Stiff-Elongated Balance of PLA-Based Polymer Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Melt Blending
2.3. Binary Blends Characterization
2.3.1. Tensile Tests
2.3.2. Statistical Analysis
2.4. Ternary Blends Characterization
2.4.1. Dynamic Mechanical Thermal Analysis
2.4.2. Rheological Measurements
2.4.3. Thermogravimetric Analysis
2.4.4. Scanning Electron Microscopy
3. Results and Discussion
3.1. Binary Blends
3.1.1. Poly(lactic acid)-Elongated Polymer
3.1.2. Poly(lactic acid)-Stiff Polymer
3.2. Ternary Blends
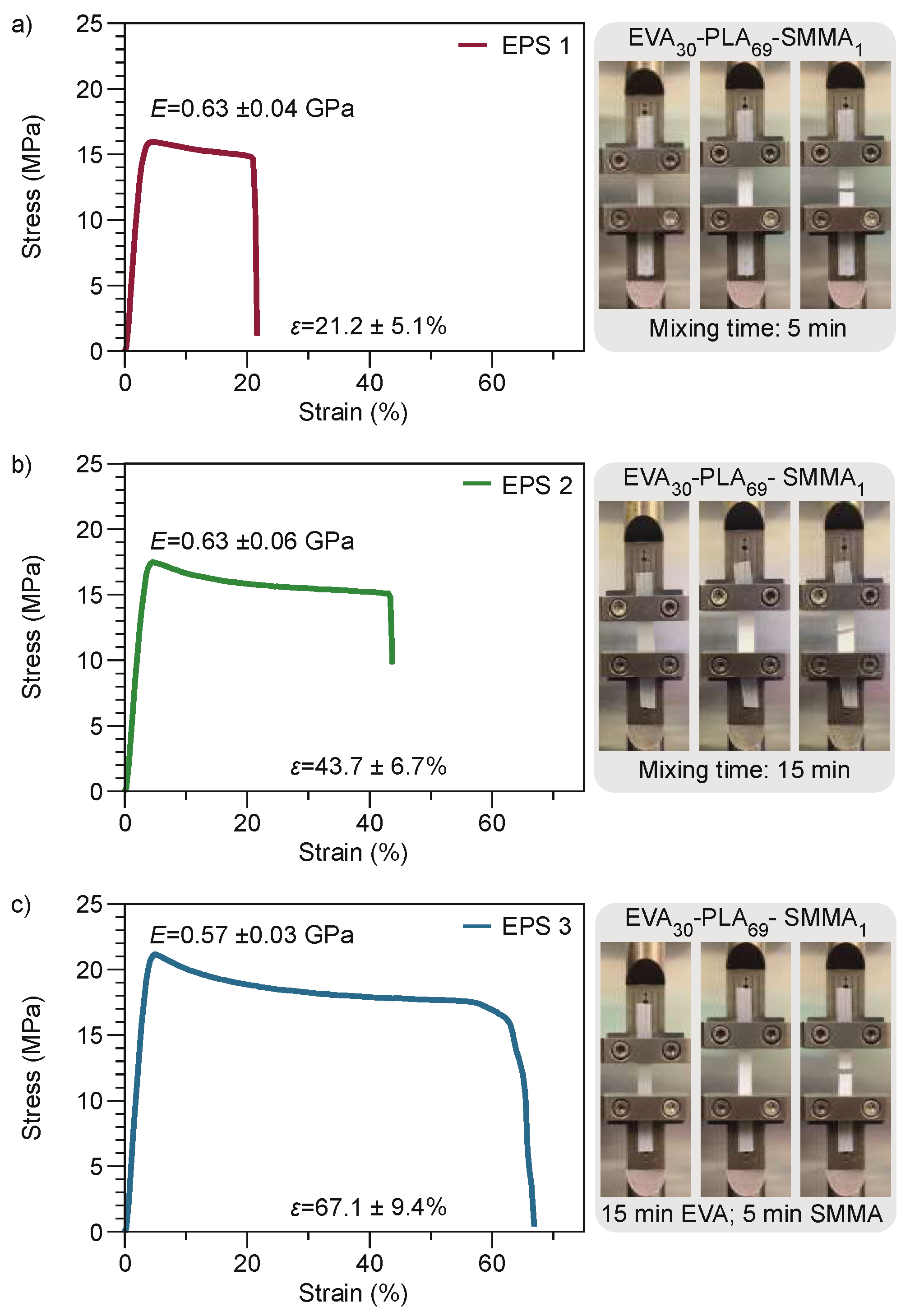
3.2.1. Tensile Tests
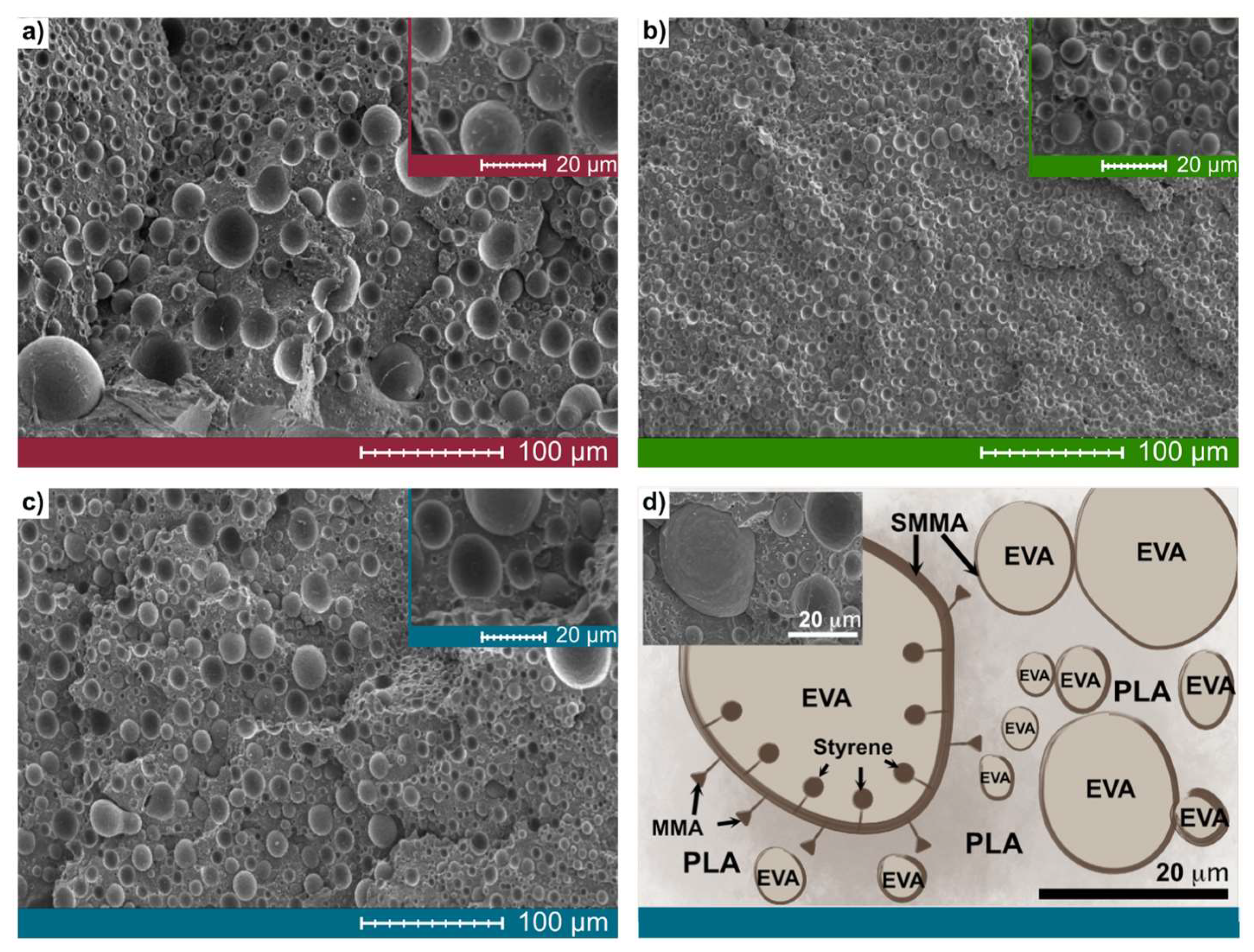
3.2.2. Morphology of Ternary Blends
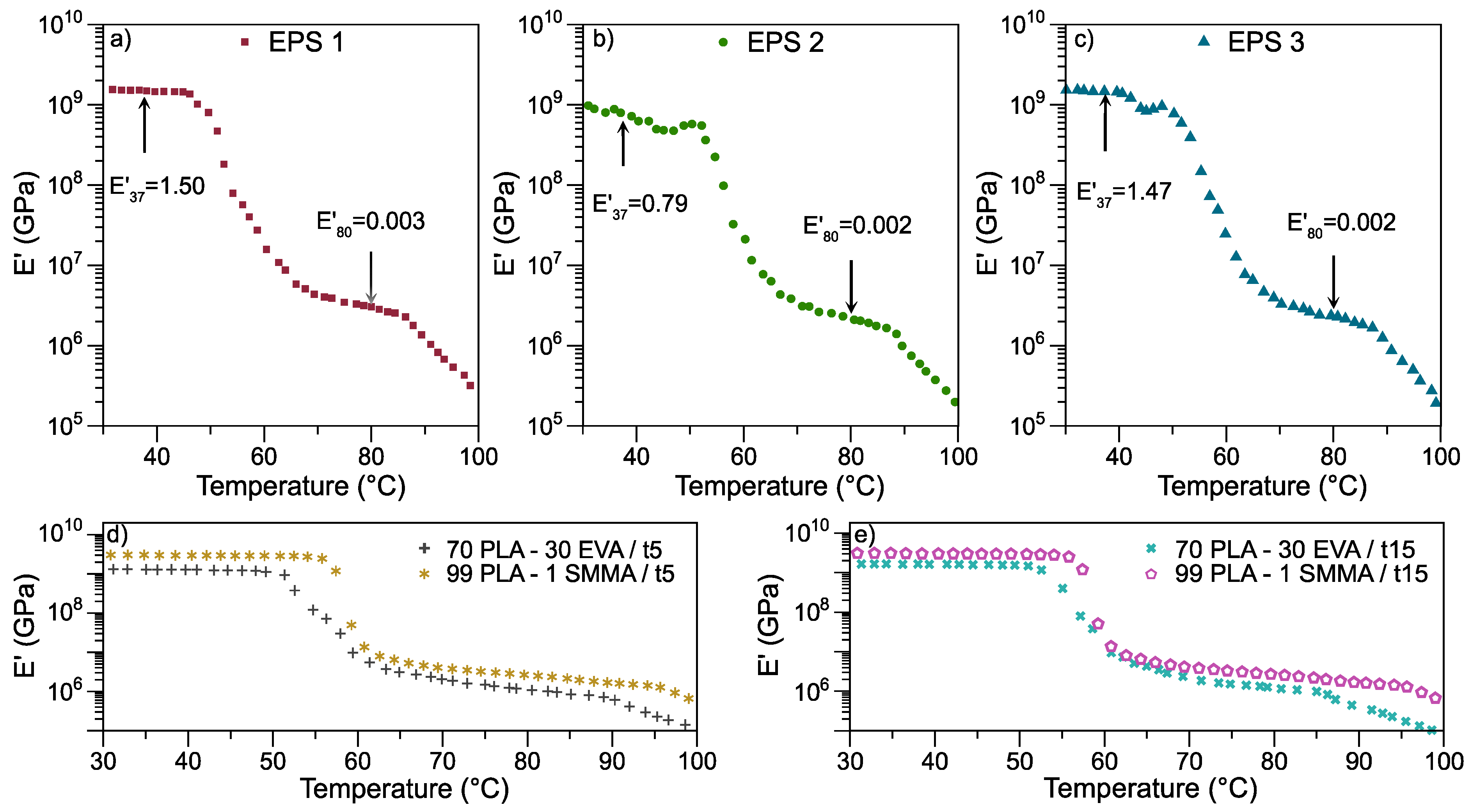
3.2.3. Dynamic Mechanical Thermal Analysis
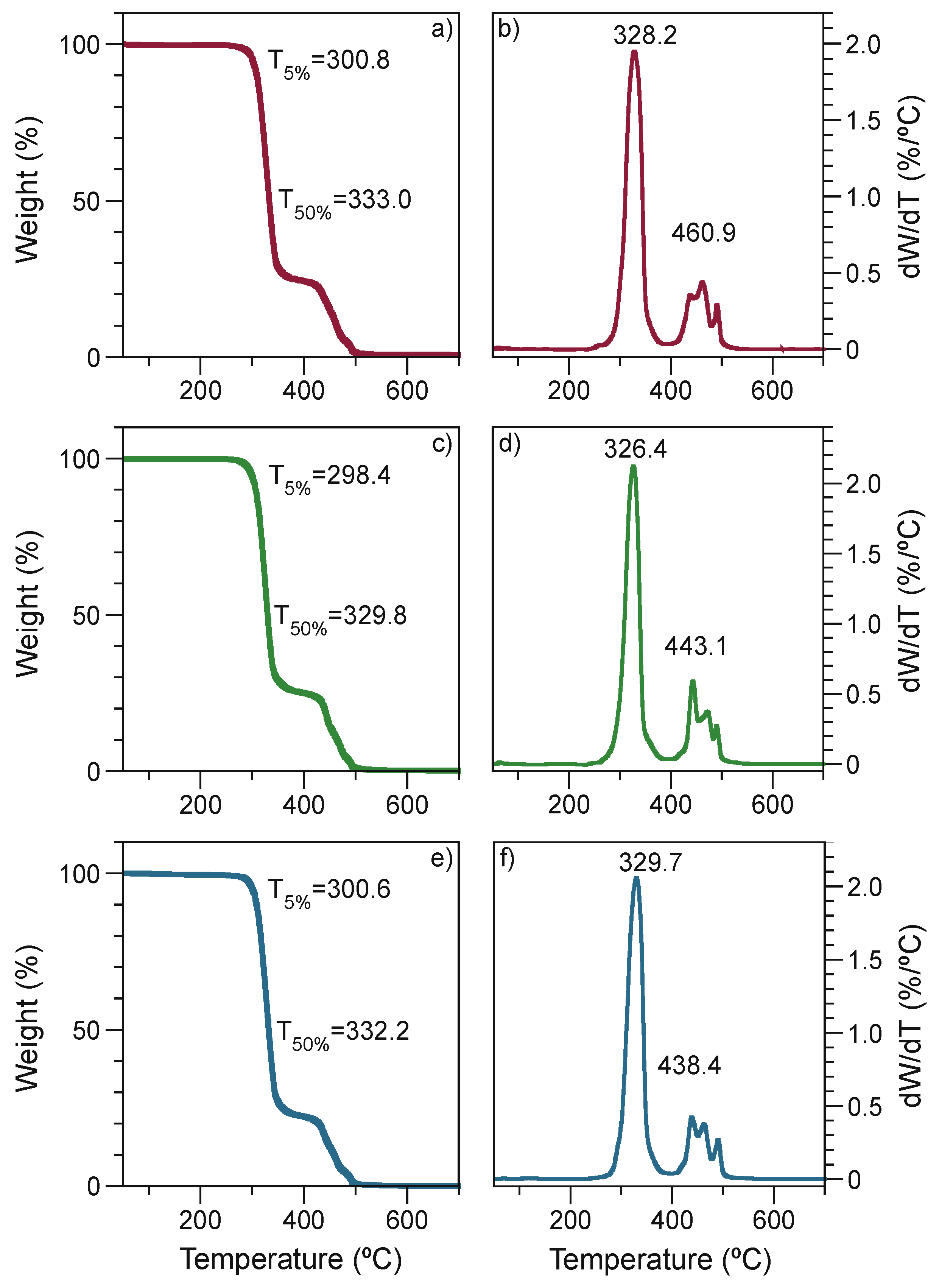
3.2.4. Thermogravimetric Analysis
3.2.5. Rheological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asad, M.I.; Ahmed, N.; Ur-Rehman, A.; Khan, G.M. Polylactide: The polymer revolutionizing the biomedical field. In Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers, 1st ed.; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 215–251. [Google Scholar] [CrossRef]
- Abdulghani, S.O.; Salih, S.I.; Razzeg, K.H. The effect of type and ratios of secondary materials on some mechanical properties of the ternary polymer blends based on the unsaturated polyester. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Haneef, I.N.H.M.; Buys, Y.F.; Shaffiar, N.M.; Shaharuddin, S.I.S.; Nor Khairusshima, M.K. Miscibility, mechanical, and thermal properties of polylactic acid/polypropylene carbonate (PLA/PPC) blends prepared by melt-mixing method. Mater. Today Proc. 2019, 17, 534–542. [Google Scholar] [CrossRef]
- Fredi, G.; Rigotti, D.; Bikiaris, D.N.; Dorigato, A. Tuning thermo-mechanical properties of poly(lactic acid) films through blending with bioderived poly(alkylene furanoate)s with different alkyl chain length for sustainable packaging. Polymer 2021, 218, 123527. [Google Scholar] [CrossRef]
- Kivade, S.B.; Gunge, A.; Nagamadhu, M.; Rajole, S. Mechanical and dynamic mechanical behavior of acetylation-treated plain woven banana reinforced biodegradable composites. Adv. Compos. Hybrid Mater. 2021, in press. [Google Scholar] [CrossRef]
- Zhu, E.Q.; Xu, G.F.; Sun, S.F.; Yang, J.; Yang, H.Y.; Wang, D.W.; Guo, Z.H.; Shi, Z.J.; Deng, J. Rosin acid modification of bamboo powder and thermoplasticity of its products based on hydrothermal pretreatment. Adv. Compos. Hybrid Mater. 2021, 4, 584–590. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. Au. 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties–From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Alsaheb, R.A.A.; Aladdin, A.; Othman, N.Z.; Malek, R.A.; Leng, O.M.; Aziz, R.; El Enshasy, H.A. Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res. 2015, 7, 51–63. [Google Scholar]
- Holt, A.; Ke, Y.; Bramhall, J.A.; Crane, G.; Grubbs, J.B.; White, E.M.; Horn, J.; Locklin, J. Blends of Poly(butylene glutarate) and Poly(lactic acid) with Enhanced Ductility and Composting Performance. ACS Appl. Polym. Mater. 2021, 3, 1652–1663. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Kontárová, S.; Prikryl, R.; Melcová, V.; Mencik, P.; Horálek, M. Printability, Mechanical and Thermal Properties of Poly(3-Hydroxybutyrate)-Poly(Lactic Acid)-Plasticizer Blends for Three-Dimensional (3D) Printing. Materials 2020, 13, 4736. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, Y.; Feng, J.; Zhang, X.; Zhang, C.; Tang, Z.; Zhu, J. Preparation and characterization of poly(lactic acid)/starch composites toughened with epoxidized soybean oil. Carbohydr. Polym. 2013, 92, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shi, J.; Mao, L.; Wang, Z.; Zhang, L. Improvement of Compatibility and Mechanical Performances of PLA/PBAT Composites with Epoxidized Soybean Oil as Compatibilizer. Ind. Eng. Chem. Res. 2020, 59, 21779–21790. [Google Scholar] [CrossRef]
- Huang, Y.; MuÌller, M.T.; Boldt, R.; Zschech, C.; Gohs, U.; Wieaner, S. Improved Rheology, Crystallization, and Mechanical Performance of PLA/mPCL Blends Prepared by Electron-Induced Reactive Processing. ACS Sustain. Chem. Eng. 2021, 9, 3478–3489. [Google Scholar] [CrossRef]
- Chen, J.; Rong, C.; Lin, T.; Chen, Y.; Wu, J.; You, J.; Wang, H.; Li, Y. Stable Co-Continuous PLA/PBAT Blends Compatibilized by Interfacial Stereocomplex Crystallites: Toward Full Biodegradable Polymer Blends with Simultaneously Enhanced Mechanical Properties and Crystallization Rates. Macromolecules 2021, 54, 2852–2861. [Google Scholar] [CrossRef]
- Kolařík, J.; Lednický, F.; Locati, G.; Fambri, L. Ultimate properties of polycarbonate blends: Effects of inclusion plastic deformation and of matrix phase continuity. Polym. Eng. Sci. 1997, 37, 128–137. [Google Scholar] [CrossRef]
- Ishida, S.; Nagasaki, R.; Chino, K.; Dong, T.; Inoue, Y. Toughening of Poly(L-lactide) by Melt Blending with Rubbers. J. Appl. Polym. Sci. 2009, 113, 558–566. [Google Scholar] [CrossRef]
- Meredith, J.C.; Amis, E.J. LCST phase separation in biodegradable polymer blends: Poly(D,L-lactide) and poly(ε-caprolactone). Macromol. Chem. Phys. 2000, 201, 733–739. [Google Scholar] [CrossRef]
- Wang, M.; Liang, X.; Wu, H.; Huang, L.; Jin, G. Super toughed poly (lactic acid)/poly (ethylene vinyl acetate) blends compatibilized by ethylene-methyl acrylate-glycidyl methacrylate copolymer. Polym. Degrad. Stab. 2021, 193, 109705. [Google Scholar] [CrossRef]
- Lohrasbi, P.; Yeganeh, J.K. Synergistic toughening of poly(lactic acid)/poly(ethylene vinyl acetate) (PLA/EVA) by dynamic vulcanization and presence of hydrophobic nanoparticles. Polym. Adv. Technol. 2021, 32, 4326–4339. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Melt Rheology of Poly(Lactic Acid)/Low Density Polyethylene Polymer Blends. Adv. Chem. Eng. Sci. 2011, 1, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.M.; Garcia-Garcia, D.; Rayón, E.; Samper, M.D.; Balart, R. Compatibilization and characterization of polylactide and biopolyethylene binary blends by non-reactive and reactive compatibilization approaches. Polymers 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, A.; Massardier, V.; Santulli, C.; Valente, M. Optimization of Thermoplastic Blend Matrix HDPE/PLA with Different Types and Levels of Coupling Agents. Materials 2018, 11, 2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Dorigato, A. Recycling of polymer blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Sastri, V.R. Plastics in Medical Devices: Properties, Requirements, and Applications, 1st ed.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Solorio, L.; Vega, A. Filament Extrusion and Its 3D Printing of Poly(Lactic Acid )/Poly(Styrene-co-Methyl Methacrylate ) Blends. Appl. Sci. 2019, 9, 5153. [Google Scholar] [CrossRef] [Green Version]
- Mihai, M.; Canada, C. Extrusion Foaming of Polylactide. In Polymeric Foams: Innovations in Process, Technologies, and Products, 1st ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2017; p. 52. [Google Scholar]
- Shabani, A.; Babaei, A.; Zanjanijam, A.R. Does nanoclay addition always lead to amelioration? Dual effects of the nanoclay on the PA-6/EVOH/SEBS ternary blends. Polym. Adv. Technol. 2019, 30, 983–997. [Google Scholar] [CrossRef]
- Liu, X.; Lei, L.; Hou, J.-W.; Tang, M.-F.; Guo, S.-R.; Wang, Z.-M.; Chen, K.-M. Evaluation of two polymeric blends (EVA/PLA and EVA/PEG) as coating film materials for paclitaxel-eluting stent application. J. Mater. Sci. Mater. Med. 2011, 22, 327–337. [Google Scholar] [CrossRef]
- Sharma, R.; Maiti, S.N. Modification of tensile and impact properties of poly(butylene terephthlate) by incorporation of styrene-ethylene-butylene-styrene and maleic anhydride- grafted -SEBS (SEBS-g-MA) terpolymer. Polym. Eng. Sci. 2013, 53, 2242–2253. [Google Scholar] [CrossRef]
- Sharma, R.; Maiti, S.N. Effects of Crystallinity of PP and Flexibility of SEBS-g-MA Copolymer on the mechanical properties of PP/SEBS-g-MA blends. Polym. Plast. Technol. Eng. 2014, 53, 229–238. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Anis Sakinah, Z.A.; Ratnam, C.T.; Luqman Chuah, A.; Yaw, C.S. Effect of mixing conditions on the tensile properties of ethylene vinyl acetate/waste tire dust (EVA/WTD) blend. Polym. Plast. Technol. Eng. 2009, 48, 1139–1142. [Google Scholar] [CrossRef]
- Zuo, X.; Xue, Y.; Wang, L.; Zhou, Y.; Yin, Y.; Chuang, Y.C.; Chang, C.C.; Yin, R.; Rafailovich, M.H.; Guo, Y. Engineering Styrenic Blends with Poly(lactic acid). Macromolecules 2019, 52, 7547–7556. [Google Scholar] [CrossRef]
- Kugimoto, D.; Kouda, S.; Yamaguchi, M. Improvement of mechanical toughness of poly(lactic acid) by addition of ethylene-vinyl acetate copolymer. Polym. Test. 2019, 80, 106021. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of biodegradable polylactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Komalan, C.; George, K.E.; Kumar, P.A.S.; Varughese, K.T.; Thomas, S. Dynamic mechanical analysis of binary and ternary polymer blends based on nylon copolymer/EPDM rubber and EPM grafted maleic anhydride compatibilizer. Express. Polym. Lett. 2007, 1, 641–653. [Google Scholar] [CrossRef]
- Singla, R.K.; Zafar, M.T.; Maiti, S.N.; Ghosh, A.K. Physical blends of PLA with high vinyl acetate containing EVA and their rheological, thermo-mechanical and morphological responses. Polym. Test. 2017, 63, 398–406. [Google Scholar] [CrossRef]
- Costache, M.C.; Jiang, D.D.; Wilkie, C.A. Thermal degradation of ethylene-vinyl acetate coplymer nanocomposites. Polymers 2005, 46, 6947–6958. [Google Scholar] [CrossRef]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Tomić, N.Z.; Marinković, A.D.; Veljović, Đ.; Trifković, K.; Lević, S.; Radojević, V.; Jančić Heinemann, R. A new approach to compatibilization study of EVA/PMMA polymer blend used as an optical fibers adhesive: Mechanical, morphological and thermal properties. Int. J. Adhes. Adhes. 2018, 81, 11–20. [Google Scholar] [CrossRef]
- Tomić, N.Z. Thermal studies of compatibilized polymers blends. In Compatibilization of Polymer Blends Micro and Nano Scale Phase Morphologies, Interphase Characterization, and Properties, 2nd ed.; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 489–510. [Google Scholar]
- Yamaguchi, M.; Shigehiko, A. LLDPE/LDPE blends. I. Rheological, thermal, and mechanical properties. J. Appl. Polym. Sci. 1999, 74, 3153–3159. [Google Scholar] [CrossRef]
- Jaggi, H.S.; Satapathy, B.K.; Ray, A.R. Viscoelastic properties correlations to morphological and mechanical response of HDPE/UHMWPE blends. J. Polym. Res. 2014, 21, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. Part B Eng. 2019, 173, 107028–107038. [Google Scholar] [CrossRef]
- Numin, M.S.; Jumbri, K.; Ramli, A.; Borhan, N. Microemulsion rheological analysis of alkaline, surfactant, and polymer in oil-water interface. Processes 2020, 8, 762. [Google Scholar] [CrossRef]
- Chauhan, G.; Ojha, K. Synthesis of a bio-polymer nanocomposite fracturing fluid for HTHP application. In Proceedings of the Society of Petroleum Engineers, Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar]
- Petisco-Ferrero, S.; Fernández, J.; Martín, M.M.F.S.; Ibarburu, P.A.S.; Oiz, J.R.S. The relevance of molecular weight in the design of amorphous biodegradable polymers with optimized shape memory effect. J. Mech. Behav. Biomed. Mater. 2016, 61, 541–553. [Google Scholar] [CrossRef]
- Das, D.; Satapathy, B.K. Microstructure-rheological percolation-mechanical properties correlation of melt-processed polypropylene-multiwall carbon nanotube nanocomposites: Influence of matrix tacticity combination. Mater. Chem. Phys. 2014, 147, 127–140. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, C. Rheological and conductive percolation laws for syndiotactic polystyrene composites filled with carbon nanocapsules and carbon nanotubes. Carbon 2011, 49, 2334–2344. [Google Scholar] [CrossRef]
- Hoseini, M.; Haghtalab, A.; Famili, M.H.N. Rheology and morphology study of immiscible linear low-density polyethylene/poly(lactic acid) blends filled with nanosilica particles. J. Appl. Polym. Sci. 2017, 134, 45526–45538. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, Z.; Zhang, Y.; Zhang, Y.; Zhang, X.; Zhou, W. Rheological behavior of polypropylene/novolac blends. J. Appl. Polym. Sci. 2007, 106, 811–816. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, Y.; Zou, H.; Liang, M.; Cao, Y. structural and rheological property relationship of bimodal polyethylene with improved environment. Polym. Sci. Ser. A 2014, 56, 671–680. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Simon, G.; Kukaleva, N. Rheological and viscoelastic behavior of HDPE/Octamethyl-POSS nanocomposites. Macromolecules 2006, 39, 1839–1849. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Chen, F.-Q.; Wu, Z.-H.; Feng, Y.-H.; Qu, J.-P. Morphology, mechanical, and rheological properties of poly(lactic acid)/ethylene acrylic acid copolymer blends processing via vane extruder. J. Appl. Polym. Sci. 2014, 131, 40146. [Google Scholar] [CrossRef]
- Li, R.; Yu, W.; Zhou, C.; Zhou, A.C. Rheological characterization of droplet-matrix versus co-continuous morphology. J. Macromol. Sci. Part B Phys. 2006, 45, 889–898. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, M.; Song, Y.; Yan, X.; Zheng, Q. Dynamic rheology and dielectric relaxation of poly(vinylidene fluoride)/poly(methyl methacrylate) blends. Compos. Sci. Technol. 2015, 106, 39–46. [Google Scholar] [CrossRef]
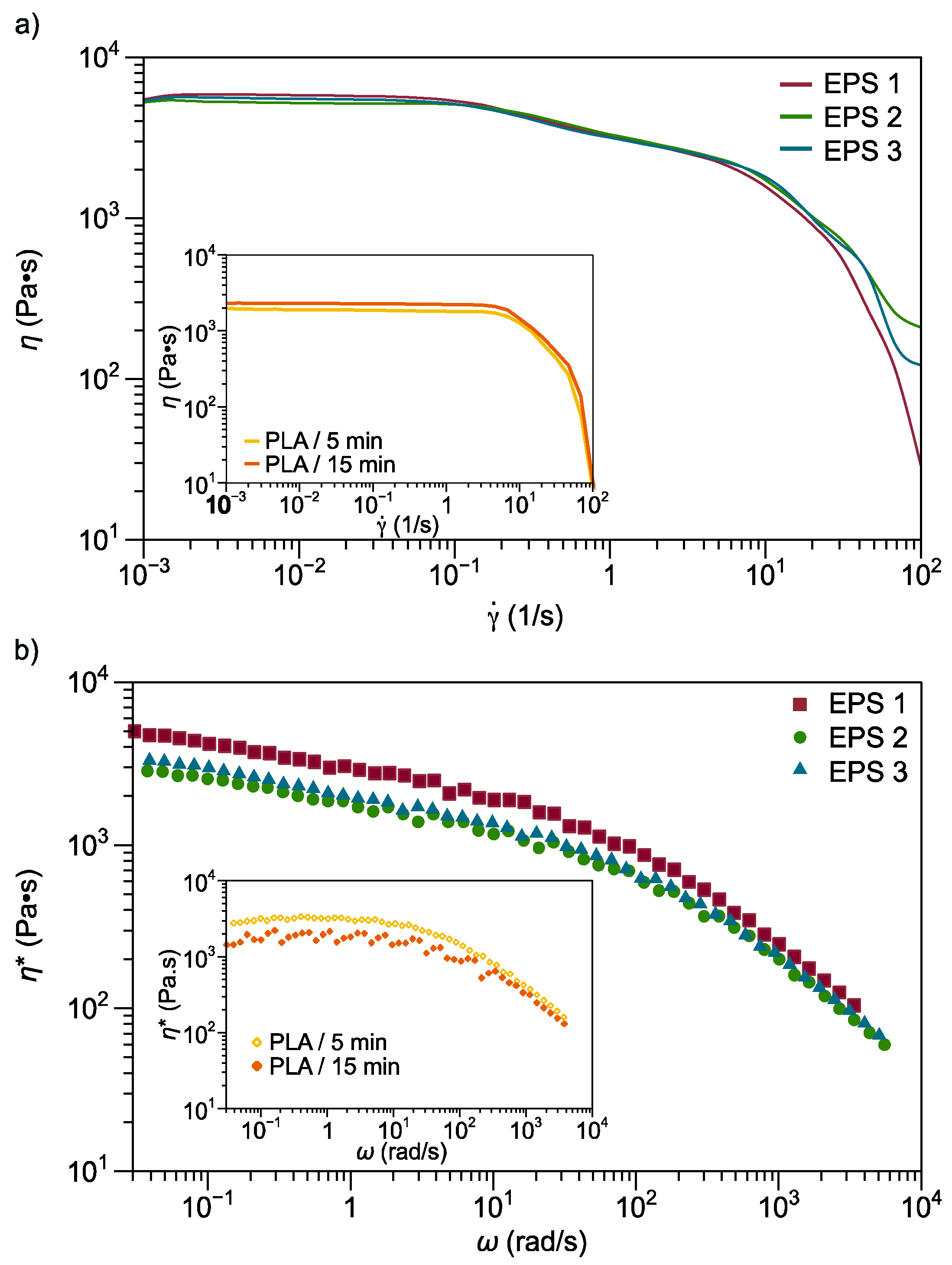

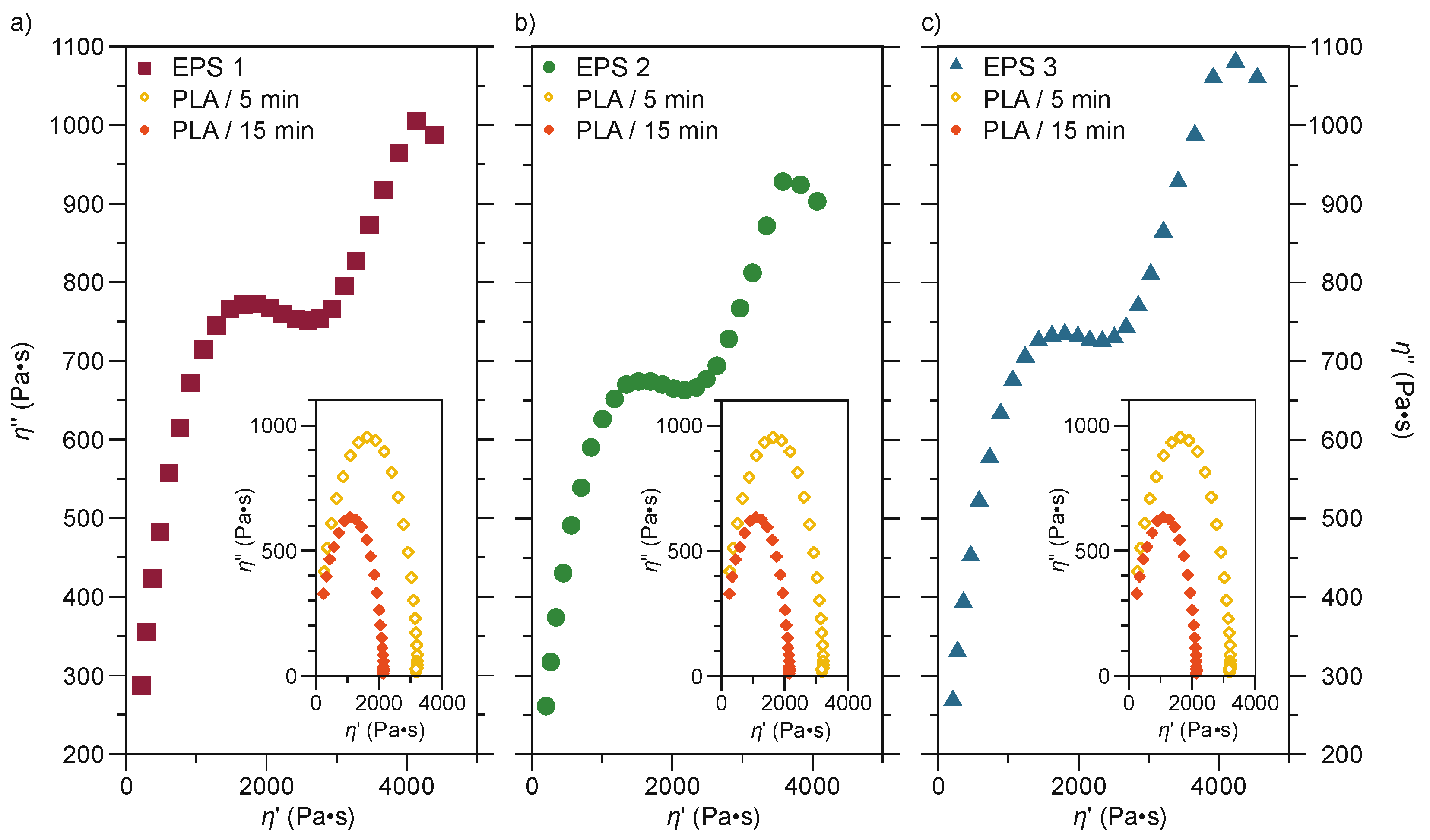
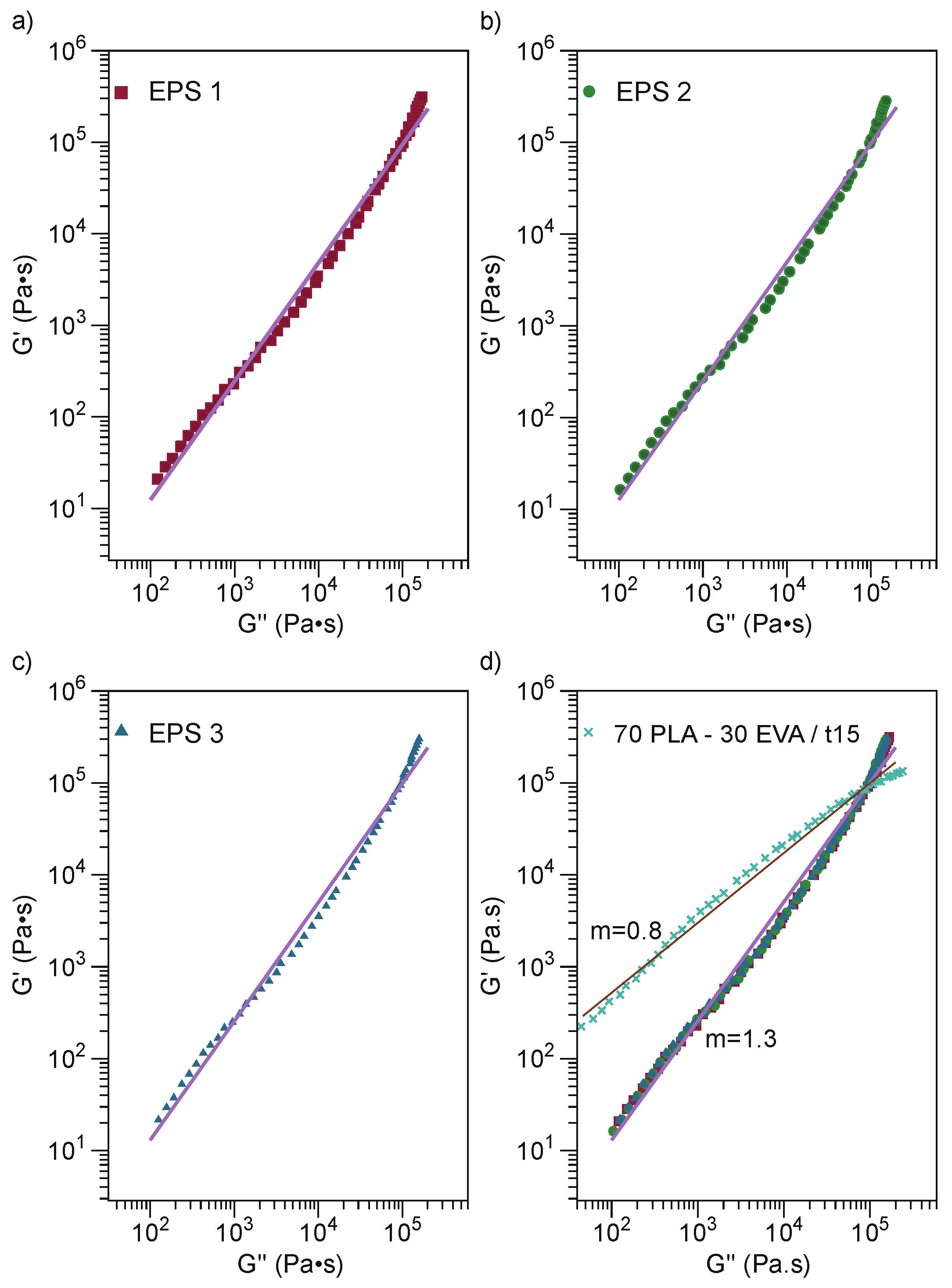
| No. | Sample | PLA Content/% | Mixing Time/min |
|---|---|---|---|
| 1 | neat PLA/t5 | 100 | 5 |
| 2 | neat PLA/t15 | 100 | 15 |
| 3 | 99PLA-1LDPE/t5 | 99 | 5 |
| 4 | 70PLA-30LDPE/t5 | 70 | 5 |
| 5 | 99PLA-1LDPE/t15 | 99 | 15 |
| 6 | 70PLA-30 LDPE/t15 | 70 | 15 |
| 7 | 99PLA-1EVA/t5 | 99 | 5 |
| 8 | 70PLA-30EVA/t5 | 70 | 5 |
| 9 | 99PLA-1EVA/t15 | 99 | 15 |
| 10 | 70PLA-30EVA/t15 | 70 | 15 |
| 11 | 99PLA-1PS/t5 | 99 | 5 |
| 12 | 70PLA-30PS/t5 | 70 | 5 |
| 13 | 99PLA-1PS/t15 | 99 | 15 |
| 14 | 70PLA-30PS/t15 | 70 | 15 |
| 15 | 99PLA-1SMMA/t5 | 99 | 5 |
| 16 | 70PLA-30SMMA/t5 | 70 | 5 |
| 17 | 99PLA-1SMMA/t15 | 99 | 15 |
| 18 | 70PLA-30SMMA/t15 | 70 | 15 |
| No. | Sample | Mixing Time/min | εb/% | E/GPa |
|---|---|---|---|---|
| 1 | neat PLA/t5 | 5 | 5.30 ± 1.3 | 0.50 ± 0.09 |
| 2 | neat PLA/t15 | 15 | 4.70 ± 0.1 | 0.62 ± 0.04 |
| 3 | 99PLA-1LDPE/t5 | 5 | 5.02 ± 1.0 | 0.61 ± 0.04 |
| 4 | 70PLA-30LDPE/t5 | 5 | 10.30 ± 2.3 | 0.51 ± 0.06 |
| 5 | 99PLA-1LDPE/t15 | 15 | 6.13 ± 1.7 | 0.66 ± 0.06 |
| 6 | 70PLA-30LDPE/t15 | 15 | 13.20 ± 6.3 | 0.56 ± 0.16 |
| 7 | 99PLA-1EVA/t5 | 5 | 8.20 ± 3.8 | 0.60 ± 0.19 |
| 8 | 70PLA-30EVA/t5 | 5 | 18.10 ± 4.8 | 0.29 ± 0.01 |
| 9 | 99PLA-1EVA/t15 | 15 | 11.90 ± 0.07 | 0.92 ± 0.18 |
| 10 | 70PLA-30EVA/t15 | 15 | 19.00 ± 8.5 | 0.43 ± 0.03 |
| No. | Sample | Mixing Time/min | E/GPa |
|---|---|---|---|
| 11 | 99 PLA-1 PS/t5 | 5 | 0.64 ± 0.05 |
| 12 | 70 PLA-30 PS/t5 | 5 | 0.75 ± 0.1 |
| 13 | 99 PLA-1 PS/t15 | 15 | 0.67 ± 0.05 |
| 14 | 70 PLA-30 PS/t15 | 15 | 0.57 ± 0.01 |
| 15 | 99 PLA-1 SMMA/t5 | 5 | 0.81 ± 0.13 |
| 16 | 70 PLA-30 SMMA/t5 | 5 | 0.76 ± 0.02 |
| 17 | 99 PLA-1 SMMA/t15 | 15 | 0.65 ± 0.05 |
| 18 | 70 PLA-30 SMMA/t15 | 15 | 0.69 ± 0.09 |
| Sample | EVA Content/% | PLA Content/% | SMMA Content/% | Mixing Time/min |
|---|---|---|---|---|
| EPS 1 | 30 | 69 | 1 | 5 |
| EPS 2 | 30 | 69 | 1 | 15 |
| EPS 3 | 30 | 69 | 1 | * 15, 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Duarte, M.E.; Estrada-Moreno, I.A.; García-Casillas, P.E.; Vega-Rios, A. Stiff-Elongated Balance of PLA-Based Polymer Blends. Polymers 2021, 13, 4279. https://doi.org/10.3390/polym13244279
Mendoza-Duarte ME, Estrada-Moreno IA, García-Casillas PE, Vega-Rios A. Stiff-Elongated Balance of PLA-Based Polymer Blends. Polymers. 2021; 13(24):4279. https://doi.org/10.3390/polym13244279
Chicago/Turabian StyleMendoza-Duarte, Mónica Elvira, Iván Alziri Estrada-Moreno, Perla Elvia García-Casillas, and Alejandro Vega-Rios. 2021. "Stiff-Elongated Balance of PLA-Based Polymer Blends" Polymers 13, no. 24: 4279. https://doi.org/10.3390/polym13244279
APA StyleMendoza-Duarte, M. E., Estrada-Moreno, I. A., García-Casillas, P. E., & Vega-Rios, A. (2021). Stiff-Elongated Balance of PLA-Based Polymer Blends. Polymers, 13(24), 4279. https://doi.org/10.3390/polym13244279










