Material Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) Antibacterial Filaments—A Case Study of Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Manufacturing
2.3. 2D Digital Radiography
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Antimicrobial Properties
- (1)
- a standard method to assess the level of biofilm formation using the non-specific ability of crystal violet to bind to bacterial biomass [26];
- (2)
- a method using the reduction of colorless tetrazolium chloride to red formazan crystals in the presence of the living, metabolically active microorganisms [27];
- (3)
- quantitative cultures in an anaerobic atmosphere [28].
3. Results and Discussion
3.1. 2D Digital Radiography
3.2. SEM–EDS Analysis
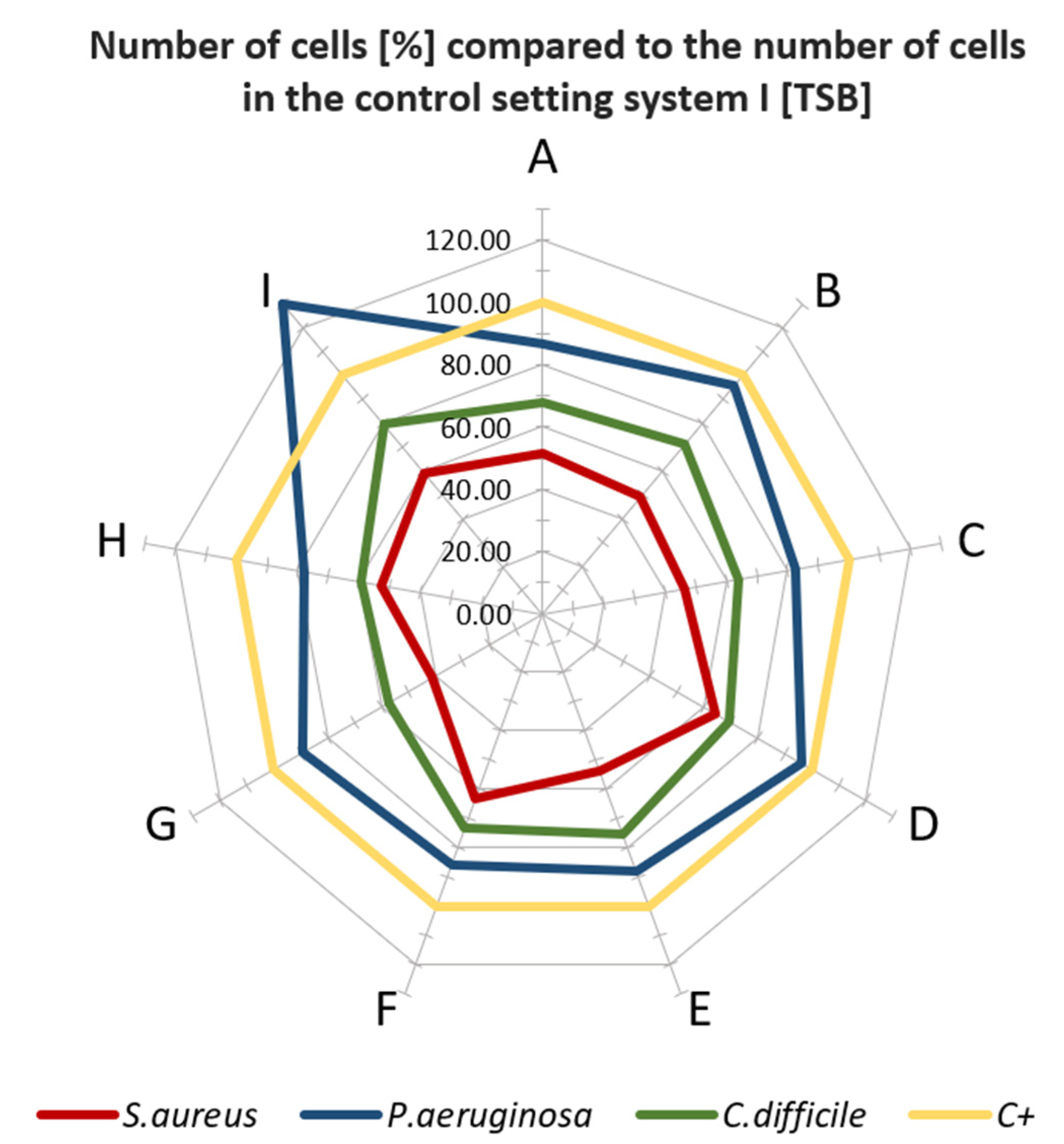
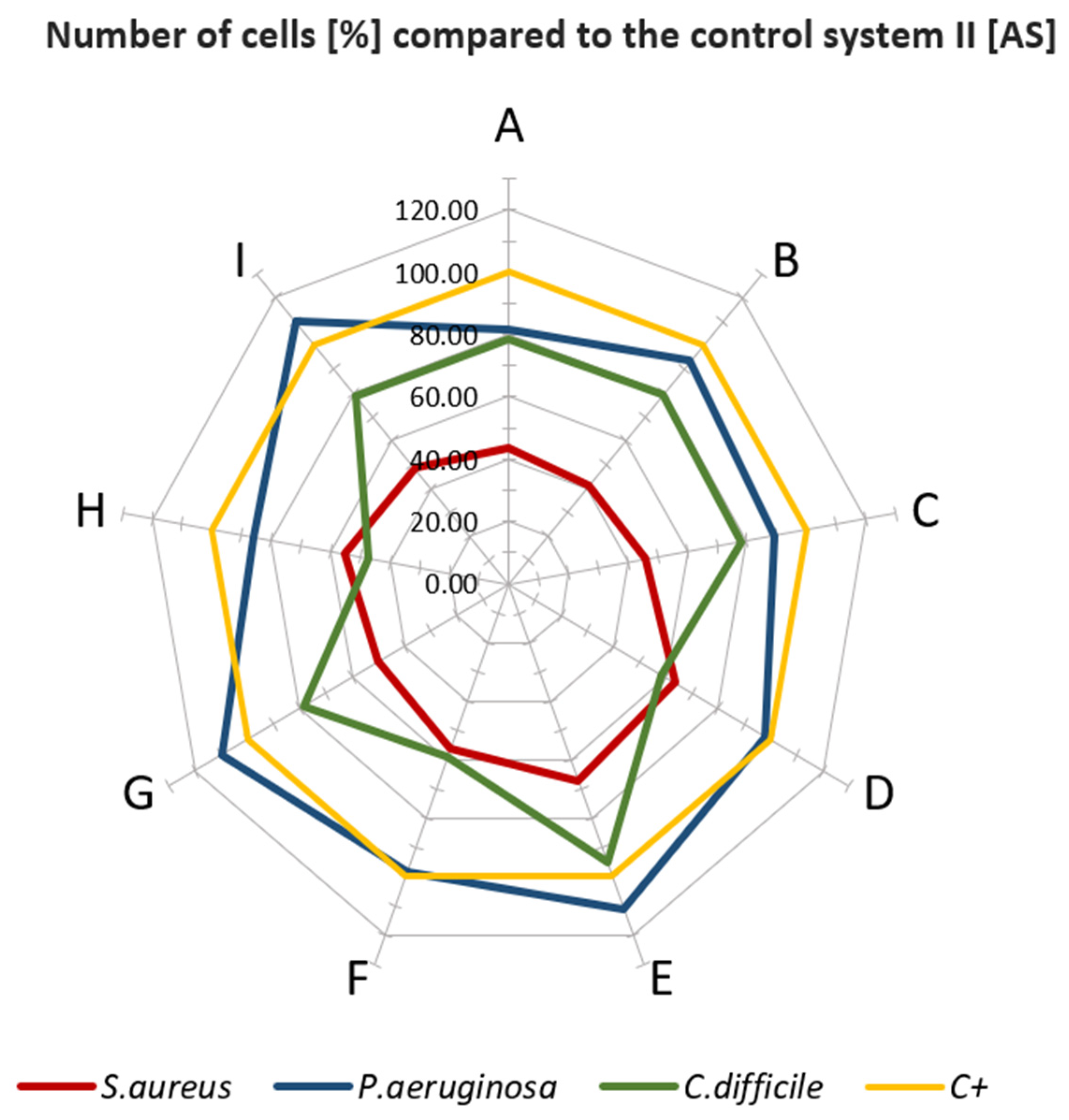
3.3. Microbiological Properties of Tested Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- COVID-19 Supply Chain Update: Importation of Vital Food and Medical Products|FDA. Available online: https://www.fda.gov/news-events/fda-voices/covid-19-supply-chain-update-importation-vital-food-and-medical-products (accessed on 23 November 2021).
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef] [PubMed]
- Mostaghimi, A.; Antonini, M.J.; Plana, D.; Anderson, P.D.; Beller, B.; Boyer, E.W.; Fannin, A.; Freake, J.; Oakley, R.; Sinha, M.S.; et al. Regulatory and Safety Considerations in Deploying a Locally Fabricated, Reusable Face Shield in a Hospital Responding to the COVID-19 Pandemic. Med 2020, 1, 139–151.e4. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budinoff, H.D.; Bushra, J.; Shafae, M. Community-driven PPE production using additive manufacturing during the COVID-19 pandemic: Survey and lessons learned. J. Manuf. Syst. 2021, 60, 799–810. [Google Scholar] [CrossRef]
- Maillard, J.Y. Testing the Effectiveness of Disinfectants and Sanitizers. Handb. Hyg. Control Food Ind. Second Ed. 2016, 569–586. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties-from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Armijo, P.R.; Markin, N.W.; Nguyen, S.; Ho, D.H.; Horseman, T.S.; Lisco, S.J.; Schiller, A.M. 3D printing of face shields to meet the immediate need for PPE in an anesthesiology department during the COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 302–308. [Google Scholar] [CrossRef]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef]
- Ghozali, M.; Fahmiati, S.; Triwulandari, E.; Restu, W.K.; Farhan, D.; Wulansari, M.; Fatriasari, W. PLA/metal oxide biocomposites for antimicrobial packaging application. Polym. Technol. Mater. 2020, 59, 1332–1342. [Google Scholar] [CrossRef]
- Psochia, E.; Papadopoulos, L.; Gkiliopoulos, D.J.; Francone, A.; Grigora, M.-E.; Tzetzis, D.; de Castro, J.V.; Neves, N.M.; Triantafyllidis, K.S.; Torres, C.M.S.; et al. Bottom-Up Development of Nanoimprinted PLLA Composite Films with Enhanced Antibacterial Properties for Smart Packaging Applications. Macromolecules 2021, 1, 5. [Google Scholar] [CrossRef]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.; Gadjanski, I.; Vidić, J. Antimicrobial nanoparticles and biodegradable polymer composites for active food packaging applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Rezanova, V.G.; Rezanova, N.M.; Plavan, V.P.; Viltsaniuk, O.O. The influence of nano-additives on the formation of matrix-fibrillar structure in the polymer mixture melts and on the properties of complex threads. Fibres Text. 2017, 24, 37–42. [Google Scholar]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Nerantzaki, M.; Kehagias, N.; Francone, A.; Fernández, A.; Sotomayor Torres, C.M.; Papi, R.; Choli-Papadopoulou, T.; Bikiaris, D.N. Design of a Multifunctional Nanoengineered PLLA Surface by Maximizing the Synergies between Biochemical and Surface Design Bactericidal Effects. ACS Omega 2018, 3, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M. 3D Printed Antibacterial Prostheses. Appl. Sci. 2018, 8, 1651. [Google Scholar] [CrossRef] [Green Version]
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymer 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.J. Digital Applications of Radiography. In Proceedings of the 3rd MENDT—East Nondestructive Testing Conference & Exhibition, Manama, Bahrain, 13–16 September 2005; Available online: www.ndt.net (accessed on 23 November 2021).
- Ewert, U. Current Developments in Digital Radiography and Computed Tomography from nm to macro scale. In Proceedings of the 12th European Conference on Non-Destructive Testing (ECNDT 2018), Gothenburg, Sweden, 11–15 June 2018. [Google Scholar]
- Junka, A.; Szymczyk, P.; Ziółkowski, G.; Karuga-Kuzniewska, E.; Smutnicka, D.; Bil-Lula, I.; Bartoszewicz, M.; Mahabady, S.; Sedghizadeh, P.P. Bad to the Bone: On In Vitro and Ex Vivo Microbial Biofilm Ability to Directly Destroy Colonized Bone Surfaces without Participation of Host Immunity or Osteoclastogenesis. PLoS ONE 2017, 12, e0169565. [Google Scholar] [CrossRef] [PubMed]
- Coffey, B.M.; Anderson, G.G. Biofilm formation in the 96-well microtiter plate. Methods Mol. Biol. 2014, 1149, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Naaber, P.; Štšepetova, J.; Smidt, I.; Rätsep, M.; Kõljalg, S.; Lõivukene, K.; Jaanimäe, L.; Löhr, I.H.; Natås, O.B.; Truusalu, K.; et al. Quantification of Clostridium difficile in antibiotic-associated-diarrhea patients. J. Clin. Microbiol. 2011, 49, 3656–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppmann, T.; Yatsenko, S.; Leonhardt, S.; Krampe, E.; Radovanovic, I.; Bastian, M.; Wintermantel, E. Antimicrobial polymers—The antibacterial effect of photoactivated nano titanium dioxide polymer composites. AIP Conf. Proc. 2015, 1593, 440. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Jarmo, O.; Veli-Jukka, A.; Eero, M. Treatment of Clostridioides (Clostridium) difficile infection. Ann. Med. 2019, 52, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Lertwimol, T.; Kosorn, W.; Hankamolsiri, W.; Nampichai, N.; Janvikul, W. 3D-printing antibacterial composite filaments containing synergistic antibacterial activity of green tea and tannic acid. Polym. Adv. Technol. 2021, 32, 4733–4744. [Google Scholar] [CrossRef]
- Maróti, P.; Kocsis, B.; Ferencz, A.; Nyitrai, M.; Lőrinczy, D. Differential thermal analysis of the antibacterial effect of PLA-based materials planned for 3D printing. J. Therm. Anal. Calorim. 2020, 139, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Mania, S.; Ryl, J.; Jinn, J.R.; Wang, Y.J.; Michałowska, A.; Tylingo, R. The Production Possibility of the Antimicrobial Filaments by Co-Extrusion of the PLA Pellet with Chitosan Powder for FDM 3D Printing Technology. Polymer 2019, 11, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Cabezas, M.A.; Martínez-García, A.; Varela-Gandía, F.J. Innovative functionalized monofilaments for 3D printing using fused deposition modeling for the toy industry. Procedia Manuf. 2017, 13, 738–745. [Google Scholar] [CrossRef]
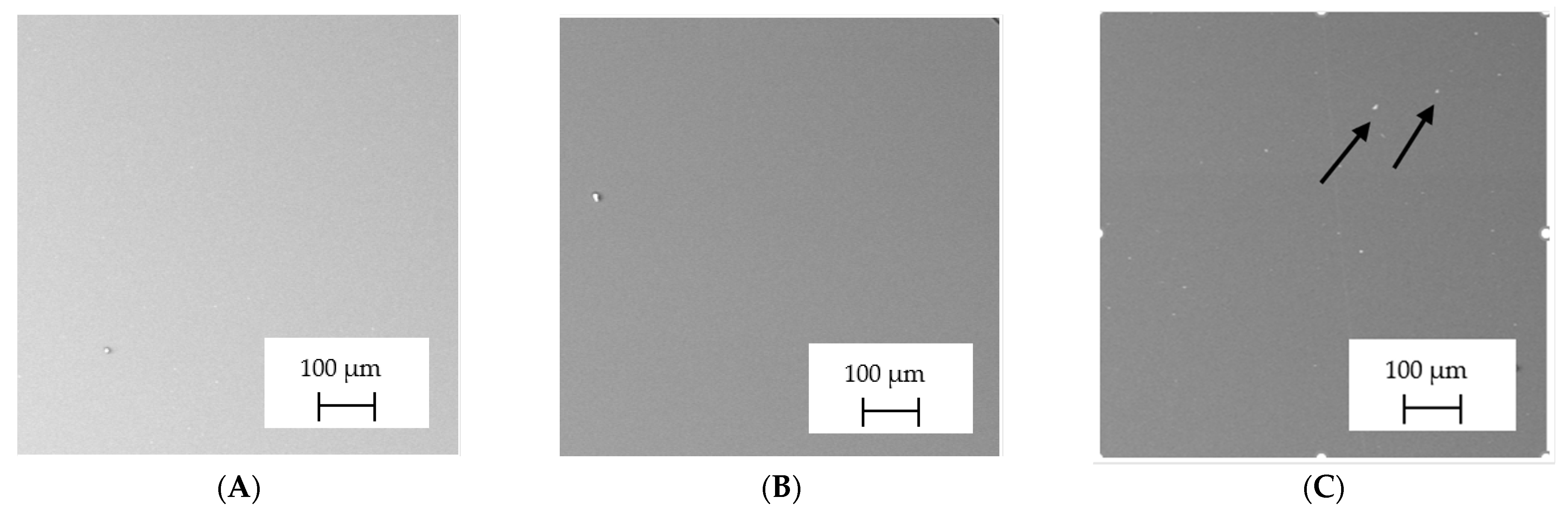
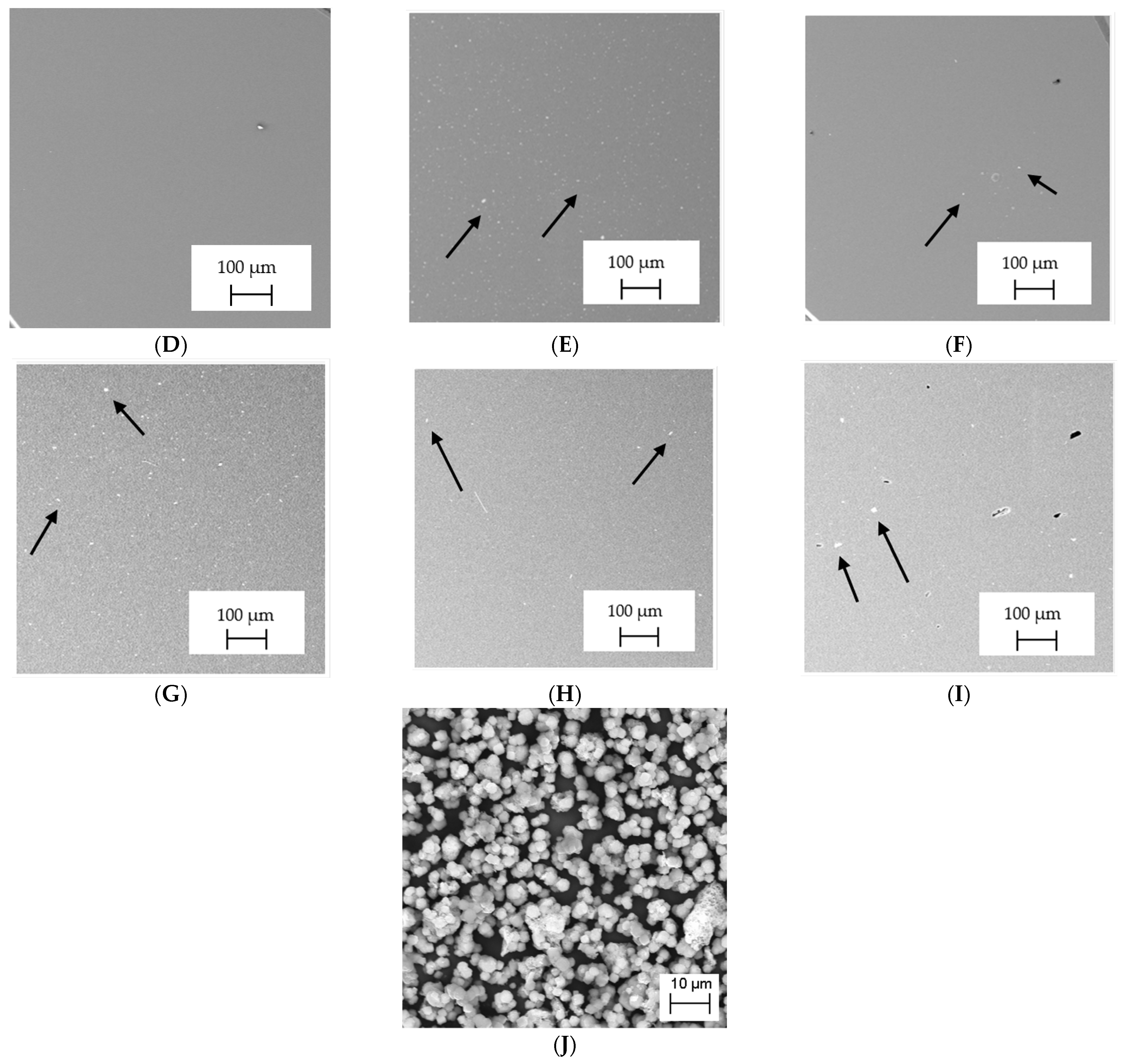
| Name in the Manuscript | Trade Name | Manufacturer | Chemical Composition | LOT; S/N; Batch No. |
|---|---|---|---|---|
| A | AbFil PLA 850 | 3D Fils (Elche, Spain) | PLA with silver additives | 20042908DIJ |
| B | Mega 3D Antibacterial PLA | FiberForce (Treviso, Italy) | Based on PLA, manufacturer doesn’t specify additives | FX-100-30 |
| C | NanoCICLA | Cicla3D (Bío, Argentina) | PLA with copper nanoparticles | 0000000503 |
| D | PLA Antibacterial | Philament/Filaticum (Miskolc, Hungary) | PLA with metal additives | N/S * |
| E | PLActive AN1 | Copper3D (Santiago, Chile) | PLA with Nano-Copper additive | 16708001 |
| F | PrimaSelect PLA AntiBac | PrimaCreator (Malmö, Sweden) | Based on PLA, manufacturer doesn’t specify additives | FB0195 |
| G | Smartfil | SMART MATERIALS 3D (Alcalá la Real (Jaén), Spain) | PLA with silver nanoparticles | 129417002085 |
| H | Tarfuse® PLA AM | Grupa Azoty S.A. (Tarnów, Polska) | Based on PLA, manufacturer doesn’t specify additives | N/S * |
| I | Antibacterial PLA | XYZ Printing Inc. (New Taipei City, Taiwan) | PLA with silver additives | RFPLK-FPE-B6W-TH-92K-0364 |
| Process Parameters | ||||
|---|---|---|---|---|
| Layer Thickness (mm) | Infill (%) | Cooling Fan Speed (%) | Perimeter Speed (mm/s | Infill Speed (mm/s) |
| 0.2 | 100 | 100 | 45 | 80 |
| Material Specific Process Parameters | ||||
| Material Name | Printhead Temperature (°C) | Bed Temperature (°C) | ||
| A | 210 | 50 | ||
| B | 210 | 55 | ||
| C | 200 | 60 | ||
| D | 210 | 60 | ||
| E | 200 | 60 | ||
| F | 210 | 50 | ||
| G | 220 | 60 | ||
| H | 220 | 60 | ||
| I | 205 | 50 | ||
| Material Name | RTG of Filament | RTG of FFF Sample |
|---|---|---|
| A | 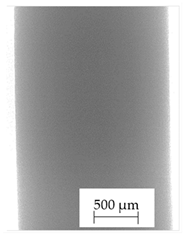 |  |
| B |  | 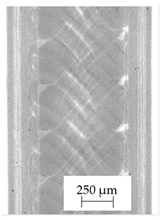 |
| C |  |  |
| D | 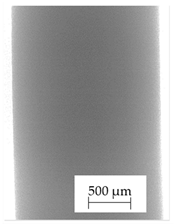 |  |
| E |  | 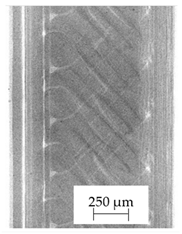 |
| F |  |  |
| G |  |  |
| H |  |  |
| I |  |  |
| Material Name | Elements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | Si | Ag | Ca | Cu | P | Mg | Na | Ti | Cl | |
| A | 66.55 ± 16.25 | 19.3 ± 9.8 | 0.45± 0.05 | 6.35 ± 5.55 | - | 6.70 ± 0.01 | - | - | 0.50 ± 0.01 | 1.10 ± 0.01 | 6.50± 0.01 | - |
| B | 88.37 ± 5.76 | 5.40 ± 1.49 | 0.30 ± 0.20 | 0.70 ± 0.16 | - | - | - | - | 0.10 ± 0.01 | 0.15± 0.05 | 1.10 ± 0.20 | 13.20 ± 0.10 |
| C | 90.50 ± 0.92 | 7.73 ± 0.75 | - | 0.60 ± 0.01 | - | 1.50 ± 0.01 | 0.10 ± 0.01 | 1.10 ± 0.01 | 0.40 ± 0.01 | - | - | - |
| D | 91.50 ± 1.28 | 7.37 ± 1.22 | 0.30 ± 0.08 | 0.67 ± 0.24 | - | - | - | - | 0.25 ± 0.05 | 0.20 ± 0.01 | - | - |
| E | 90.03 ± 1.27 | 7.97 ± 0.73 | 0.90 ± 0.22 | 0.83 ± 0.17 | - | - | 0.50 ± 0.10 | - | - | - | - | - |
| F | 91.43 ± 1.47 | 6.73 ± 0.90 | 0.43 ± 0.05 | 0.40 ± 0.08 | - | - | - | - | 0.30 ± 0.10 | 0.40 ± 0.01 | 2.10 ± 0.01 | - |
| G | 76.17 ± 9.38 | 9.10 ± 0.21 | 1.16 ± 0.24 | 4.36 ± 2.64 | 0.03 ± 0.05 | 9.13 ± 10.9 | - | - | - | - | - | - |
| H | 88.17 ± 6.41 | 6.70 ± 0.22 | 2.60 ± 3.18 | 2.53 ± 2.95 | - | - | 0.10 ± 0.01 | - | - | - | - | - |
| I | 86.64 ± 4.52 | 10.60 ± 3.29 | 0.40 ± 0.22 | 0.80 ± 0.36 | - | - | - | - | 1.20 ± 0.54 | 1.20 ± 0.01 | - | - |
| J | 3.52 ± 0.68 | 29.17 ± 3.50 | 3.74 ± 0.23 | 2.56 ± 0.11 | - | - | 57.65 ± 1.58 | - | - | - | - | 4.33 ± 0.23 |
| Material Name | Producer Report According to Antimicrobial Activity |
|---|---|
| A | N/A |
| B | N/A |
| C | Method based on ISO 22196. Effectiveness on E. coli ATCC 8739 after 8 h—99.97181% Effectiveness on E. coli ATCC 8739 after 24 h—99.98909% |
| D | N/A |
| E | Effectiveness on S. aureus MRSA after 8 h >98%; after 24 h > 99.99% Effectiveness on E. coli DH5 after 8 h > 98% after 24 h > 99.99%. |
| F | Method based on ISO 22196. Effectiveness on S. aureus after 24 h 99.59% Effectiveness on E. coli DH5 after 24 h 88.43% |
| G | Method based on JIS Z 2801 (ISO 22196). Effectiveness on S. aureus CECT 240, ATCC 6538 P after 24 h—99.99% Effectiveness on E. coli CECT 516, ATCC 8739 after 24 h—99.99% |
| H | The antibacterial additives used in the H filament are approved for marketing in the European Union—they comply with the European Regulation on biocidal products (BPR, Regulation (EU) 528/2012 and with the requirements of the American Environmental Protection Agency—Antimicrobial Division of the Environmental Protection Agency (EPA).The antibacterial additives used are included in the list of chemical compounds approved by OEKO-TEX. |
| I | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, P.; Hoppe, V.; Grochowska, E.; Paleczny, J.; Junka, A.; Smolina, I.; Kurzynowski, T. Material Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) Antibacterial Filaments—A Case Study of Antimicrobial Properties. Polymers 2021, 13, 4337. https://doi.org/10.3390/polym13244337
Gruber P, Hoppe V, Grochowska E, Paleczny J, Junka A, Smolina I, Kurzynowski T. Material Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) Antibacterial Filaments—A Case Study of Antimicrobial Properties. Polymers. 2021; 13(24):4337. https://doi.org/10.3390/polym13244337
Chicago/Turabian StyleGruber, Piotr, Viktoria Hoppe, Emilia Grochowska, Justyna Paleczny, Adam Junka, Irina Smolina, and Tomasz Kurzynowski. 2021. "Material Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) Antibacterial Filaments—A Case Study of Antimicrobial Properties" Polymers 13, no. 24: 4337. https://doi.org/10.3390/polym13244337
APA StyleGruber, P., Hoppe, V., Grochowska, E., Paleczny, J., Junka, A., Smolina, I., & Kurzynowski, T. (2021). Material Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) Antibacterial Filaments—A Case Study of Antimicrobial Properties. Polymers, 13(24), 4337. https://doi.org/10.3390/polym13244337








