Fabrication of High-Performance CNT Reinforced Polymer Composite for Additive Manufacturing by Phase Inversion Technique
Abstract
:1. Introduction
2. Current Methods of CNT Nanocomposite Formation
2.1. CNT’s Modifications
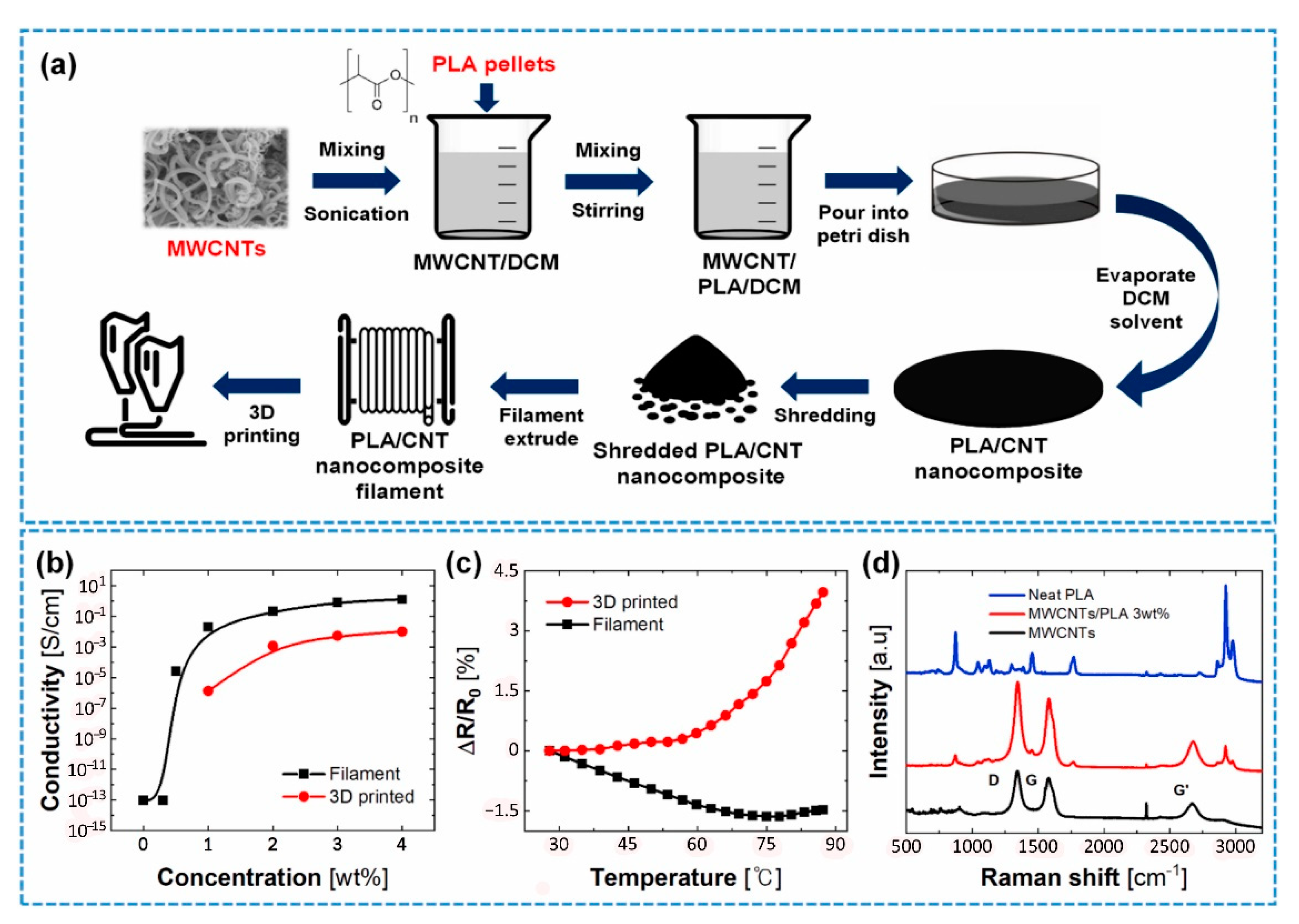
2.2. 3D Printing of Modified CNT Nanocomposites
3. An Alternative to Improve Nanocomposite Properties
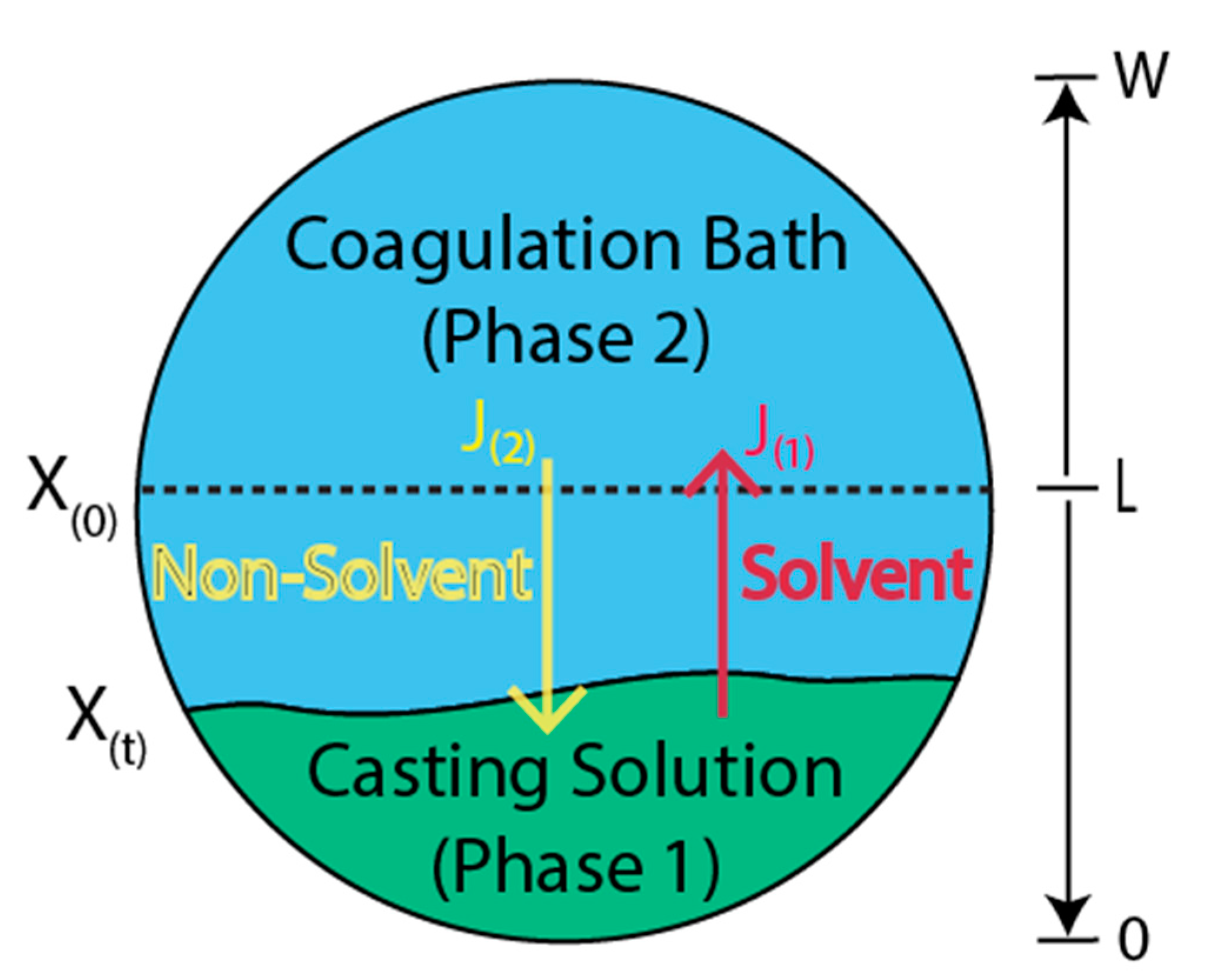
3.1. Phase Inversion Technique
- (a)
- Immersion precipitation. A polymer solution is soaked in nonsolvent coagulation or precipitation bath (commonly water) in this method. The polymer coagulation occurs through the desorption of the solvent in the precipitation bath.
- (b)
- Thermally-induced phase separation. This technique relies on diminishing the solvent’s efficiency by temperature reduction. Subsequently, the solvent is removed by freeze-drying, evaporation, or extraction.
- (c)
- Evaporation-induced phase separation. The polymer solution is produced in a solvent or a combination of volatile nonsolvent, and the evaporation of the solvent results in precipitation or demixing/precipitation. This method is also called solution casting.
- (d)
- Vapor-induced phase separation. The polymer solution is exposed to an atmosphere containing nonsolvent vapor (usually water). Therefore, demixing/precipitation occurs by the absorption of nonsolvent.
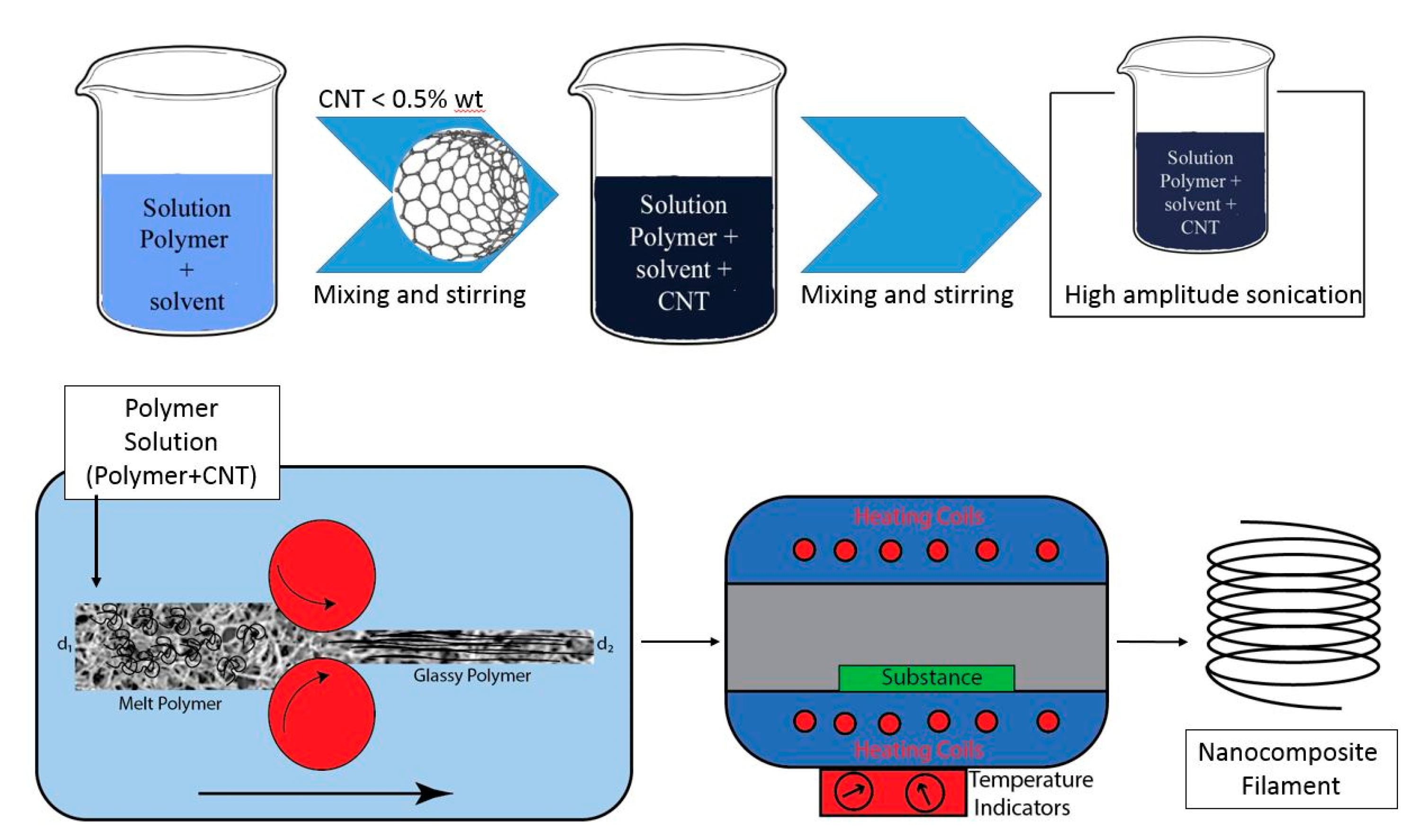
3.2. Phase Inversion for CNT Nanocomposite Fabrication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Parandoush, P.; Lin, D. A review on additive manufacturing of polymer-fiber composites. Compos. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Berman, B. 3-D Printing: The New Industrial Revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Podsiadły, B.; Matuszewski, P.; Skalski, A.; Słoma, M. Carbon Nanotube-Based Composite Filaments for 3D Printing of Structural and Conductive Elements. Appl. Sci. 2021, 11, 1272. [Google Scholar] [CrossRef]
- Van de Werken, N.; Tekinalp, H.; Khanbolouki, P.; Ozcan, S.; Williams, A.; Tehrani, M. Additively manufactured carbon fiber-reinforced composites: State of the art and perspective. Addit. Manuf. 2020, 31, 100962. [Google Scholar] [CrossRef]
- Abeykoon, C.; Sri-Amphorn, P.; Fernando, A. Optimization of fused deposition modeling parameters for improved PLA and ABS 3D printed structures. Int. J. Lightweight Mater. Manuf. 2020, 3, 284–297. [Google Scholar] [CrossRef]
- Liao, G.; Li, Z.; Cheng, Y.; Xu, D.; Zhu, D.; Jiang, S.; Guo, J.; Chen, X.; Xu, G.; Zhu, Y. Properties of Oriented Carbon Fiber/Polyamide 12 Composite Parts Fabricated by Fused Deposition Modeling. Mater. Des. 2018, 139, 283–292. [Google Scholar] [CrossRef]
- Sodeifian, G.; Ghaseminejad, S.; Yousefi, A.A. Preparation of Polypropylene/Short Glass Fiber Composite as Fused Deposition Modeling (FDM) Filament. Results Phys. 2019, 12, 205–222. [Google Scholar] [CrossRef]
- Zhang, W.; Cotton, C.; Sun, J.; Heider, D.; Gu, B.; Sun, B.; Chou, T.-W. Interfacial Bonding Strength of Short Carbon Fiber/Acrylonitrile-Butadiene-Styrene Composites Fabricated by Fused Deposition Modeling. Compos. Part B Eng. 2018, 137, 51–59. [Google Scholar] [CrossRef]
- Ahmadi, M.; Zabihi, O.; Masoomi, M.; Naebe, M. Synergistic effect of MWCNTs functionalization on interfacial and mechanical properties of multi-scale UHMWPE fibre reinforced epoxy composites. Compos. Sci. Technol. 2016, 134, 1–11. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Muhammad Asyraf, M.R.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: An overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Guo, F.; Kang, T.; Liu, Z.; Tong, B.; Guo, L.; Wang, Y.; Liu, C.; Chen, X.; Zhao, Y.; Shen, Y.; et al. Advanced Lithium Metal-Carbon Nanotube Composite Anode for High-Performance Lithium-Oxygen Batteries. Nano Lett. 2019, 19, 6377–6384. [Google Scholar] [CrossRef]
- Chen, M.; Shao, L.L.; Lv, X.W.; Wang, G.C.; Yang, W.Q.; Yuan, Z.Y.; Qian, X.; Han, Y.Y.; Ding, A.X. In situ growth of Ni-encapsulated and N-doped carbon nanotubes on N-doped ordered mesoporous carbon for high-efficiency triiodide reduction in dye-sensitized solar cells. Chem. Eng. J. 2020, 390, 124633. [Google Scholar] [CrossRef]
- Mo, Z.; Yang, R.; Lu, D.; Yang, L.; Hu, Q.; Li, H.; Zhu, H.; Tang, Z.; Gui, X. Lightweight, three-dimensional carbon Nanotube@TiO 2 sponge with enhanced microwave absorption performance. Carbon 2019, 144, 433–439. [Google Scholar] [CrossRef]
- Zainol Abidin, M.S.; Herceg, T.; Greenhalgh, E.S.; Shaffer, M.; Bismarck, A. Enhanced fracture toughness of hierarchical carbon nanotube reinforced carbon fibre epoxy composites with engineered matrix microstructure. Compos. Sci. Technol. 2019, 170, 85–92. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Harussani, M.M.; Siti Zulaikha, N.D.; Norhana, A.H.; Imran Syakir, M.; Norli, A. Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors—An overview. Polimery 2021, 66, 85–97. [Google Scholar] [CrossRef]
- Souto, L.F.C.; Soares, B.G. Polyaniline/carbon nanotube hybrids modified with ionic liquids as anticorrosive additive in epoxy coatings. Prog. Org. Coat. 2020, 143, 105598. [Google Scholar] [CrossRef]
- Peng-ChengMa, A.; Siddiquia, N.; Gad, M.; Jang-Kyo, K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Bahun, G.J.; Wang, C.; Adronov, A. Solubilizing single-walled carbon nanotubes with pyrene-functionalized block copolymers. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1941–1951. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of halloysite, imogolite, and boron nitride nanotubes in solvent media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Riggs, J.E.; Walker, D.B.; Carroll, D.L.; Sun, Y.P. Optical limiting properties of suspended and solubilized carbon nanotubes. J. Phys. Chem. B 2000, 104, 7071–7076. [Google Scholar] [CrossRef]
- Shaffer, M.S.P.; Koziol, K. Polystyrene grafted multi-walled carbon nanotubes. ChemComm 2002, 18, 2074–2075. [Google Scholar] [CrossRef]
- Shaffer, M.S.; Fan, X.; Windle, A.H. Dispersion and packing of carbon nanotubes. Carbon 1998, 36, 1603–1612. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Yu, H.; Ding, W.; Li, Y.; Wang, J. High-performance sodium polyacrylate nanocomposites developed by using ionic liquid covalently modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2020, 137, 49161. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, J.; Deng, L.; Li, L.; Li, J.; Yang, Y.; Khashab, N.M. Polyetherimide/bucky gels nanocomposites with superior conductivity and thermal stability. ACS Appl. Mater. Interfaces 2013, 5, 7478–7484. [Google Scholar] [CrossRef]
- Han, J.Y.; Chen, T.; Mu, Q.; Baird, D.G. Thermotropic liquid crystalline polymer reinforced polypropylene composites enhanced with carbon nanotubes for use in fused filament fabrication. Polym. Compos. 2021, 42, 4115–4127. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, E.; Giamberini, M.; D’Amore, A.; Priola, A.; Malucelli, G. December. The effect of prepolymer composition of amino-hardened liquid crystalline epoxy resins on physical properties of cured thermoset. Macromol. Symp. 1999, 148, 197–209. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Baird, D.G. An improved technique for exfoliating and dispersing nanoclay particles into polymer matrices using supercritical carbon dioxide. Polymer 2007, 48, 6923–6933. [Google Scholar] [CrossRef]
- Love, L.J.; Kunc, V.; Rios, O.; Duty, C.E.; Elliott, A.M.; Post, B.K.; Smith, R.J.; Blue, C.A. The importance of carbon fiber to polymer additive manufacturing. Mater. Res. 2014, 29, 1893–1898. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.Y.; Lauke, B.; Mäder, E.; Yue, C.Y.; Hu, X. Tensile properties of short-glass-fiber-and short-carbon-fiber-reinforced polypropylene composites. Compos. Part A Appl. Sci. 2000, 31, 1117–1125. [Google Scholar] [CrossRef]
- Loos, M.R.; Yang, J.; Feke, D.L.; Manas-Zloczower, I. Effect of block-copolymer dispersants on properties of carbon nanotube/epoxy systems. Compos Sci Technol. 2012, 72, 482–488. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shimizu, H. Conductive PVDF/PA6/CNTs nanocomposites fabricated by dual formation of cocontinuous and nanodispersion structures. Macromolecules 2008, 41, 5339–5344. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey Jr, J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Stano, G.; Di Nisio, A.; Lanzolla, A.M.; Ragolia, M.; Percoco, G. Fused filament fabrication of commercial conductive filaments: Experimental study on the process parameters aimed at the minimization, repeatability and thermal characterization of electrical resistance. Int. J. Adv. Manuf. Syst. 2020, 111, 2971–2986. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, L.; Liu, X.; Zhou, Y.; Zhang, M.; Wang, Y.; Yang, L.; Wei, D. Wide linear range and highly sensitive flexible pressure sensor based on multistage sensing process for health monitoring and human-machine interfaces. Chem. Eng. J. 2021, 412, 128649. [Google Scholar] [CrossRef]
- Kim, H.G.; Hajra, S.; Oh, D.; Kim, N.; Kim, H.J. Additive manufacturing of high-performance carbon-composites: An integrated multi-axis pressure and temperature monitoring sensor. Compos. B. Eng. 2021, 222, 109079. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Park, J.; Jang, I.R.; Lee, K.; Kim, H.J. High efficiency crumpled carbon nanotube heaters for low drift hydrogen sensing. Sensors 2019, 19, 3878. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Panes, A.; Claver, J.; Camacho, A.M. The influence of manufacturing parameters on the mechanical behaviour of PLA and ABS pieces manufactured by FDM: A comparative analysis. Materials 2018, 11, 1333. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.G.; Barbera, L.; Masania, K.; Studart, A.R. Three-dimensional printing of multicomponent glasses using phase-separating resins. Nat. Mater. 2020, 19, 212–217. [Google Scholar] [CrossRef]
- Hahn, V.; Kiefer, P.; Frenzel, T.; Qu, J.; Blasco, E.; Barner-Kowollik, C.; Wegener, M. Rapid assembly of small materials building blocks (voxels) into large functional 3D metamaterials. Adv. Funct. Mater. 2020, 30, 1907795. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D printing of inherently nanoporous polymers via polymerization-induced phase separation. Nat. Commun. 2021, 12, 247. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Kinetic modeling and simulation of nonsolvent induced phase separation: Immersion precipitation of PVC-based casting solution in a finite salt coagulation bath. Polymer 2020, 199, 122527. [Google Scholar] [CrossRef]
- Fernandes, G.R.; Pinto, J.C.; Nobrega, R. Modeling and simulation of the phase-inversion process during membrane preparation. J. Appl. Polym. Sci. 2001, 82, 3036–3051. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ford, D.M.; Qian, X.; Cervellere, M.R.; Millett, P.C.; Wang, X. A review on models and simulations of membrane formation via phase inversion processes. J. Membr. Sci. 2021, 640, 119810. [Google Scholar] [CrossRef]
- de Lannoy, C.F.; Soyer, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D.R. Thermodynamics of polymer/diluent systems for thermally induced phase separation: 1. Determination of equation of state parameters. Polymer 1992, 33, 1026–1035. [Google Scholar] [CrossRef]
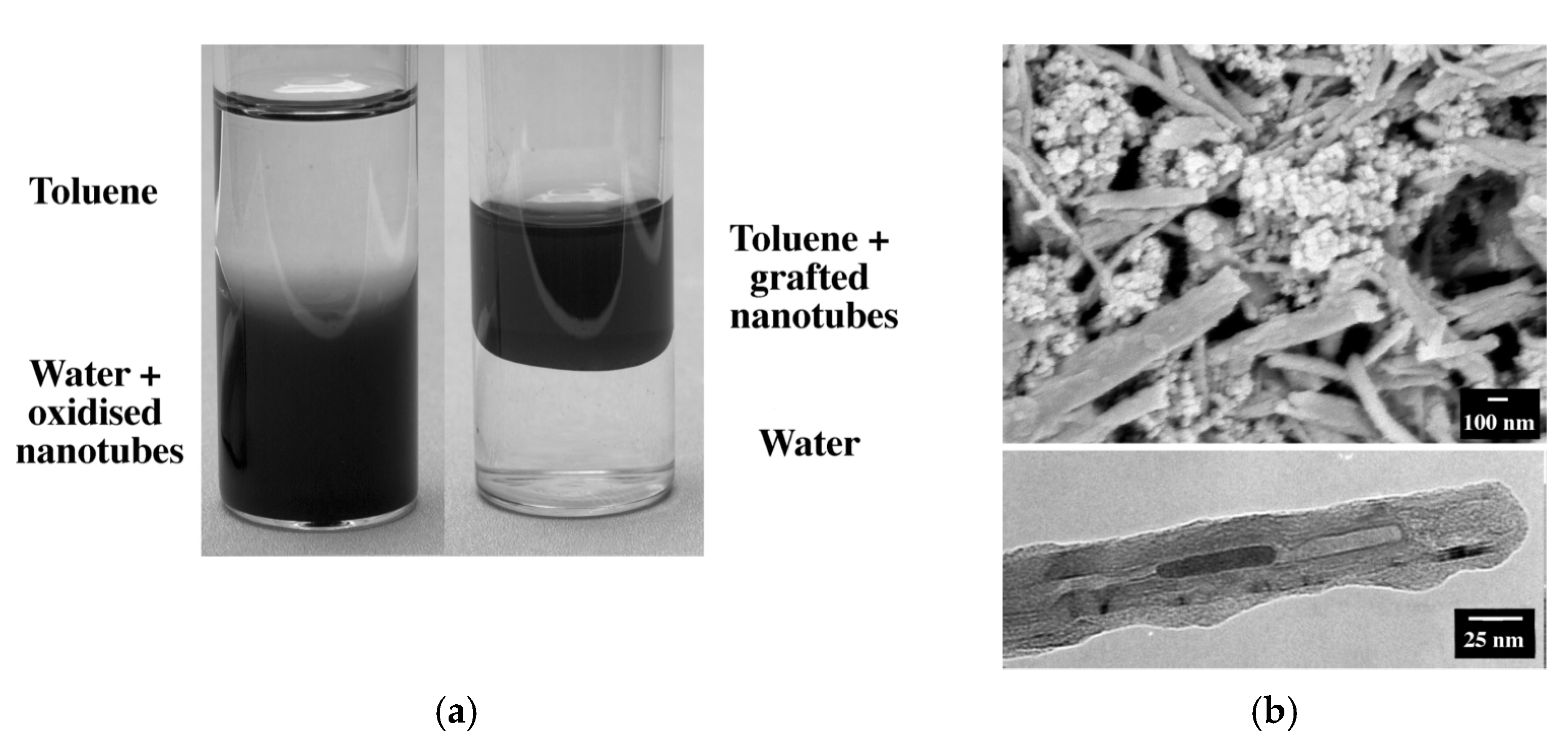



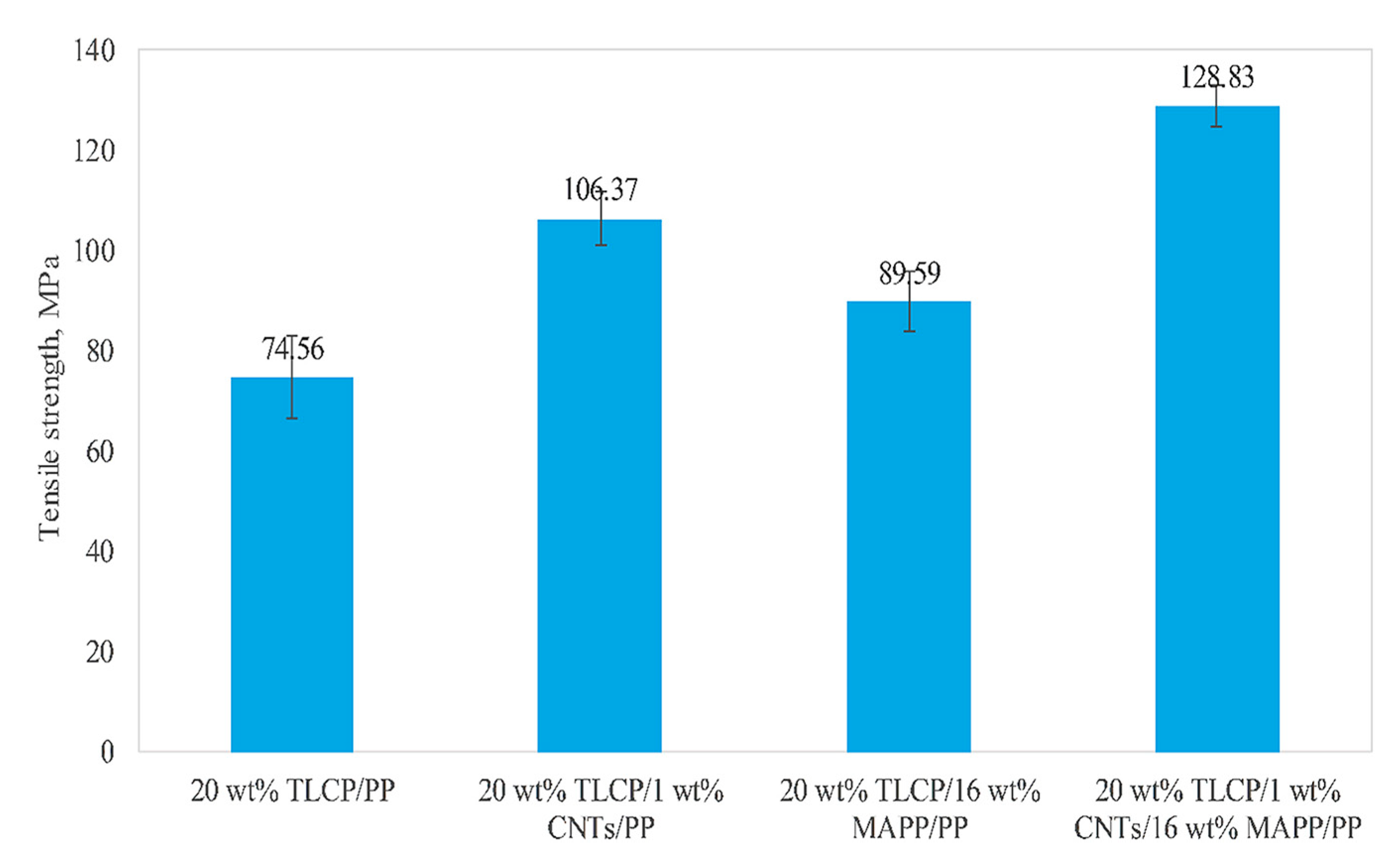
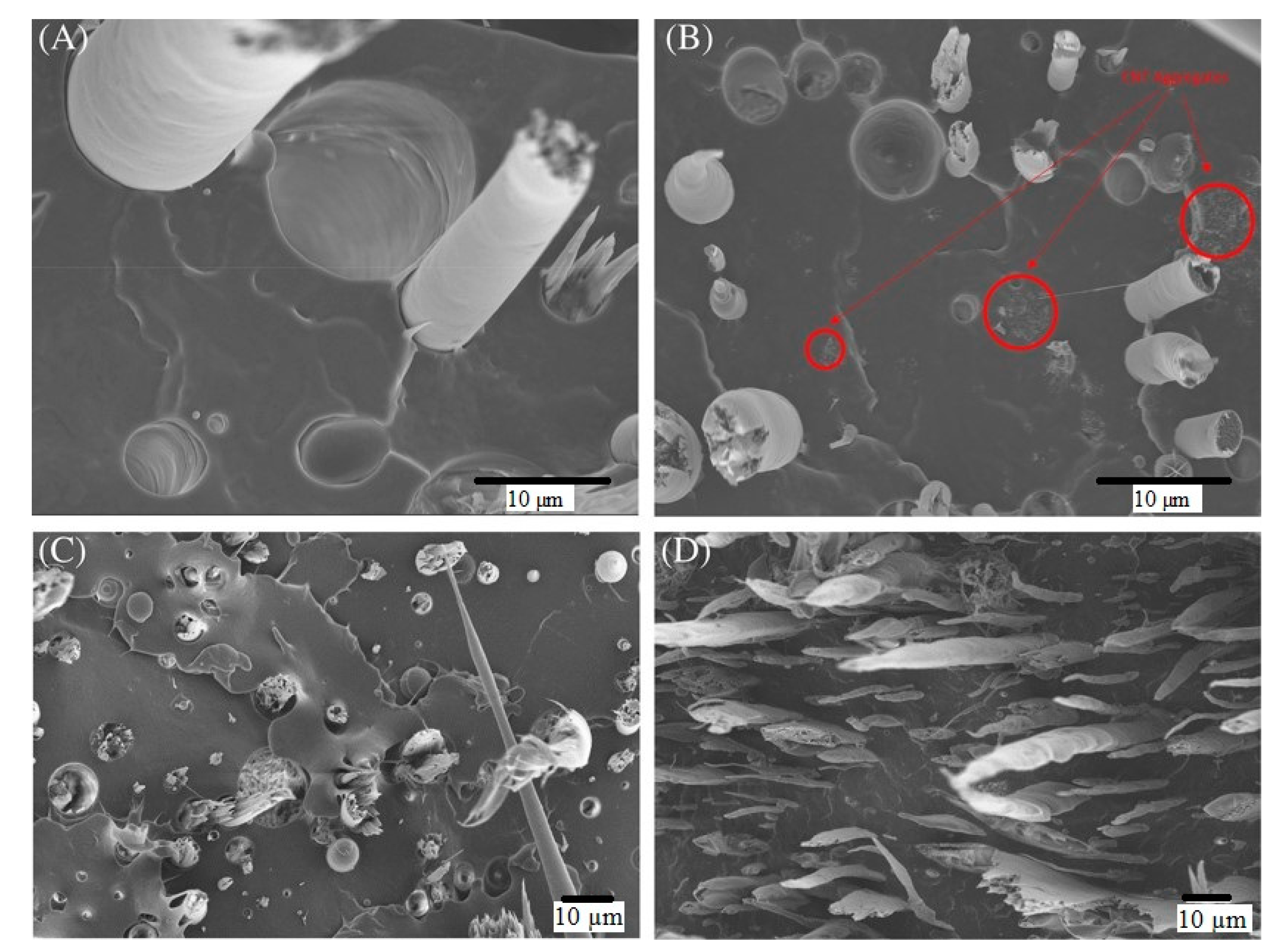
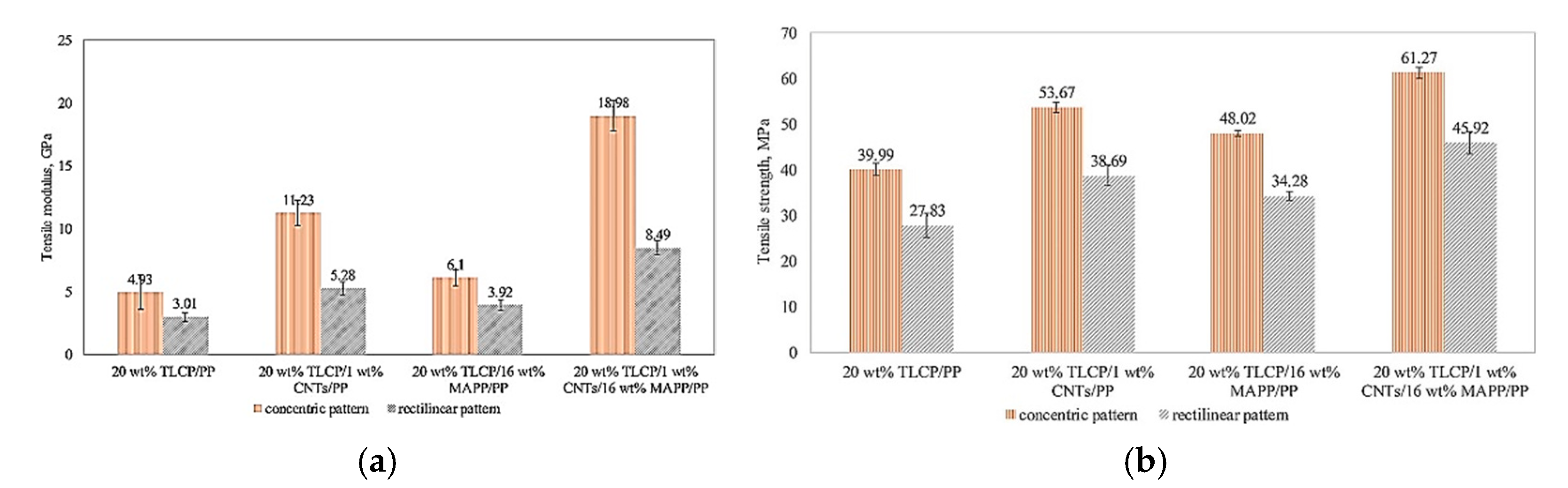

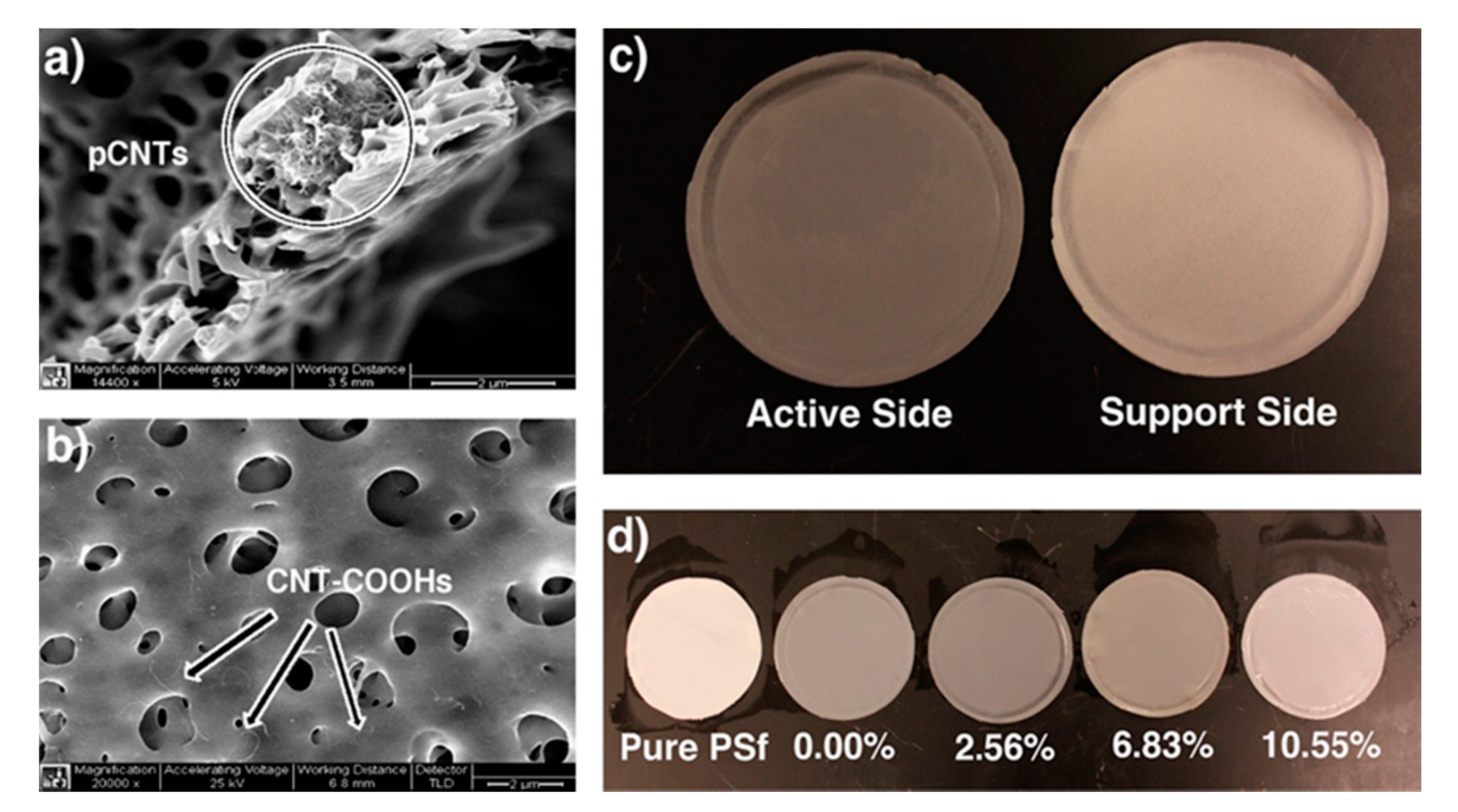
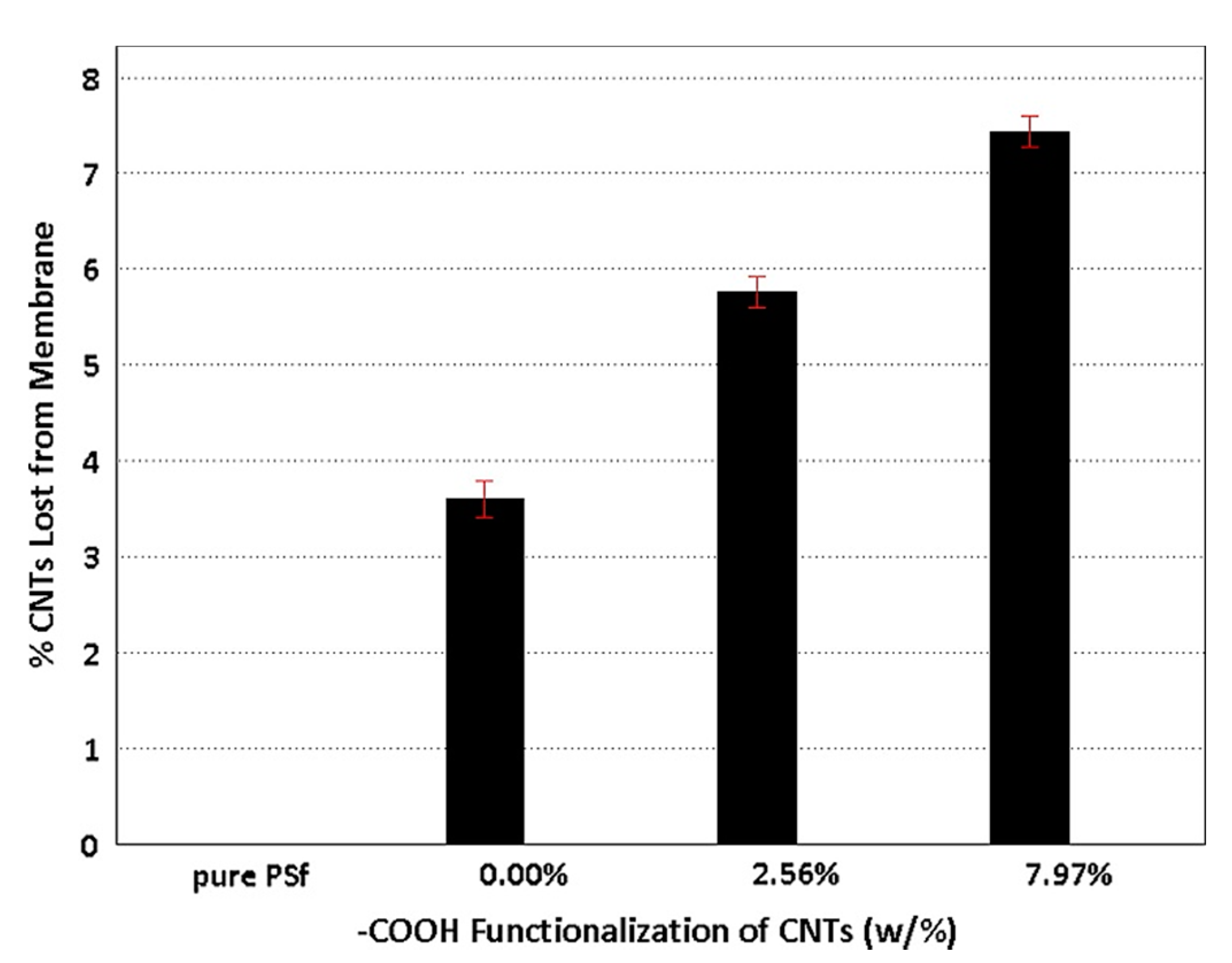
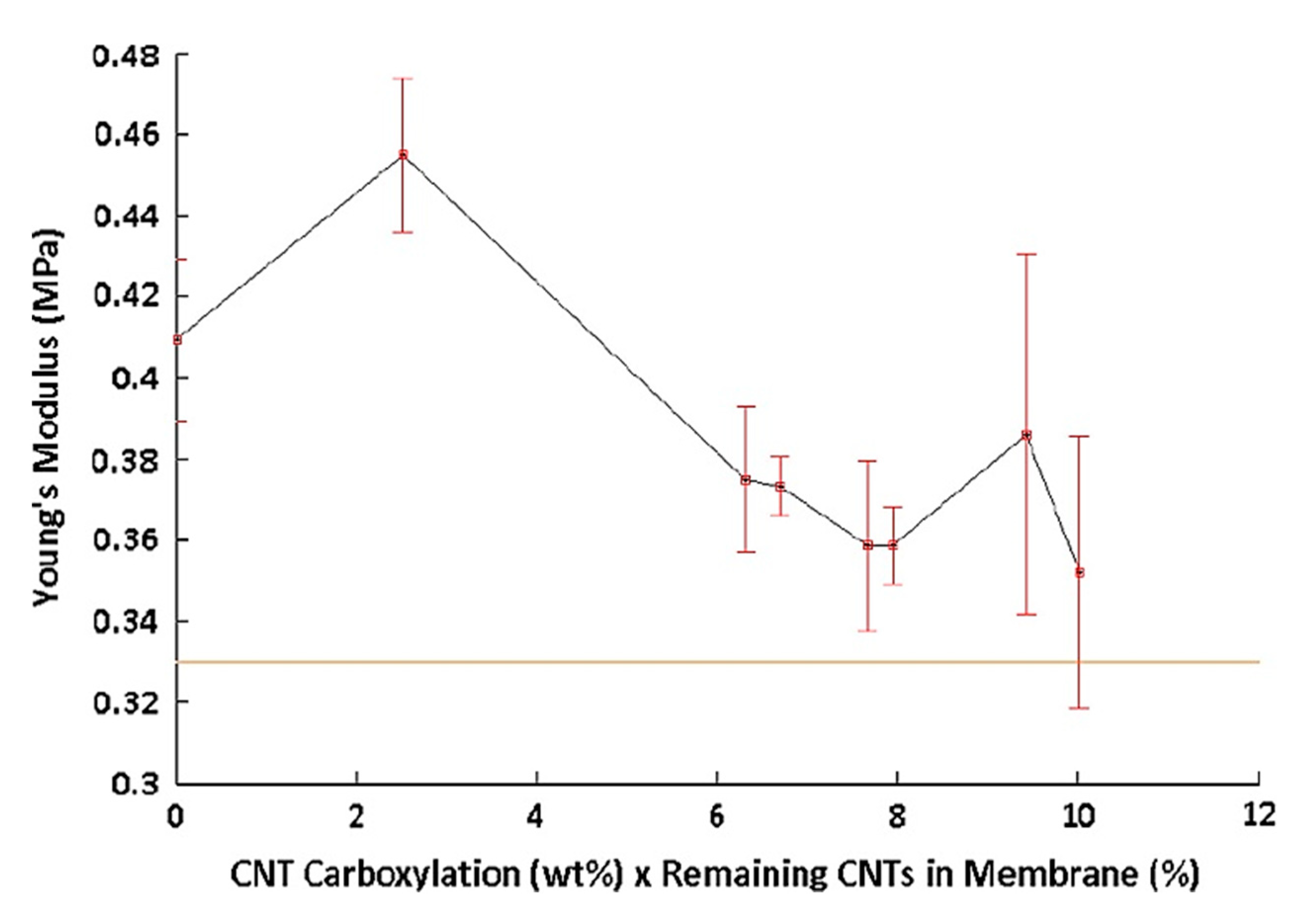
| Carboxylation Degree (wt%) | 0 | 6.53 | 6.94 | 7.97 | 8.29 | 9.91 | 10.55 |
|---|---|---|---|---|---|---|---|
| Reaction time (h) | 0 | 1 | 2 | 4 | 4 | 8 | 8 |
| Volume of acids to mass of CNTs (mL/g) | 0 | 150 | 150 | 75 | 150 | 150 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnian, P.; D’Amore, A. Fabrication of High-Performance CNT Reinforced Polymer Composite for Additive Manufacturing by Phase Inversion Technique. Polymers 2021, 13, 4007. https://doi.org/10.3390/polym13224007
Parnian P, D’Amore A. Fabrication of High-Performance CNT Reinforced Polymer Composite for Additive Manufacturing by Phase Inversion Technique. Polymers. 2021; 13(22):4007. https://doi.org/10.3390/polym13224007
Chicago/Turabian StyleParnian, Pooyan, and Alberto D’Amore. 2021. "Fabrication of High-Performance CNT Reinforced Polymer Composite for Additive Manufacturing by Phase Inversion Technique" Polymers 13, no. 22: 4007. https://doi.org/10.3390/polym13224007
APA StyleParnian, P., & D’Amore, A. (2021). Fabrication of High-Performance CNT Reinforced Polymer Composite for Additive Manufacturing by Phase Inversion Technique. Polymers, 13(22), 4007. https://doi.org/10.3390/polym13224007






