeric Materials Encyclopedia; CRC Press: New York, NY, USA, 1996; pp. 1113–1118. [Google Scholar]
- Nichifor, M.; Stanciu, M.C.; Simionescu, B.C. New cationic hydrophilic and amphiphilic polysaccharides synthesized by one pot procedure. Carbohydr. Polym. 2010, 82, 965–975. [Google Scholar] [CrossRef]
- Bierbrauer, K.L.; Alasino, R.V.; Strumia, M.C.; Beltramo, D.M. Cationic cellulose and its interaction with chondroitin sulfate. Rheological properties of the polyelectrolyte complex. Eur. Polym. J. 2014, 50, 142–149. [Google Scholar] [CrossRef]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Interactions of ibuprofen with cationic polysaccharides in aqueous dispersions and hydrogels: Rheological and diffusional implications. Eur. J. Pharm. Sci. 2003, 20, 429–438. [Google Scholar] [CrossRef]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Cont. Rel. 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Denuziere, A.; Ferrier, C.; Domard, A. Chitosan-chondroitin and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carb. Polym. 1996, 29, 317–323. [Google Scholar] [CrossRef]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Rheological evaluation of the interactions between cationic celluloses and carbopol 974P in water. Biomacromolecules 2001, 2, 886–893. [Google Scholar] [CrossRef]
- Yoshioka, M.; Coutts, R.D.; Amiel, D.; Hacker, S.A. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthr. Cartil. 1996, 4, 87–98. [Google Scholar] [CrossRef]
- Shimizu, G.; Yoshioka, M.; Coutts, R.D.; Harwood, F.L.; Kubo, T.; Hirasawa, Y.; Amiel, D. Long-term effects of hyaluronan on experimental osteoarthritis in the rabbit knee. Osteoarthr. Cartil. 1998, 6, 1–9. [Google Scholar] [CrossRef][Green Version]
- Laverty, S.; Girard, C.A.; Williams, J.M.; Hunziker, E.B.; Pritzker, K.P.H. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthr. Cartil. 2010, 18, S53–S65. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, S.; Funakoshiy, T.; Sasazaway, F.; Todoh, M.; Tadano, S.; Iwasaki, N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthr. Cartil. 2014, 22, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Mathieu, P.; Rinaudo, M. Mannitol preserves the viscoelasctic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol. Ther. 2014, 1, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.E.; Dursema, H.; Skrabut, E.M. Clearance kinetics of a single injection crosslinked hylan-based viscosupplement in a rabbit model. Osteoarthr. Cartil. 2007, 15, C64. [Google Scholar] [CrossRef][Green Version]
- Edsman, K.; Hjelm, R.; Lärkner, H.; Nord, L.I.; Karlsson, A.; Wiebensjö, A.; Urban Höglund, A.; Helander Kenne, A.; Näsström, J. Intra-articular duration of DurolaneTM after single injection into the rabbit knee. Cartilage 2011, 2, 384–388. [Google Scholar] [CrossRef]
- Maheu, E.; Avouac, B.; Dreiser, R.L.; Bardin, T. A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan G-F 20 in the treatment of symptomatic knee osteoarthritis: A 6-month, multicenter, randomized, controlled non-inferiority trial. PLoS ONE 2019, 14, e0226007. [Google Scholar] [CrossRef]



| Group | R-LC | R-MC | L-LC | L-MC | Time |
|---|---|---|---|---|---|
| A | 1.586 ± 0.615 | 1.131 ± 0.223 | 1.564 ± 0.680 | 1.122 ± 0.171 | 0 |
| B | 1.199 ± 0.478 | 1.039 ± 0.139 | 1.232 ± 0.402 | 1.034 ± 0.328 | 0 |
| A | 0.744 ± 0.340 | 1.221 ± 0.157 | 0.859 ± 0.324 | 1.369 ± 0.319 | 28 d 1 |
| B | 1.171 ± 0.908 | 1.082 ± 0.382 | 0.866 ± 0.797 | 0.992 ± 0.206 | 28 d |
| A | 1.601 ± 0.291 | 1.226 ± 0.366 | 1.312 ± 0.348 | 1.115 ± 0.394 | 3 m 1 |
| B | 1.569 ± 0.921 | 1.080 ± 0.375 | 1.486 ± 1.022 | 0.996 ± 0.451 | 3 m |
| A | 1.510 ± 0.440 | 1.149 ± 0.430 | 1.724 ± 0.517 | 0.838 ± 0.400 | 6 m |
| B | 1.220 ± 0.477 | 1.001 ± 0.503 | 1.313 ± 0.490 | 0.673 ± 0.181 | 6 m |
| A | 0.909 ± 0.354 | 0.589 ± 0.155 | 2.662 ± 3.510 | 0.812 ± 0.459 | 12 m |
| B | 0.971 ± 0.094 | 1.006 ± 0.179 | 1.080 ± 0.270 | 0.656 ± 0.473 | 12 m |
| C | 1.362 ± 0.438 | 1.150 ± 0.239 | 1.302 ± 0.472 | 1.155 ± 0.271 | 0 |
| C | 0.982 ± 0.265 | 1.143 ± 0.230 | 1.178 ± 0.531 | 0.953 ± 0.169 | 28 d |
| C | 1.782 ± 0.390 | 0.866 ± 0.611 | 1.634 ± 0.587 | 1.115 ± 0.573 | 3 m |
| C | 1.074 ± 0.395 | 0.843 ± 0.354 | 1.223 ± 0.412 | 1.050 ± 0.108 | 6 m |
| C | 0.629 ± 0.161 | 0.601 ± 0.209 | 0.782 ± 0.262 | 0.869 ± 0.366 | 12 m |
| Group | Average Score 1 | Time (Months) | |
|---|---|---|---|
| Right Knee | Left Knee | ||
| A | 2.00 ± 1.22 | 2.40 ± 1.14 | 3 |
| B | 2.66 ± 0.81 | 2.00 ± 1.41 | 3 |
| A | 3.16 ± 0.98 | 2.17 ± 1.47 | 6 |
| B | 3.20 ± 1.64 | 2.16 ± 1.17 | 6 |
| A | 3.83 ± 0.40 | 2.00 ± 1.09 | 12 |
| B | 3.00 ± 1.09 | 1.33 ± 0.51 | 12 |
| C | 2.80 ± 1.30 | 3.00 ± 1.41 | 6 |
| C | 2.33 ± 1.50 # | 3.33 ± 0.81 # | 12 |
| Criteria | 12 Months | Group A | Group B | Group C | |||
|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | ||
| Synoviocytes | Proliferation | 0.50 ± 0.55 1 | 0.33 ± 0.52 1 | 0 1 | 0.17 ± 0.41 1 | 1.00 ± 0.63 | 1.17 ± 0.75 |
| Hypertrophy | 0.50 ± 0.55 | 0.67 ± 0.52 | 0.67 ± 0.52 | 0.67 ± 0.52 | 0.67 ± 0.52 | 0.83 ± 0.41 | |
| Inflammatory infiltrate | Granulocytic infiltrate | 0 | 0 | 0 | 0 | 0 | 0 |
| Fibrinous exudate | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymphoplasmacytic infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymphoplasmacytic aggregates/follicles | 0 | 0 | 0 | 0.17 ± 0.41 | 0 | 0 | |
| Synovial stroma | Villous hyperplasia | 1.17 ± 0.98 | 1.50 ± 0.55 | 1.17 ± 0.98 | 0.83 ± 0.75 | 2.33 ± 0.52 | 2.00 ± 1.10 |
| Proliferation of fibroblasts/fibrocytes | 2.33 ± 0.82 | 1.83 ± 0.411 | 2.00 ± 0.89 | 2.67 ± 0.522 | 2.00 ± 0.63 | 2.50 ± 0.55 | |
| Proliferation of blood vessels | 1.00 ± 0.63 | 0.83 ± 0.75 | 0.50 ± 0.55 | 0.83 ± 0.41 | 0.83 ± 0.41 | 0.83 ± 0.41 | |
| Cartilage/bone-detritus | 0 | 0.17 ± 0.41 | 0.50 ± 1.22 | 0.33 ± 0.82 | 0 | 0.50 ± 1.22 | |
| Hemosiderosis | 0 | 0 | 0 | 0 | 0 | 0 | |
| Group | Time (Months) | Average Score | |
|---|---|---|---|
| Right Knee | Left Knee | ||
| A | 3 | 55.17 ± 21.08 | 50.00 ± 10.41 |
| B | 3 | 65.00 ± 16.19 | 54.67 ± 8.33 |
| A | 6 | 76.50 ± 25.70 | 58.17 ± 17.55 |
| B | 6 | 78.83 ± 16.92 | 65.80 ± 15.00 |
| A | 12 | 96.00 ± 20.13 | 48.83 ± 15.38 |
| B | 12 | 65.50 ± 19.37 | 53.67 ± 22.18 |
| C | 6 | 77.17 ± 17.51 | 86.00 ± 14.64 |
| C | 12 | 68.00 ± 31.20 | 92.67 ± 23.83 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierbrauer, K.L.; Alasino, R.V.; Barclay, F.E.; Belotti, E.M.; Ortega, H.H.; Beltramo, D.M. Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model. Polymers 2021, 13, 4426. https://doi.org/10.3390/polym13244426
Bierbrauer KL, Alasino RV, Barclay FE, Belotti EM, Ortega HH, Beltramo DM. Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model. Polymers. 2021; 13(24):4426. https://doi.org/10.3390/polym13244426
Chicago/Turabian StyleBierbrauer, Karina L., Roxana V. Alasino, Fernando E. Barclay, Eduardo M. Belotti, Hugo H. Ortega, and Dante M. Beltramo. 2021. "Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model" Polymers 13, no. 24: 4426. https://doi.org/10.3390/polym13244426
APA StyleBierbrauer, K. L., Alasino, R. V., Barclay, F. E., Belotti, E. M., Ortega, H. H., & Beltramo, D. M. (2021). Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model. Polymers, 13(24), 4426. https://doi.org/10.3390/polym13244426