Development of a New Formulation Based on In Situ Photopolymerized Polymer for the Treatment of Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extract
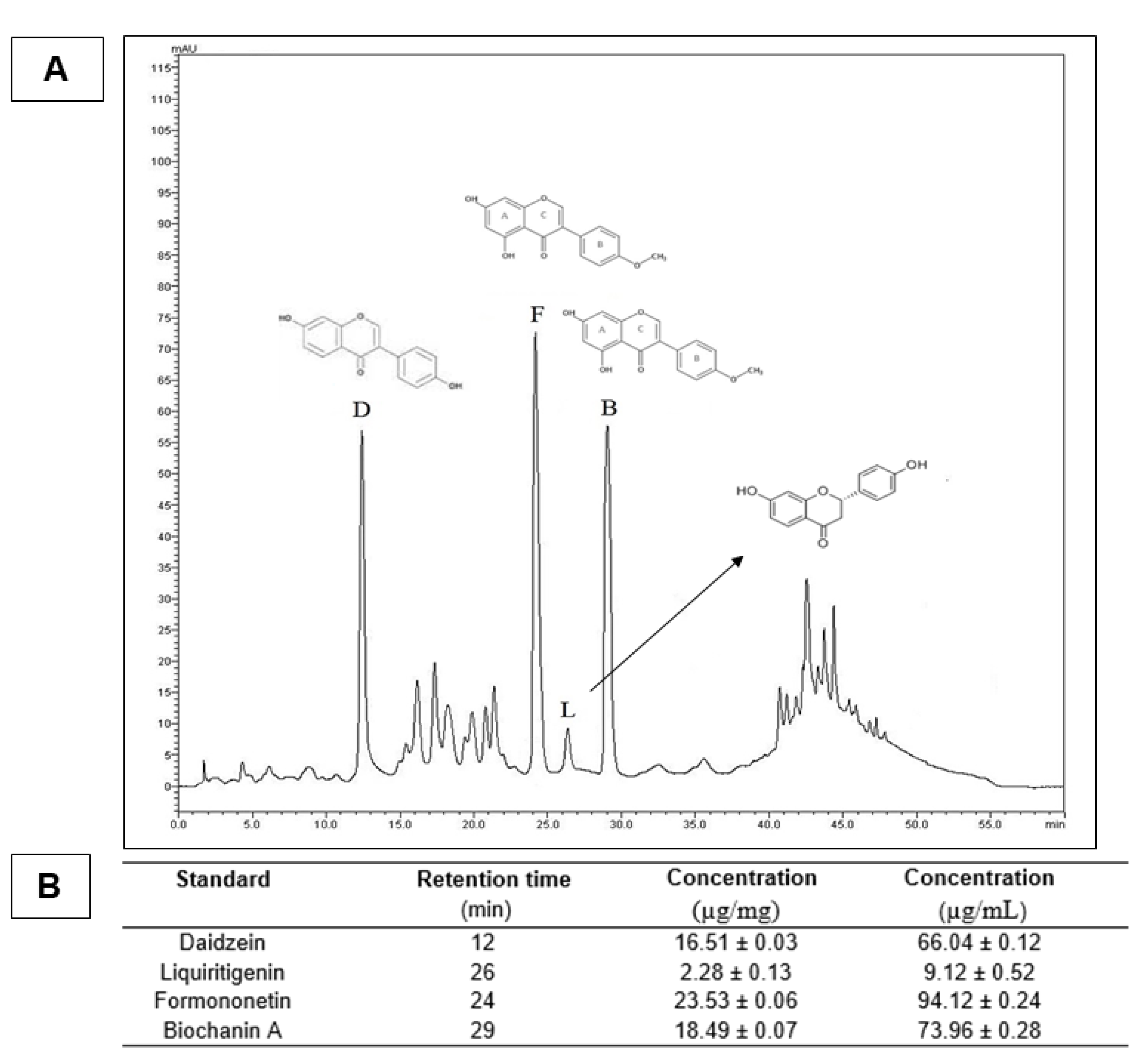
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Methacrylated Gelatin Synthesis
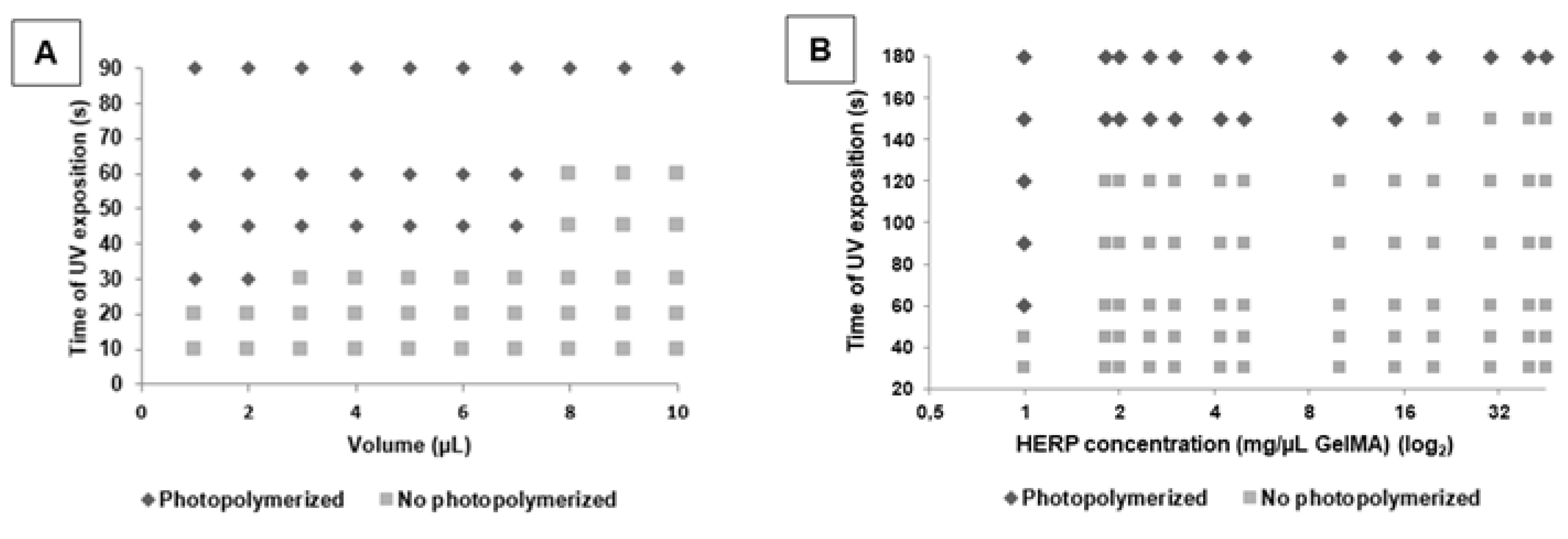
2.5. Development of the GelMA Hydrogel with and without Extract
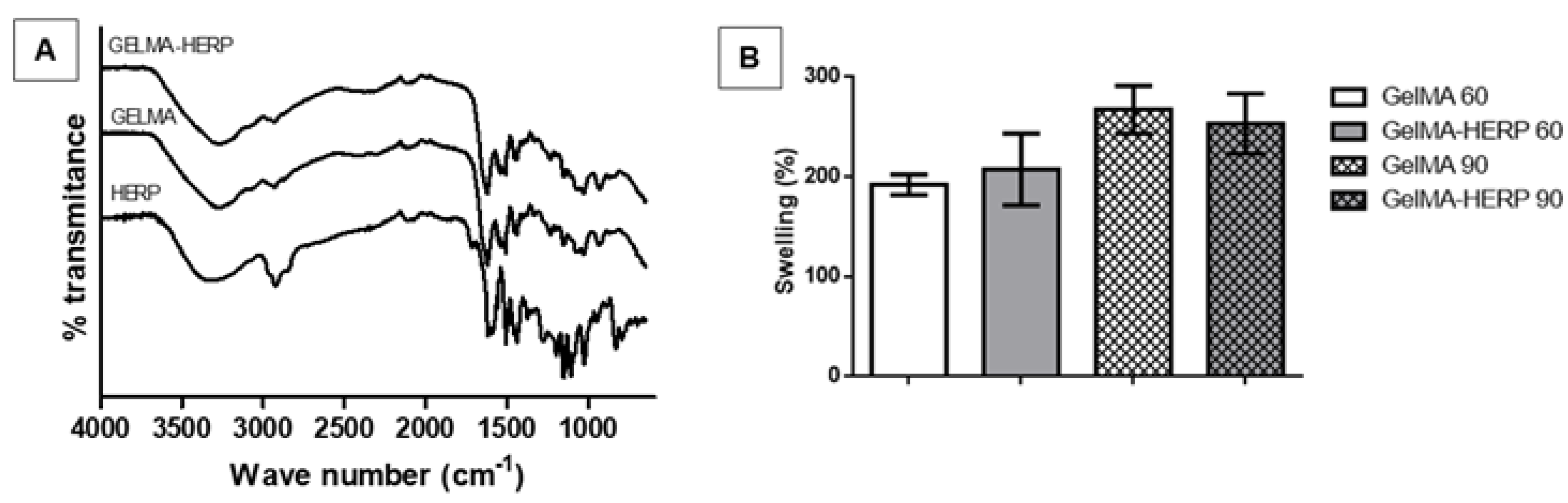
2.6. Hydrogel Swelling Analysis
2.7. GelMA Biodegradation
2.8. Fourier Transform Infrared Spectroscopy
2.9. In Vivo Experiments
2.9.1. Experimental Groups and Spinal Cord Injury Surgery
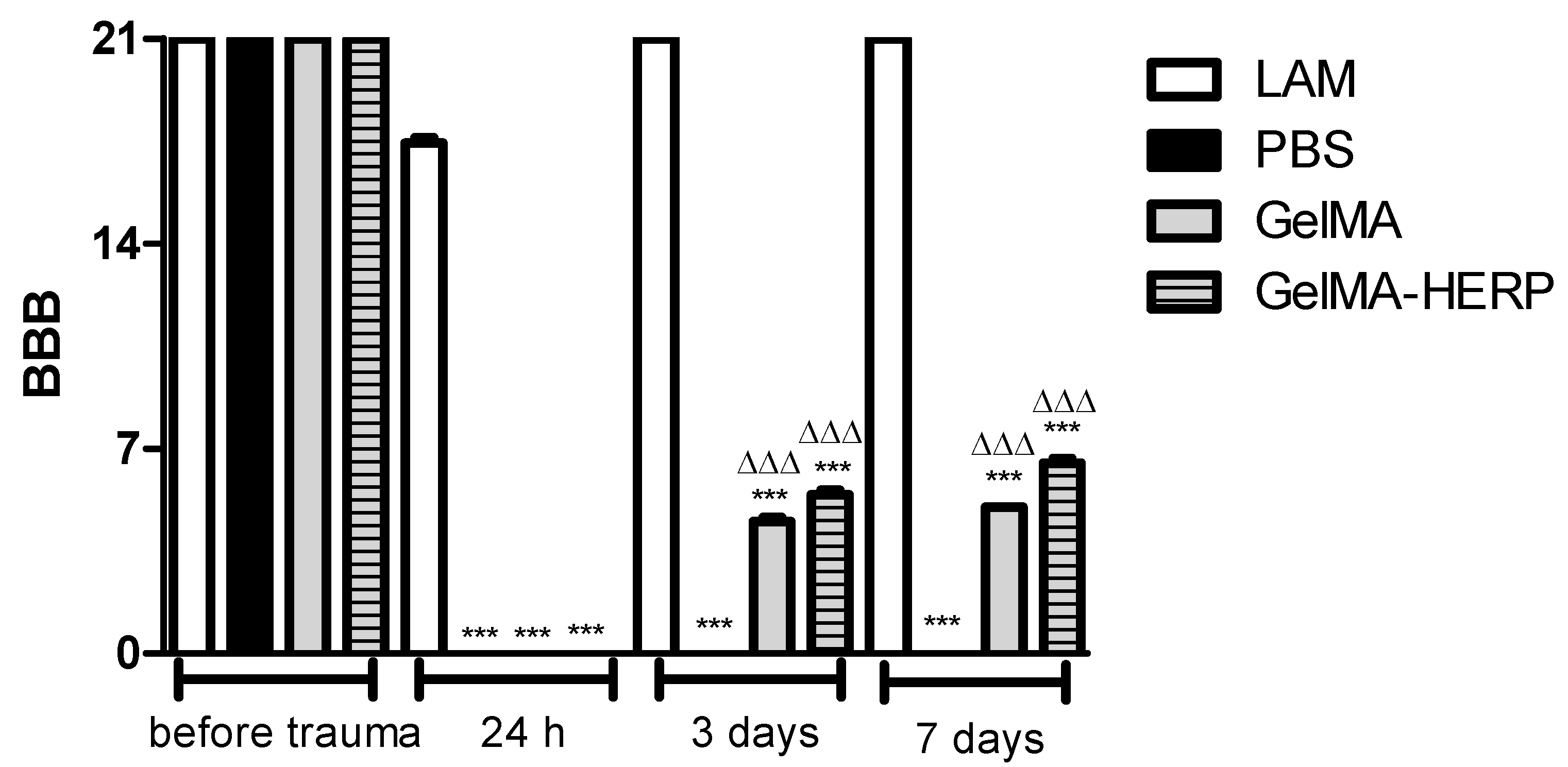
2.9.2. Assessment of Recovery of Locomotion
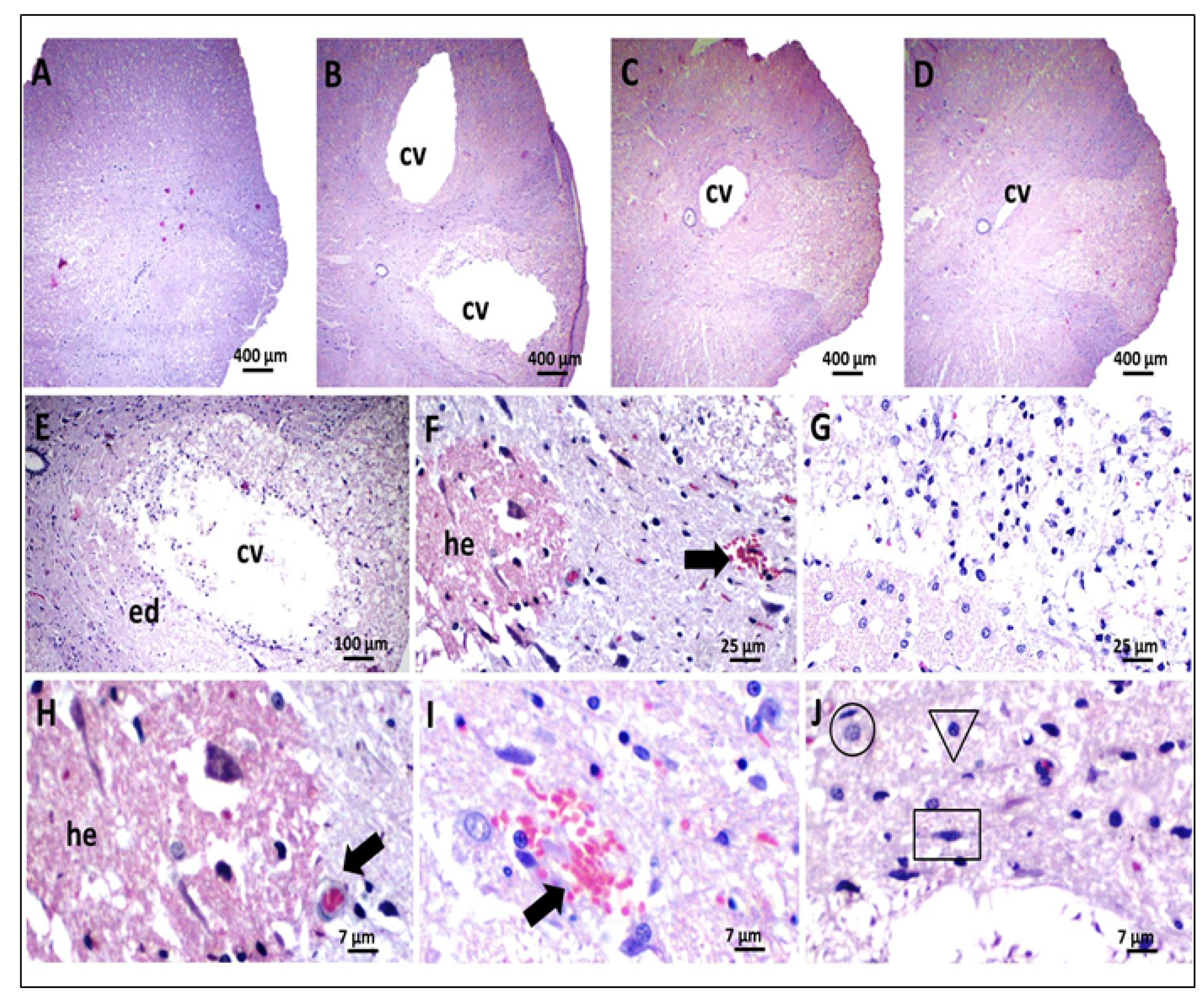
2.9.3. Histological Analysis
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- He, Z.; Zang, H.; Zhu, L.; Huang, K.; Yi, T.; Zhang, S.; Cheng, S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomed. 2019, 14, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2018, 10, 17742–17755. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Sámano, C.; Nistri, A. Mechanism of Neuroprotection against Experimental Spinal Cord Injury by Riluzole or Methylprednisolone. Neurochem. Res. 2019, 44, 200–213. [Google Scholar] [CrossRef]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Poluyi, E.O.; Owolabi, B.S.; Kanu, O.O.; Popoola, M.O. Surgical decompression for traumatic spinal cord injury in a tertiary center. Niger. J. Clin. Pract. 2017, 20, 1455–1460. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Gao, H.; Zhang, M.; Chen, B.; Yang, H. Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury. Med. Sci. Monit. 2017, 23, 1800–1811. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.; Ogata, T.; Nagao, M.; Sawada, Y.; Nishimura, R.; Fujita, N. Effects of Treadmill Training Combined with Serotonergic Interventions on Spasticity after Contusive Spinal Cord Injury. J. Neurotrauma 2018, 35, 1358–1366. [Google Scholar] [CrossRef]
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic spinal cord injury: Current concepts and treatment update. Arq. Neuro-Psiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Mbori, N.J.; Chuan, X.Y.; Feng, Q.X.; Alizada, M.; Zhan, J. Evaluation of the Combination of Methylprednisolone and Tranilast after Spinal Cord Injury in Rat Models. J. Korean Neurosurg. Soc. 2016, 59, 334–340. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.M.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Bastos, E.C.; et al. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, K.C.; Barbosa, T.C.; Nery, M.; Chaud, M.V.; da Silva, C.F.; Andrade, L.N.; Correa, C.B.; Jaguer, A.; Padilha, F.F.; Cardoso, J.C.; et al. Antibacterial activity of chitosan/collagen membranes containing red propolis extract. Pharmazie 2020, 75, 75–81. [Google Scholar] [CrossRef]
- Picolotto, A.; Pergher, D.; Pereira, G.P.; Machado, K.G.; da Silva Barud, H.; Roesch-Ely, M.; Gonzalez, M.H.; Tasso, L.; Figueiredo, J.G.; Moura, S. Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus. Biomed. Pharmacother. 2019, 112, 108640. [Google Scholar] [CrossRef] [PubMed]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.A.; Rebouças Silva, J.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Barbosa, R.A.; Nunes, T.L.; Nunes, T.L.; da Paixão, A.O.; Belo Neto, R.; Moura, S.; Albuquerque Junior, R.L.; Cândido, E.A.; Padilha, F.F.; Quintans-Júnior, L.J.; et al. Hydroalcoholic extract of red propolis promotes functional recovery and axon repair after sciatic nerve injury in rats. Pharm. Biol. 2016, 54, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.B.; Tang, P.F.; Zhang, W.; Zhao, Y.P.; Zhang, L.C.; Zhang, H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene 2016, 592, 128–133. [Google Scholar] [CrossRef]
- Morais, R.P.; Novais, G.B.; Sangenito, L.S.; Santos, A.L.S.; Priefer, R.; Morsink, M.; Mendonca, M.C.; Souto, E.B.; Severino, P.; Cardoso, J.C. Naringenin-Functionalized Multi-Walled Carbon Nanotubes: A Potential Approach for Site-Specific Remote-Controlled Anticancer Delivery for the Treatment of Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4557. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Cheng, Q.; Zeng, Y.; Xu, X.; Guan, G. Naringenin promotes SDF-1/CXCR4 signaling pathway in BMSCs osteogenic differentiation. Folia Histochem. Cytobiol. 2021, 59, 66–73. [Google Scholar] [CrossRef]
- Boisserand, L.S.; Kodama, T.; Papassin, J.; Auzely, R.; Moisan, A.; Rome, C.; Detante, O. Biomaterial Applications in Cell-Based Therapy in Experimental Stroke. Stem Cells Int. 2016, 2016, 6810562. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Dursun Usal, T.; Yucel, D.; Hasirci, V. A novel GelMA-pHEMA hydrogel nerve guide for the treatment of peripheral nerve damages. Int. J. Biol. Macromol. 2019, 121, 699–706. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Zhao, Y.; Xie, H.; Lin, Q.; He, Z.; Wang, X.; Li, J.; Zhang, H.; Wang, C.; et al. A Thermosensitive Heparin-Poloxamer Hydrogel Bridges aFGF to Treat Spinal Cord Injury. ACS Appl. Mater. Interfaces 2017, 9, 6725–6745. [Google Scholar] [CrossRef]
- de Mélo Silva, I.S.; do Amorim Costa Gaspar, L.M.; Rocha, A.M.O.; da Costa, L.P.; Tada, D.B.; Franceschi, E.; Padilha, F.F. Encapsulation of Red Propolis in Polymer Nanoparticles for the Destruction of Pathogenic Biofilms. AAPS PharmSciTech 2020, 21, 49. [Google Scholar] [CrossRef]
- da Silva, R.O.; Andrade, V.M.; Bullé Rêgo, E.S.; Azevedo Dória, G.A.; Santos Lima, B.D.; da Silva, F.A.; de Souza Araújo, A.A.; de Albuquerque Júnior, R.L.; Cordeiro Cardoso, J.; Zanardo Gomes, M. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. J. Ethnopharmacol. 2015, 170, 66–71. [Google Scholar] [CrossRef] [Green Version]
- de Vasconcelos, A.C.P.; Morais, R.P.; Novais, G.B.; da Barroso, S.; Menezes, L.R.O.; dos Santos, S.; da Costa, L.P.; Correa, C.B.; Severino, P.; Gomes, M.Z.; et al. In situ photocrosslinkable formulation of nanocomposites based on multi-walled carbon nanotubes and formononetin for potential application in spinal cord injury treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102272. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xia, C.; Mei, S.; Wang, J.; Shan, Z.; Lin, X.; Fan, S. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials 2016, 81, 1–13. [Google Scholar] [CrossRef]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.P.; Fan, L.H.; Nan, K.; Li, J.; Dang, X.Q.; Wang, K.Z. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J. Neuroinflamm. 2017, 14, 97. [Google Scholar] [CrossRef]
- de Almeida, E.B.; Cordeiro Cardoso, J.; Karla de Lima, A.; de Oliveira, N.L.; de Pontes-Filho, N.T.; Oliveira Lima, S.; Leal Souza, I.C.; de Albuquerque-Júnior, R.L. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar] [CrossRef]
- de Mendonca, L.S.; de Mendonca, F.M.R.; de Araujo, Y.L.F.M.; de Araujo, E.D.; Ramalho, S.A.; Narain, N.; Jain, S.; Orellana, S.C.; Padilha, F.F.; Cardoso, J.C. Chemical markers and antifungal activity of red propolis from Sergipe, Brazil. Food Sci. Tech.-Brazil 2015, 35, 291–298. [Google Scholar] [CrossRef] [Green Version]
- de Moraes Porto, I.C.C.; De Almeida, D.C.C.; de Oliveira Costa, G.V.C.; Donato, T.S.S.; Nunes, L.M.; Do Nascimento, T.G.; dos Santos Oliveira, J.M.; Da Silva, C.B.; Dos Santos, N.B.; e Silva, M.L.D.A.; et al. Mechanical and aesthetics compatibility of Brazilian red propolis micellar nanocomposite as a cavity cleaning agent. BMC Complementary Altern. Med. 2018, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Alves, A.V.F.; Queiroz, L.A.; Lima, B.S.; Filho, R.N.P.; Araújo, A.A.S.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207. [Google Scholar] [CrossRef]
- Reis, J.H.O.; Barreto, G.A.; Cerqueira, J.C.; Anjos, J.P.D.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.; Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Frozza, C.O.; Garcia, C.S.; Gambato, G.; de Souza, M.D.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative Chemistry of Propolis from Eight Brazilian Localities. Evid.-Based Complementary Altern. Med. 2013, 2013, 267878. [Google Scholar] [CrossRef] [Green Version]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Cavendish, R.L.; de Souza Santos, J.; Neto, R.B.; Paixão, A.O.; Oliveira, J.V.; de Araujo, E.D.; e Silva, A.A.B.; Thomazzi, S.M.; Cardoso, J.C.; Gomes, M.Z. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Visone, R.; Russo, L.; Cipolla, L.; Moretti, M.; Rasponi, M. VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Research. Part A 2015, 103, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Rahali, K.; Ben Messaoud, G.; Kahn, C.J.F.; Sanchez-Gonzalez, L.; Kaci, M.; Cleymand, F.; Fleutot, S.; Linder, M.; Desobry, S.; Arab-Tehrany, E. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2017, 18, 2675. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.C.; Yu, J.; Tsai, W.B. Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable crosslinking for controlled drug release. J. Mater. Chem. B 2016, 4, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; McKinney, J.; Miller, T.; Bongiorno, T.; McDevitt, T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015, 13, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.-H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 7, 1903–1911. [Google Scholar] [CrossRef]
- Ramadan, W.S.; Abdel-Hamid, G.A.; Al-Karim, S.; Abbas, A.T. Histological, immunohistochemical and ultrastructural study of secondary compressed spinal cord injury in a rat model. Folia Histochem. Cytobiol. 2017, 55, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadeh-Ardabili, P.M.; Rad, S.K.; Rad, S.K.; Khazaài, H.; Sanusi, J.; Zadeh, M.-a.-R.H. Palm vitamin E reduces locomotor dysfunction and morphological changes induced by spinal cord injury and protects against oxidative damage. Sci. Rep. 2017, 7, 14365. [Google Scholar] [CrossRef]
- Piantino, J.; Burdick, J.A.; Goldberg, D.; Langer, R.; Benowitz, L.I. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp. Neurol. 2006, 201, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Hwang, D.H.; Kim, B.G.; Go, D.H.; Park, K.D. Thermosensitive polymer-based hydrogel mixed with the anti-inflammatory agent minocycline induces axonal regeneration in hemisected spinal cord. Macromol. Res. 2010, 18, 399–403. [Google Scholar] [CrossRef]
- Jain, A.; Kim, Y.T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Forostyak, S.; Koci, Z.; Zaviskova, K.; Vackova, I.; Vyborny, K.; Sandvig, I.; Sandvig, A.; Medberry, C.J.; Badylak, S.F.; et al. Injectable Extracellular Matrix Hydrogels as Scaffolds for Spinal Cord Injury Repair. Tissue Eng. Part A 2016, 22, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, G.B.; dos Santos, S.; Santana, R.J.R.; Filho, R.N.P.; Cunha, J.L.S.; Lima, B.S.; Araújo, A.A.S.; Severino, P.; Júnior, R.L.C.A.; Cardoso, J.C.; et al. Development of a New Formulation Based on In Situ Photopolymerized Polymer for the Treatment of Spinal Cord Injury. Polymers 2021, 13, 4274. https://doi.org/10.3390/polym13244274
Novais GB, dos Santos S, Santana RJR, Filho RNP, Cunha JLS, Lima BS, Araújo AAS, Severino P, Júnior RLCA, Cardoso JC, et al. Development of a New Formulation Based on In Situ Photopolymerized Polymer for the Treatment of Spinal Cord Injury. Polymers. 2021; 13(24):4274. https://doi.org/10.3390/polym13244274
Chicago/Turabian StyleNovais, Gabrielle B., Stefane dos Santos, Robertta J. R. Santana, Rose N. P. Filho, John L. S. Cunha, Bruno S. Lima, Adriano A. S. Araújo, Patricia Severino, Ricardo L. C. Albuquerque Júnior, Juliana C. Cardoso, and et al. 2021. "Development of a New Formulation Based on In Situ Photopolymerized Polymer for the Treatment of Spinal Cord Injury" Polymers 13, no. 24: 4274. https://doi.org/10.3390/polym13244274
APA StyleNovais, G. B., dos Santos, S., Santana, R. J. R., Filho, R. N. P., Cunha, J. L. S., Lima, B. S., Araújo, A. A. S., Severino, P., Júnior, R. L. C. A., Cardoso, J. C., Souto, E. B., & Gomes, M. Z. (2021). Development of a New Formulation Based on In Situ Photopolymerized Polymer for the Treatment of Spinal Cord Injury. Polymers, 13(24), 4274. https://doi.org/10.3390/polym13244274








