Development and Characterization of Eudragit-RL-100-Based Aceclofenac Sustained-Release Matrix Pellets Prepared via Extrusion/Spheronization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Assessment of Pellet Wet Mass (Mixer Torque Rheometry (MTR))
2.3. Extrusion/Spheronization Procedures
2.4. Drug Content
2.5. Particle Size
2.6. In Vitro Release
3. Results
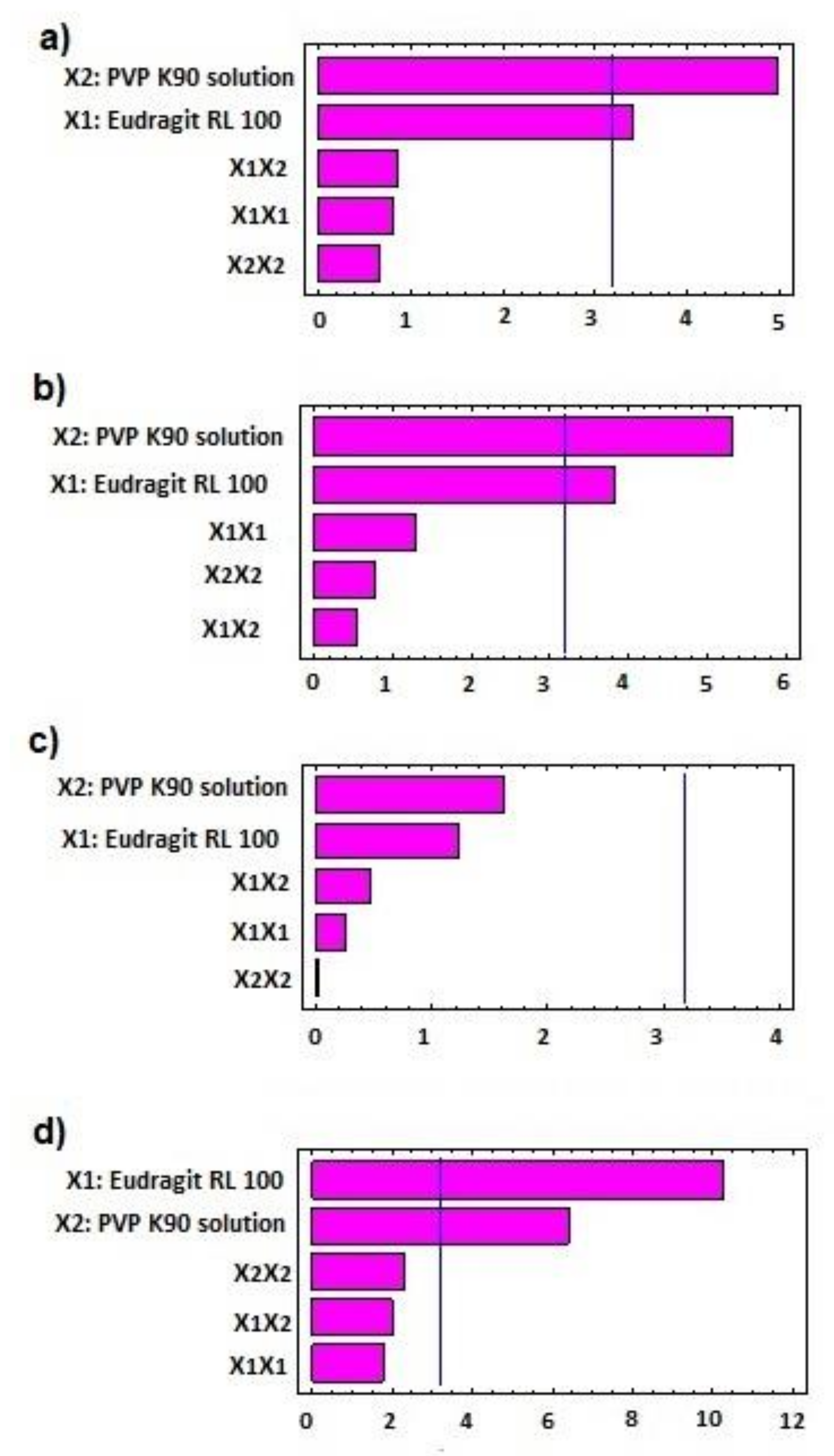
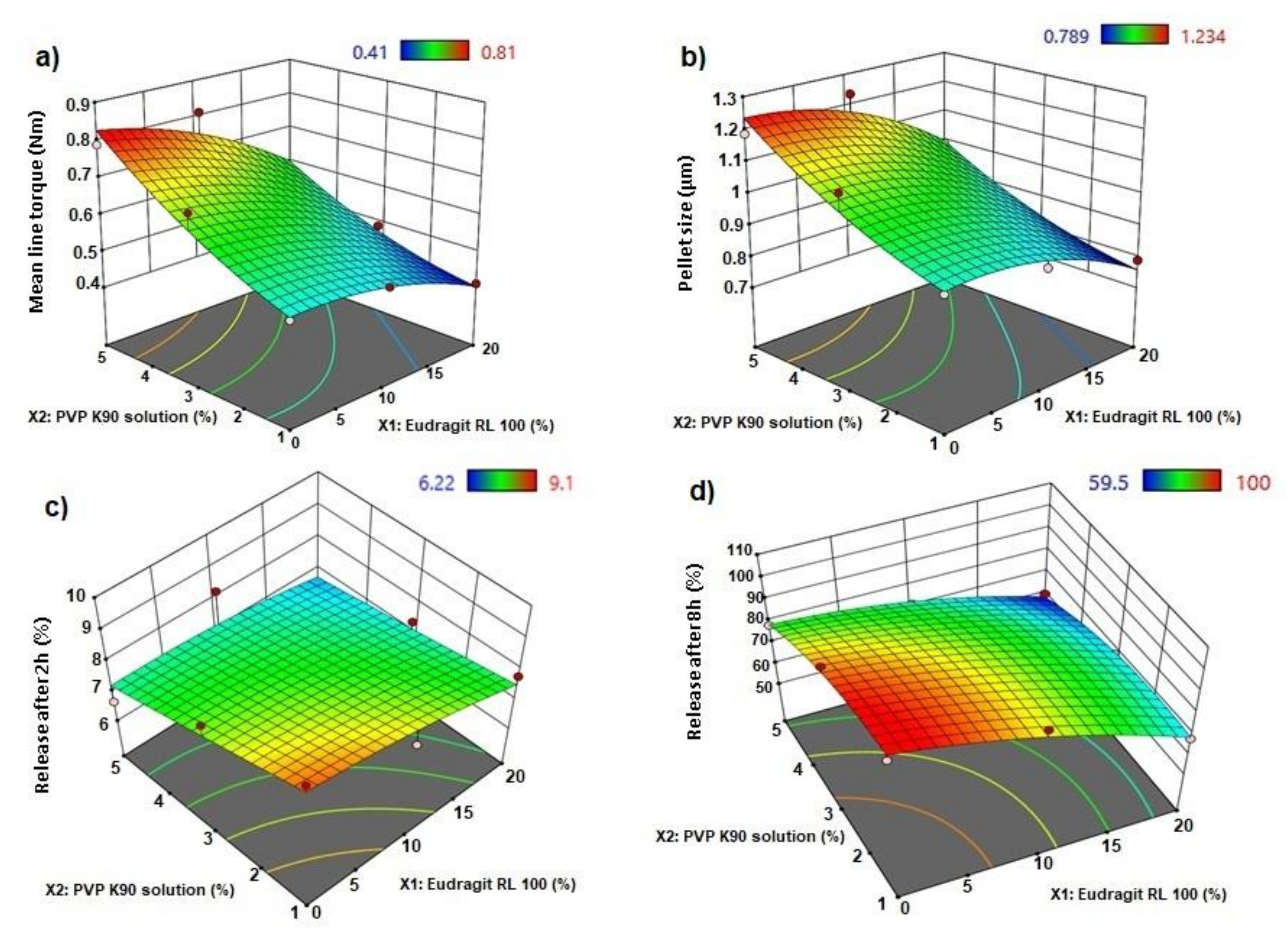
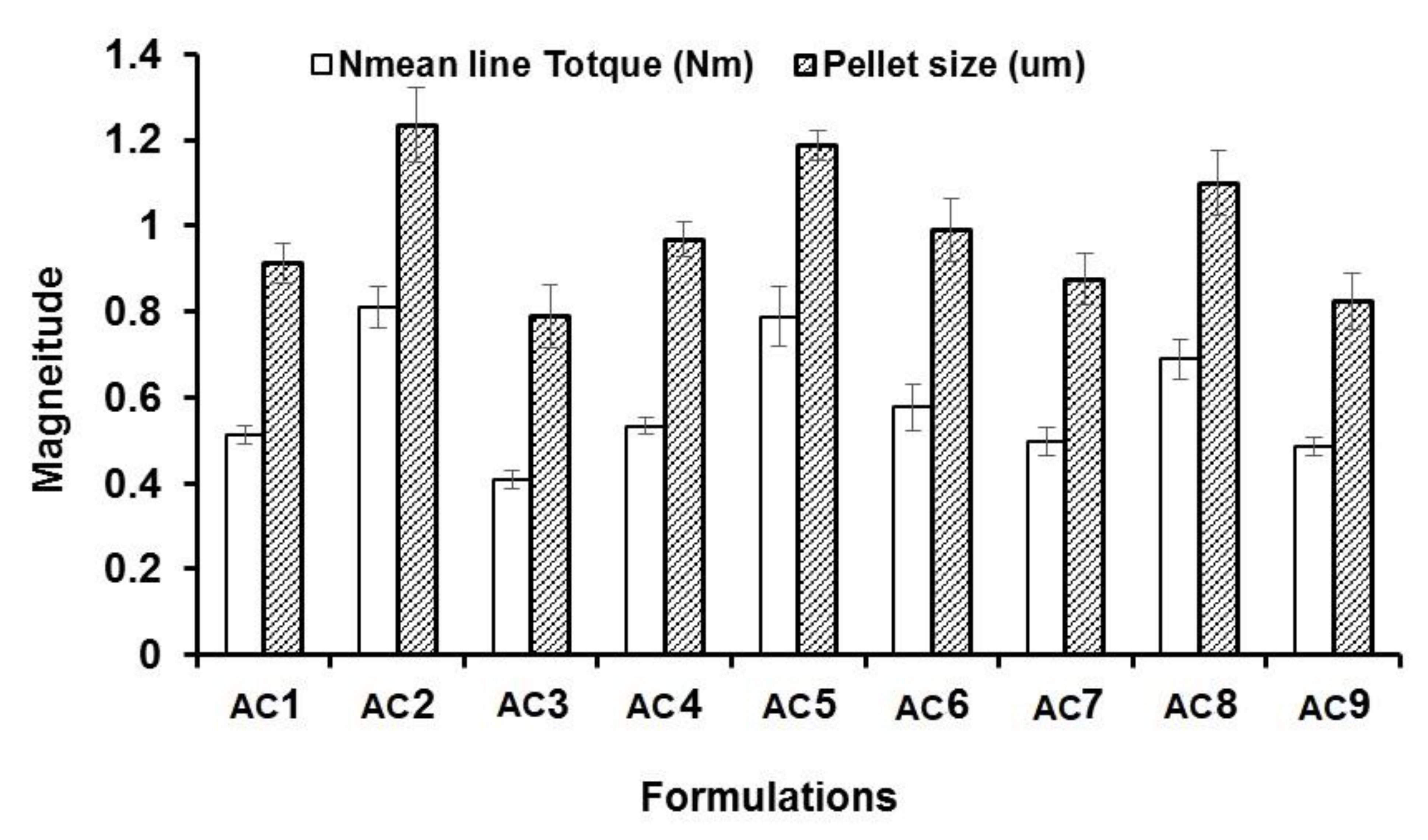
3.1. Effect of Independent Factors on Wet Mass
3.2. Drug Content
3.3. Effect on Pellet Size
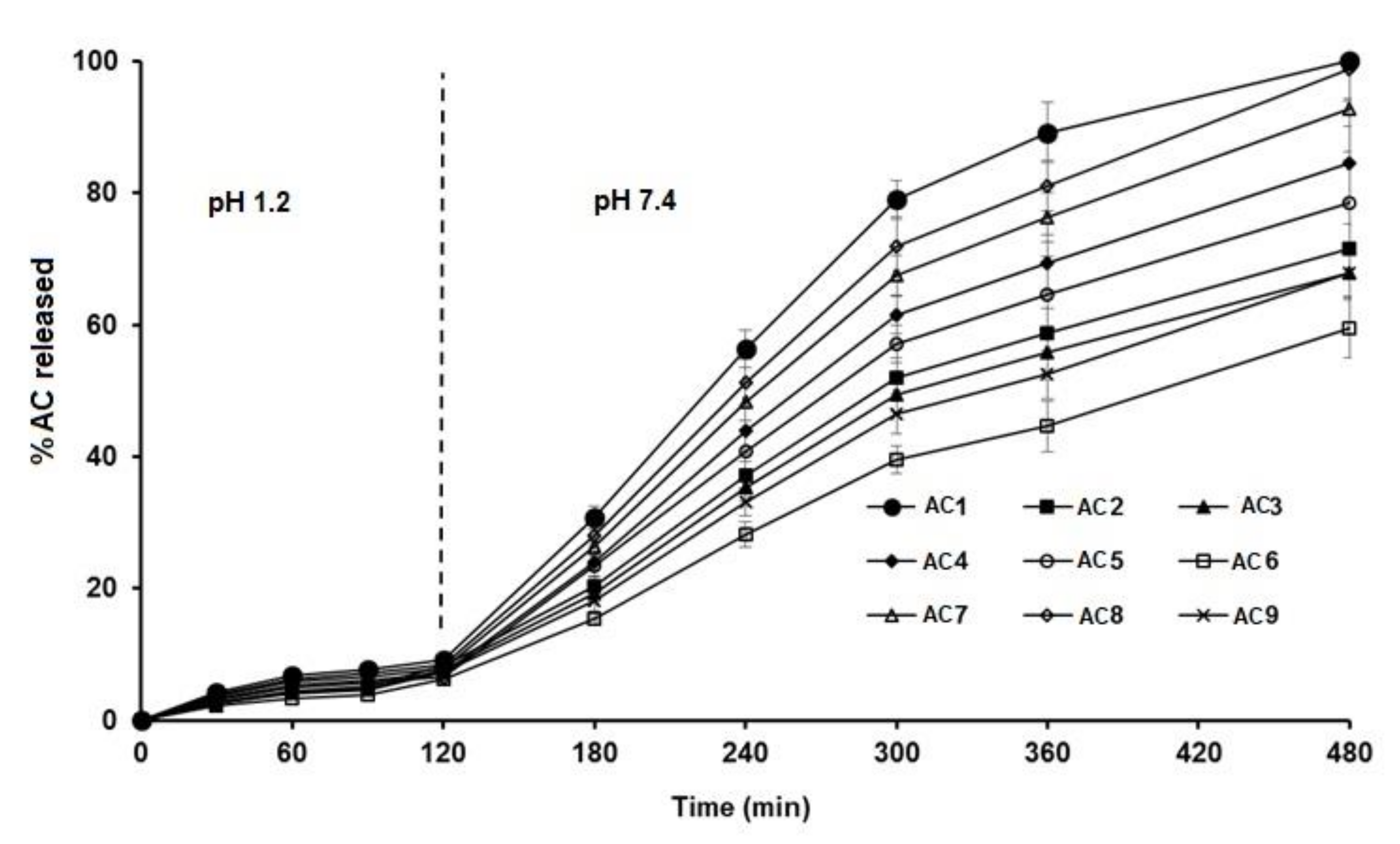
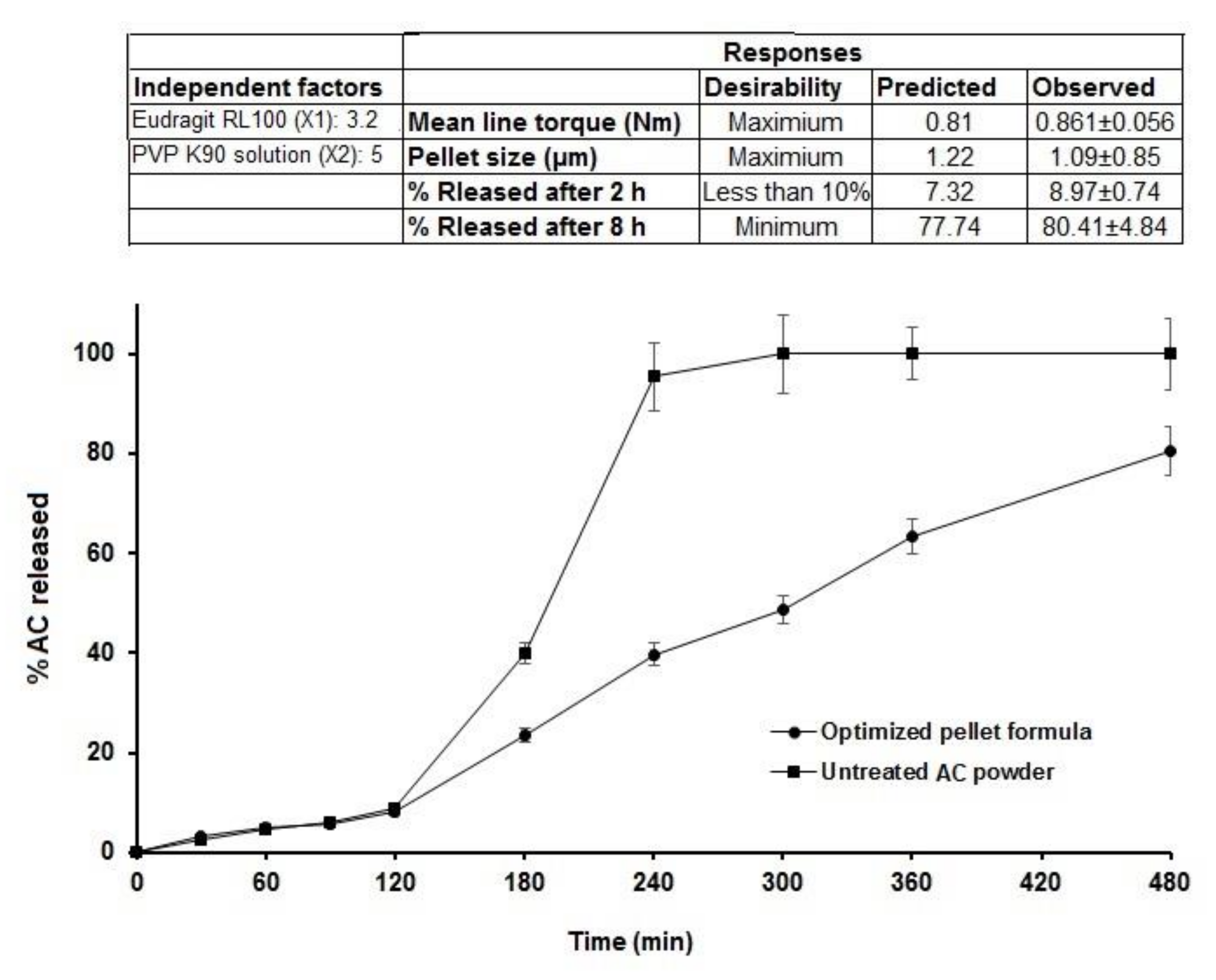
3.4. Effect on In Vitro Release
3.5. Optimization of AC Sustained-Release Matrix Pellet Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 71771, Aceclofenac. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aceclofenac (accessed on 21 September 2021).
- Dooley, M.; Spencer, C.M.; Dunn, C.J. Aceclofenac: A Reappraisal of its Use in the Management of Pain and Rheumatic Disease. Drugs 2001, 61, 1351–1378. [Google Scholar] [CrossRef]
- Walker, G. Preservex tablets. In ABPI Compendium of Data Sheets and Summaries of Product Characteristics 1999–2000; Datapharm Publications Ltd.: London, UK, 1999; pp. 1680–1681. [Google Scholar]
- Torri, G.; Vignati, C.; Agrifoglio, E.; Benvenuti, M.; Ceciliani, L.; Raschella, B.F.; Letizia, G.; Martorana, U.; Tessari, L.; Thovez, G.; et al. Aceclofenac versus piroxicam in the management of osteoarthritis of the knee: A double-blind controlled study. Curr. Ther. Res. 1994, 55, 576–583. [Google Scholar] [CrossRef]
- Díaz, C.; Rodríguez de la Serna, A.; Geli, C.; Gras, X. Efficacy and tolerability of aceclofenac versus diclofenac in the treatment of knee osteoarthritis: A multicentre study. Eur. J. Rheumatol. Inflamm. 1996, 16, 17–22. [Google Scholar]
- Martín-Mola, E.; Gijón-Baños, J.; Ansoleaga, J.J. Aceclofenac in comparison to ketoprofen in the treatment of rheumatoid arthritis. Rheumatol. Int. 1995, 15, 111–1116. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Alonso-Ruiz, A.; Ansoleaga, J.J. Comparative study of the efficacy and safety of aceclofenac and tenoxicam in rheumatoid arthritis. Clin. Rheumatol. 1996, 15, 473–477. [Google Scholar] [CrossRef]
- Ankita, S.; Tarkeshwar, S.; Dev, I.M.; Kumar, J.S. Design, Development and Evaluation of Aceclofenac Sustained Release Matrix Tablets. Int. J. Drug Dev. Res. 2011, 3, 307–313. [Google Scholar]
- Lakshmana, P.S.; Shirwaikar, A.A.; Shirwaikar, A.; Kumar, A. Formulation and evaluation of sustained release microspheres of rosin containing aceclofenac. Ars Pharm. 2009, 50, 51–62. [Google Scholar]
- Ghosh, S.; Barik, B.B. Preparation and evaluation of aceclofenac sustained release formulation and comparison of formulated and marketed product. Int. J. Med. Sci. 2009, 1, 375–382. [Google Scholar]
- Hamdani, J.; Moës, A.J.; Amighi, K. Development and evaluation of prolonged release pellets obtained by the melt pelletization process. Int. J. Pharm. 2002, 245, 167–177. [Google Scholar] [CrossRef]
- Podczeck, F.; Knight, P.E.; Newton, J.M. The evaluation of modified microcrystalline cellulose for the preparation of pellets with high drug loading by extrusion/spheronization. Int. J. Pharm. 2008, 350, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Das, P.; Das, H.; Ghosh, A. MUPS (Multiple Unit Pellet System) Tablets—A Brief Review. J. Pharm. Biomed. Sci. 2011, 12, 1–5. [Google Scholar]
- Alshora, D.H.; Ibrahim, M.A.; Ezzeldin, E.; Iqbal, M. Optimized flurbiprofen sustained-release matrix pellets prepared by extrusion/spheronization. J. Drug Deliv. Sci. Tecnol. 2020, 59, 101902. [Google Scholar] [CrossRef]
- Gupta, A.M.; Shivhare, U.D.; Suruse, P.B. Different Aspects of Pellets Formulation and their Evaluation. Int. J. Pharm. Phytopharmacol. Res. 2015, 4, 331–336. [Google Scholar]
- Raval, M.K.; Ramani, R.V.; Sheth, N.R. Formulation and evaluation of sustained release enteric-coated pellets of budesonide for intestinal delivery. Int. J. Pharm. Investig. 2013, 3, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallavi, M.; Chaudhari Pravin, D. Formulation and evaluation of multiparticulate system for chronotherapeutic delivery of salbutamol sulphate. J. Pharm. Bioallied. Sci. 2012, 4, S71–S73. [Google Scholar]
- Bölcskei, É.; Regdon, G., Jr.; Soványa, T.; Kleinebudde, P.; Pintye-Hódi, K. Optimization of preparation of matrix pellets containing Eudragit® NE 30D. Chem. Eng. Res. Design 2012, 90, 651–657. [Google Scholar] [CrossRef]
- Amin, M.J.; Pate, K.S.; Patel, D.R.; Patel, Z.P.; Bajag, J.V. Formulation and evaluation of sustained-release pellets of lornoxicam. Int. J. Appl. Pharm. 2021, 33, 221–227. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Zayed, G.M.; Alsharif, F.M.; Abdelhafez, W.A. Utilizing mixer torque rheometer in the prediction of optimal wet massing parameters for pellet formulation by extrusion/spheronization. Saudi Pharm. J. 2019, 27, 182–190. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mahrous, G.M. Effect of wet mass on dextromethorphan hydrobromide matrix pellets, Braz. J. Pharm. Sci. 2018, 54, 1–9. [Google Scholar]
- Ibrahim, M.A. Formulation and evaluation of mefenamic acid sustained release matrix pellets. Acta Pharm. 2013, 63, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, G.M.; Ibarhim, M.A.; El-Badry, M.; Al-Anazi, F.K. Indomethacin sustained release pellets prepared by extrusion/spheronization. J. Drug Deliv. Sci. Technol. 2010, 20, 119–125. [Google Scholar] [CrossRef]
- Mahrous, G.M. Ketorolac enteric matrix pellets produced by extrusion/spheronization. Bull. Pharm. Sci. Assiut Univ. 2010, 33, 51–58. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Amin, M.A.; Fetih, G.; Abou Ela, A. Formulation and evaluation of ketorolac tromethamine-Eudragit solid dispersions. STP Pharma Prat. 2010, 20, 189–200. [Google Scholar]
- Ibrahim, M.A.; Mahrous, G.M.; El-Badry, M.; Al-Anazi, F.K. Indomethacin-Loaded Pellets Prepared by Extrusion/Spheronization: Effect of Cosolvents. Farmacia 2011, 59, 483–499. [Google Scholar]
- Jani, G.K.; Shah, D.P.; Prajapatia, V.D.; Jain, V.C. Gum and mucilages: Versatile excipients for pharmaceutical formulations. Asian J. Pharm. Sci. 2009, 4, 309–323. [Google Scholar]
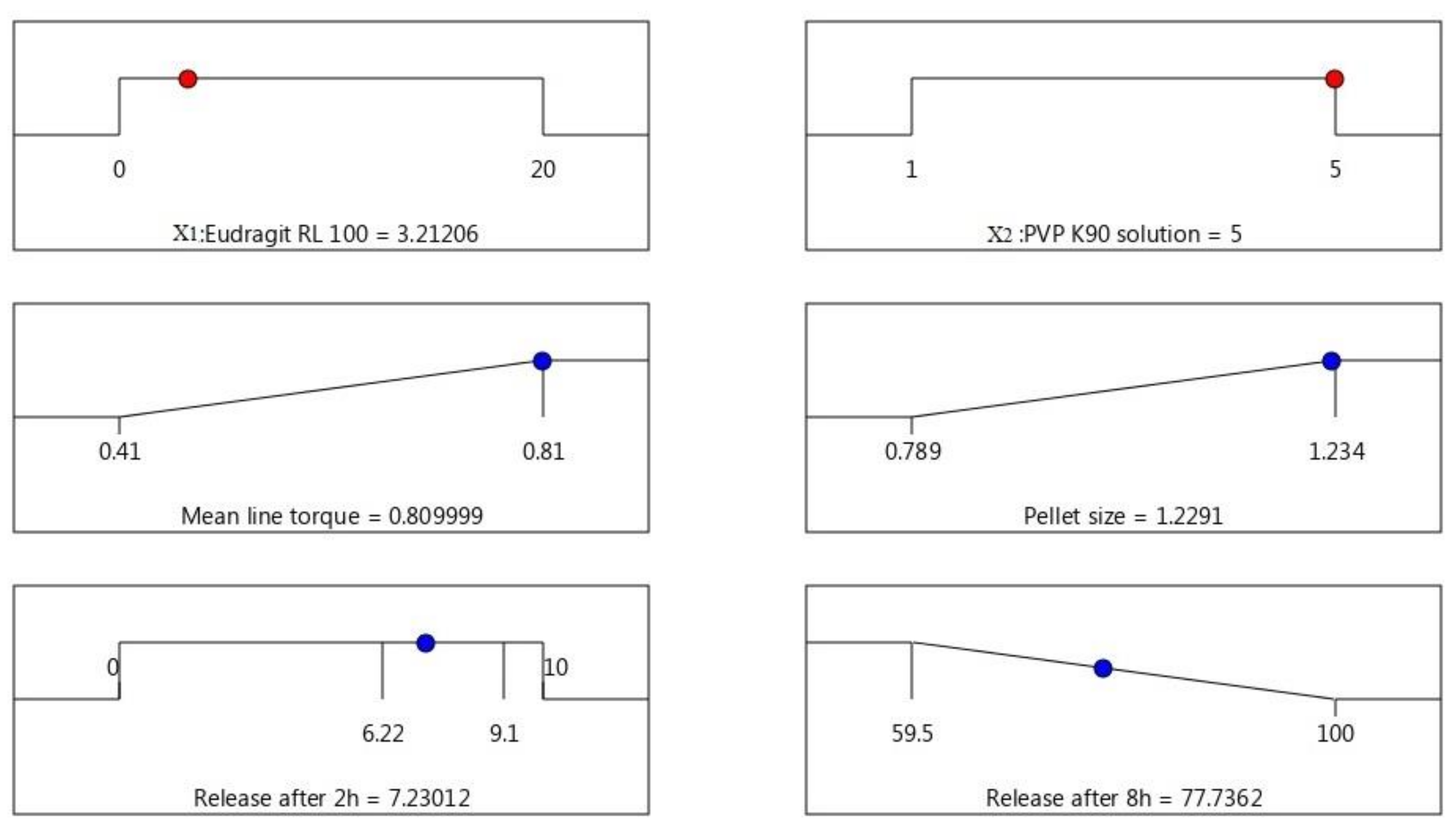
| Independent Factor | Level | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |||||||
| X1: Eudragit L100 (%) | 0 | 10 | 20 | ||||||
| X2: Polyvinylpyrrolidone K90 (PVP) (%) | 1 | 3 | 5 | ||||||
| Ingredients % | Formula | ||||||||
| AC1 | AC2 | AC3 | AC4 | AC5 | AC6 | AC7 | AC8 | AC9 | |
| Aceclofenac | 15% | ||||||||
| Eudragit L 100 | 0 | 10 | 20 | 10 | 0 | 20 | 10 | 0 | 20 |
| PVP K 90 | 1 | 5 | 1 | 3 | 5 | 5 | 1 | 3 | 3 |
| Avicel PH 101 | to 100% | ||||||||
| Responses | Source | Sum of Square | p-Value |
|---|---|---|---|
| Peak Torque | X1: Eudragit RL100 | 0.04455 | 0.0422 |
| X2: PVP K90 | 0.09526 | 0.0155 | |
| X1X2 | 0.00292 | 0.447 | |
| X12 | 0.00267 | 0.4657 | |
| X22 | 0.00180 | 0.5425 | |
| Pellet Size | X1: Eudragit RL100 | 0.05980 | 0.0309 |
| X2: PVP K90 | 0.11537 | 0.0128 | |
| X1X2 | 0.00130 | 0.6105 | |
| X12 | 0.00692 | 0.2815 | |
| X22 | 0.00240 | 0.4964 | |
| % Release after 2 h | X1: Eudragit RL100 | 1.30667 | 0.3034 |
| X2: PVP K90 | 2.25707 | 0.2019 | |
| X1X2 | 0.18923 | 0.6694 | |
| X12 | 0.05556 | 0.8148 | |
| X22 | 0.00109 | 0.9737 | |
| % Release after 8 h | X1: Eudragit RL100 | 105.15 | 0.0020 |
| X2: PVP K90 | 40.94 | 0.0077 | |
| X1X2 | 42.9025 | 0.1386 | |
| X12 | 34.445 | 0.1702 | |
| X22 | 55.8625 | 0.1061 |
| # | Dependent Factors (Responses) Mean ± Standard Deviation | ||||
|---|---|---|---|---|---|
| Peak Torque (Nm) | Pellet Size (µm) | AC Content (mg) | Release after 2 h (%) | Release after 8 h (%) | |
| AC1 | 0.513 ± 0.022 (0.933) * | 914 ± 47 | 14.85 ± 0.85 | 9.1 ± 0.84 | 100.00 ± 5.51 |
| AC2 | 0.81 ± 0.047 (0.800) | 1230 ± 87 | 13.95 ± 0.42 | 8.2 ± 0.54 | 71.45 ± 3.87 |
| AC3 | 0.41 ± 0.021 (0.667) | 789 ± 74 | 15.43 ± 0.53 | 7.79 ± 0.74 | 67.88 ± 4.21 |
| AC4 | 0.534 ± 0.018 (0.667) | 968 ± 41 | 15.62 ± 0.71 | 7.16 ± 0.41 | 84.42 ± 5.71 |
| AC5 | 0.789 ± 0.071 (0.800) | 1187 ± 35 | 14.23 ± 0.0.91 | 6.66 ± 0.45 | 78.52 ± 6.51 |
| AC6 | 0.578 ± 0.054 (0.800) | 990 ± 74 | 13.71 ± 0.62 | 6.22 ± 0.74 | 59.5 ± 4.60 |
| AC7 | 0.498 ± 0.034 (0.933) | 876 ± 58 | 15.81 ± 0.57 | 7.87 ± 0.85 | 92.78 ± 6.52 |
| AC8 | 0.689 ± 0.047 (0.800) | 1100 ± 71 | 13.95 ± 0.47 | 8.37 ± 0.98 | 98.7 ± 4.87 |
| AC9 | 0.486 ± 0.021 (0.667) | 823 ± 65 | 14.63 ± 0.79 | 7.32 ± 0.93 | 67.8 ± 3.45 |
| Release Time | Formula | Zero-Order Model | First-Order Model | Higuchi Diffusion Model | Korsmeyer–Peppas Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | Slope | r | Slope | r | Slope | r | n * | ||
| 0–2 h (pH 1.2) | AC1 | 0.957 | 0.073 | 0.961 | −0.0003 | 0.998 | 0.842 | 0.992 | 0.539 |
| AC2 | 0.979 | 0.062 | 0.979 | −0.0002 | 0.962 | 0.678 | 0.967 | 0.704 | |
| AC3 | 0.979 | 0.059 | 0.979 | −0.0002 | 0.962 | 0.644 | 0.967 | 0.704 | |
| AC4 | 0.955 | 0.054 | 0.960 | −0.0002 | 0.998 | 0.656 | 0.992 | 0.539 | |
| AC5 | 0.944 | 0.050 | 0.960 | −0.0002 | 0.998 | 0.610 | 0.992 | 0.539 | |
| AC6 | 0.979 | 0.047 | 0.979 | −0.0002 | 0.962 | 0.514 | 0.967 | 0.704 | |
| AC7 | 0.957 | 0.062 | 0.960 | −0.0002 | 0.998 | 0.720 | 0.992 | 0.539 | |
| AC8 | 0.957 | 0.066 | 0.961 | −0.0003 | 0.998 | 0.766 | 0.992 | 0.539 | |
| AC9 | 0.979 | 0.055 | 0.979 | −0.0002 | 0.962 | 0.605 | 0.967 | 0.704 | |
| 2–8 h (pH 7.4) | AC1 | 0.975 | 0.226 | 0.801 | −0.0073 | 0.940 | 4.667 | 0.946 | 1.196 |
| AC2 | 0.985 | 0.158 | 0.977 | −0.0011 | 0.936 | 3.214 | 0.963 | 1.266 | |
| AC3 | 0.985 | 0.150 | 0.979 | −0.0010 | 0.936 | 3.053 | 0.963 | 1.266 | |
| AC4 | 0.985 | 0.187 | 0.965 | −0.0017 | 0.936 | 3.798 | 0.963 | 1.266 | |
| AC5 | 0.987 | 0.173 | 0.974 | −0.0014 | 0.940 | 3.528 | 0.968 | 1.220 | |
| AC6 | 0.989 | 0.128 | 0.977 | −0.0008 | 0.925 | 2.571 | 0.976 | 1.346 | |
| AC7 | 0.985 | 0.205 | 0.942 | −0.0023 | 0.936 | 4.174 | 0.963 | 1.266 | |
| AC8 | 0.985 | 0.218 | 0.879 | −0.0036 | 0.936 | 4.440 | 0.963 | 1.266 | |
| AC9 | 0.986 | 0.154 | 0.978 | −0.0010 | 0.917 | 3.376 | 0.974 | 1.347 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.; Alshora, D.H. Development and Characterization of Eudragit-RL-100-Based Aceclofenac Sustained-Release Matrix Pellets Prepared via Extrusion/Spheronization. Polymers 2021, 13, 4034. https://doi.org/10.3390/polym13224034
Ibrahim MA, Alshora DH. Development and Characterization of Eudragit-RL-100-Based Aceclofenac Sustained-Release Matrix Pellets Prepared via Extrusion/Spheronization. Polymers. 2021; 13(22):4034. https://doi.org/10.3390/polym13224034
Chicago/Turabian StyleIbrahim, Mohamed Abbas, and Doaa Hasan Alshora. 2021. "Development and Characterization of Eudragit-RL-100-Based Aceclofenac Sustained-Release Matrix Pellets Prepared via Extrusion/Spheronization" Polymers 13, no. 22: 4034. https://doi.org/10.3390/polym13224034
APA StyleIbrahim, M. A., & Alshora, D. H. (2021). Development and Characterization of Eudragit-RL-100-Based Aceclofenac Sustained-Release Matrix Pellets Prepared via Extrusion/Spheronization. Polymers, 13(22), 4034. https://doi.org/10.3390/polym13224034







