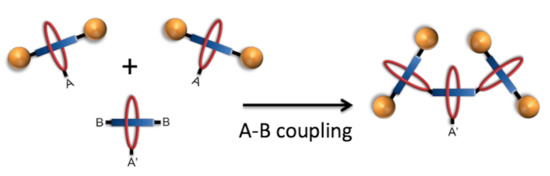
Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer
Abstract
:1. Introduction
2. Materials and Methods
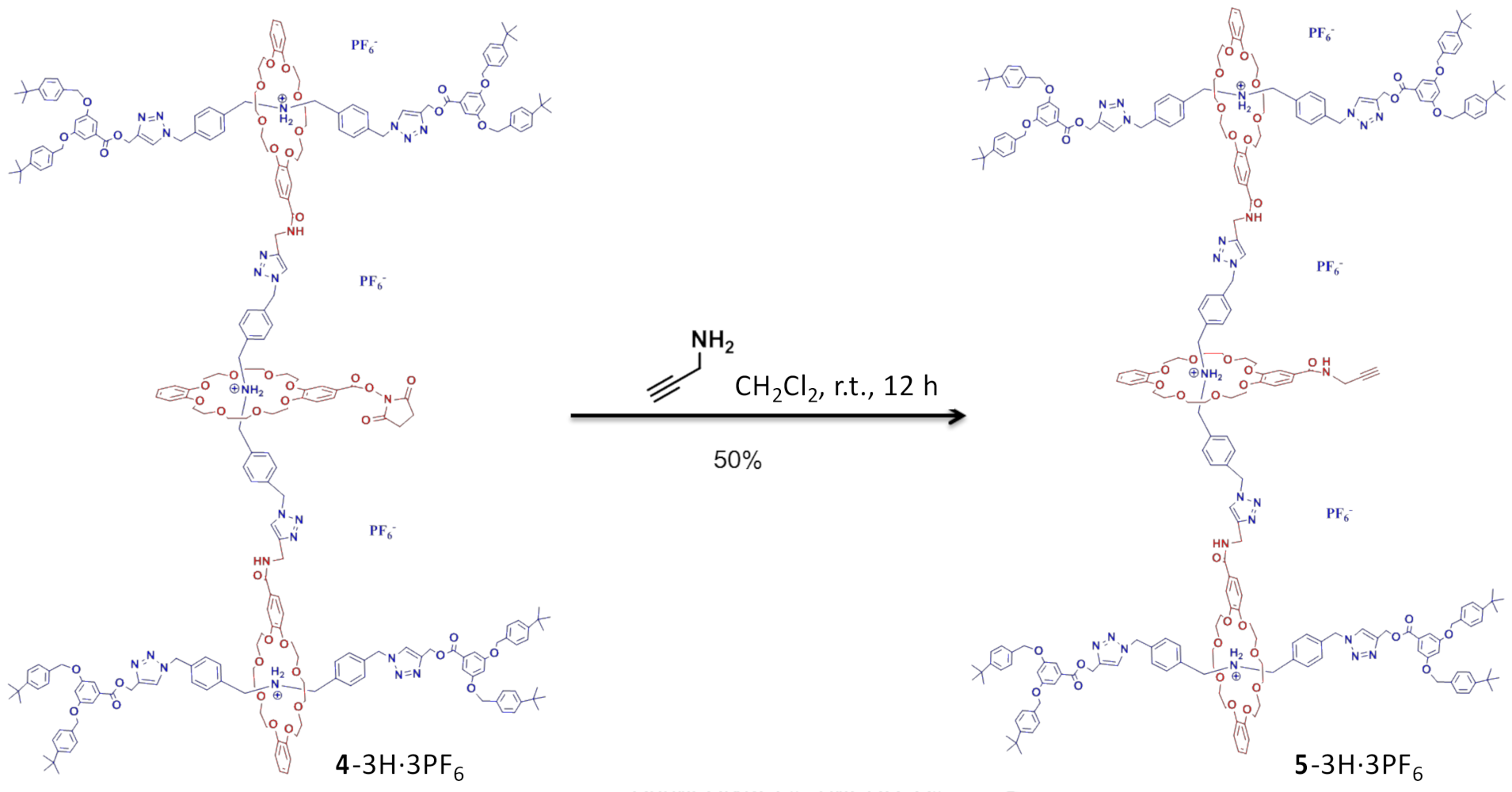
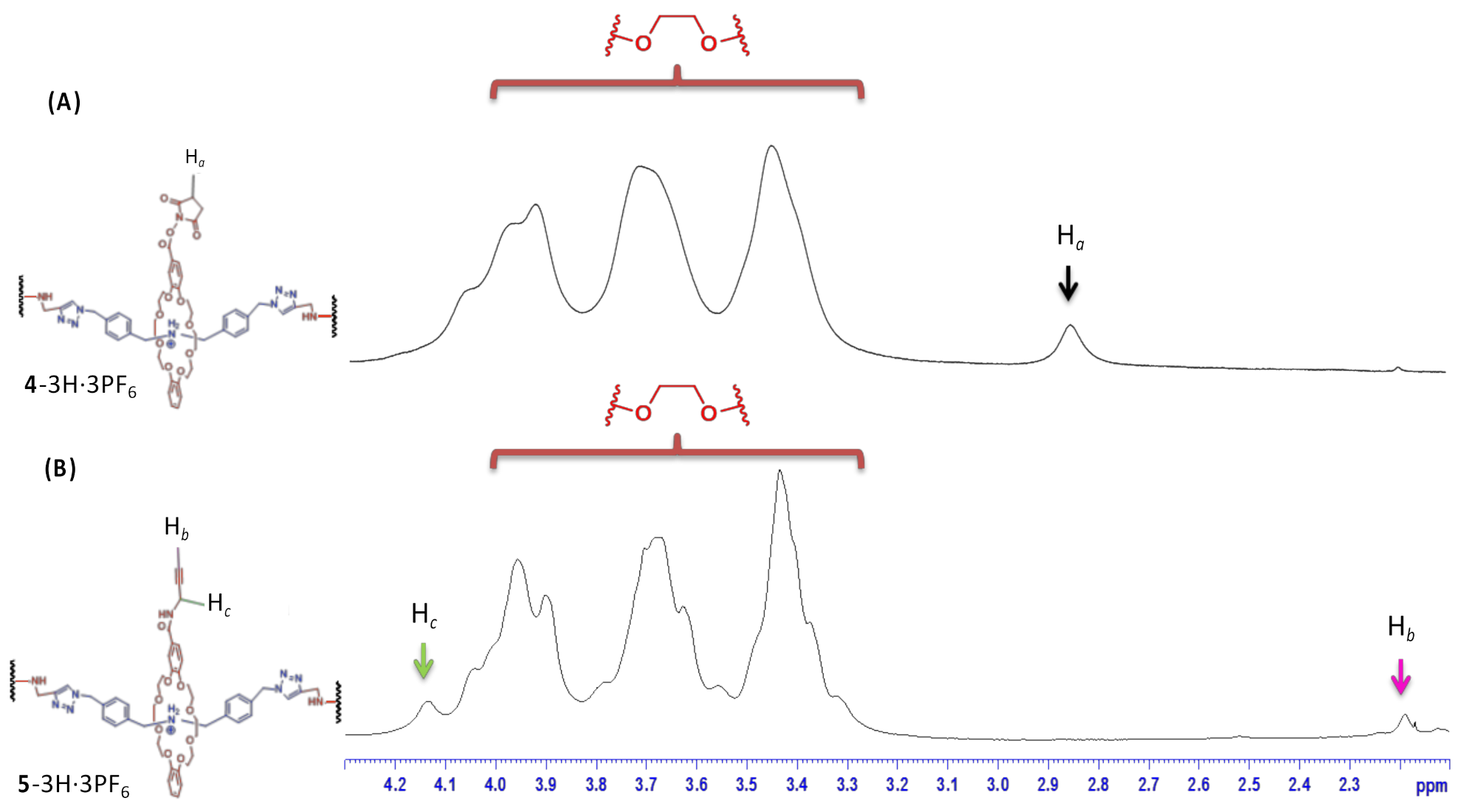
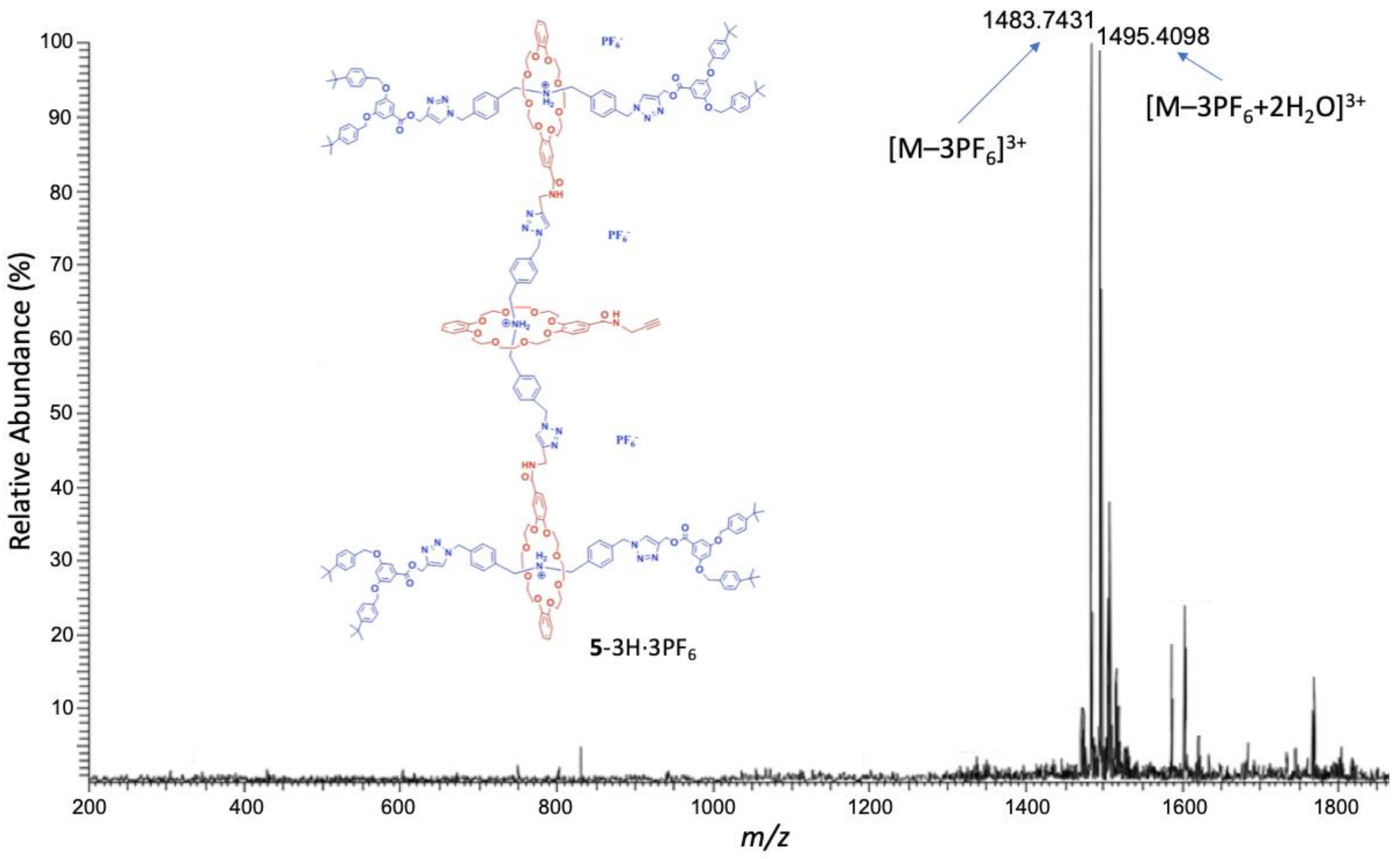
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-Based Molecular Muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, Q.-C. Recent progress on switchable rotaxanes. Chem. Soc. Rev. 2006, 35, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; McGonigal, P.R.; Schneebeli, S.T.; Li, H.; Vermeulen, N.A.; Ke, C.; Stoddart, J.F. An artificial molecular pump. Nat. Nanotechnol. 2015, 10, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Fielden, S.D.P.; Leigh, D.A. A catalysis-driven artificial molecular pump. Nature 2021, 594, 529–534. [Google Scholar] [CrossRef]
- Canton, M.; Groppi, J.; Casimiro, L.; Corra, S.; Baroncini, M.; Silvi, S.; Credi, A. Second-Generation Light-Fueled Supramolecular Pump. J. Am. Chem. Soc. 2021, 143, 10890–10894. [Google Scholar] [CrossRef]
- Wilson, M.R.; Solà, J.; Carlone, A.; Goldup, S.M.; Lebrasseur, N.; Leigh, D.A. An autonomous chemically fuelled small-molecule motor. Nature 2016, 534, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, B.; Bo, G.D.; Ward, J.W.; Papmeyer, M.; Kuschel, S.; Aldegunde, M.J.; Gramlich, P.M.E.; Heckmann, D.; Goldup, S.M.; D’Souza, D.M.; et al. Sequence-Specific Peptide Synthesis by an Artificial Small-Molecule Machine. Science 2013, 339, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Leigh, D.A.; Marcos, V.; Wilson, M.R. Rotaxane Catalysts. ACS Catal. 2014, 4, 4490–4497. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Chan, A.S.C.; Leung, K.C.-F. A Fluorescent and Switchable Rotaxane Dual Organocatalyst. Org. Lett. 2016, 18, 976–979. [Google Scholar] [CrossRef]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Liu, M.; Fréchet, J.M.J. Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 1999, 2, 393–401. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, K. Rotaxane Dendrimers. In Dendrimers V: Functional and Hyperbranched Building Blocks, Photophysical Properties, Applications in Materials and Life Sciences; Schalley, C.A., Vögtle, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 111–140. [Google Scholar]
- Leung, K.C.-F.; Lau, K.-N. Self-assembly and thermodynamic synthesis of rotaxane dendrimers and related structures. Polym. Chem. 2010, 1, 988–1000. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Leung, K.C.-F. Development and advancement of rotaxane dendrimers as switchable macromolecular machines. Mater. Chem. Front. 2020, 4, 2825–2844. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Li, W.-J.; Wang, W.; Yang, H.-B. Rotaxane Dendrimers: Alliance between Giants. Acc. Chem. Res. 2021, 54, 4091–4106. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Ashton, P.R.; Balzani, V.; Brown, C.L.; Credi, A.; Fréchet, J.M.J.; Leon, J.W.; Raymo, F.M.; Spencer, N.; Stoddart, J.F.; et al. Self-Assembly of [n]Rotaxanes Bearing Dendritic Stoppers. J. Am. Chem. Soc. 1996, 118, 12012–12020. [Google Scholar] [CrossRef]
- Leung, K.C.F.; Aricó, F.; Cantrill, S.J.; Stoddart, J.F. Template-Directed Dynamic Synthesis of Mechanically Interlocked Dendrimers. J. Am. Chem. Soc. 2005, 127, 5808–5810. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.F.; Aricó, F.; Cantrill, S.J.; Stoddart, J.F. Dynamic Mechanically Interlocked Dendrimers: Amplification in Dendritic Dynamic Combinatorial Libraries. Macromolecules 2007, 40, 3951–3959. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Zhou, Q.-F.; Chen, L.-J.; Xu, L.; Wang, C.-H.; Li, X.; Yang, H.-B. Facile construction of organometallic rotaxane-terminated dendrimers using neutral platinum–acetylides as the main scaffold. Chem. Commun. 2018, 54, 2224–2227. [Google Scholar] [CrossRef]
- Elizarov, A.M.; Chiu, S.-H.; Glink, P.T.; Stoddart, J.F. Dendrimer with Rotaxane-Like Mechanical Branching. Org. Lett. 2002, 4, 679–682. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.-J.; Wang, X.-Q.; Sun, B.; Li, X.; Zhang, Y.; Shi, J.; Yu, Y.; Zhang, L.; Liu, M.; et al. Organometallic rotaxane dendrimers with fourth-generation mechanically interlocked branches. Proc. Natl. Acad. Sci. USA 2015, 112, 5597–5601. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-J.; Hu, Z.; Xu, L.; Wang, X.-Q.; Wang, W.; Yin, G.-Q.; Zhang, D.-Y.; Sun, Z.; Li, X.; Sun, H.; et al. Rotaxane-Branched Dendrimers with Enhanced Photosensitization. J. Am. Chem. Soc. 2020, 142, 16748–16756. [Google Scholar] [CrossRef]
- Ho, W.K.W.; Lee, S.-F.; Wong, C.-H.; Zhu, X.-M.; Kwan, C.-S.; Chak, C.-P.; Mendes, P.M.; Cheng, C.H.K.; Leung, K.C.-F. Type III-B rotaxane dendrimers. Chem. Commun. 2013, 49, 10781–10783. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Zhao, R.; Van Hove, M.A.; Cai, Z.; Leung, K.C.-F. Higher-generation type III-B rotaxane dendrimers with controlling particle size in three-dimensional molecular switching. Nat. Commun. 2018, 9, 497. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Leung, K.C.-F. Hetero type III-B rotaxane dendrimers. J. Chin. Chem. Soc. 2020, 67, 1734–1741. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Wang, T.; Li, M.; Chan, A.S.C.; Cai, Z.; Leung, K.C.-F. Type III-C rotaxane dendrimers: Synthesis, dual size modulation and in vivo evaluation. Chem. Commun. 2019, 55, 13426–13429. [Google Scholar] [CrossRef]
- Wang, T.; Cai, Z.; Chen, Y.; Lee, W.K.; Kwan, C.-S.; Li, M.; Chan, A.S.C.; Chen, Z.-F.; Cheung, A.K.L.; Leung, K.C.-F. MALDI-MS Imaging Analysis of Noninflammatory Type III Rotaxane Dendrimers. J. Am. Soc. Mass Spectrom. 2020, 31, 2488–2494. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Leung, K.C.F.; Liong, M.; Pentecost, C.D.; Stoddart, J.F.; Zink, J.I. Construction of a pH-Driven Supramolecular Nanovalve. Org. Lett. 2006, 8, 3363–3366. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Li, X.-B.; Li, X.; Lee, S.-F.; Yu, J.C.; Mendes, P.M.; Hermann, K.E.; Van Hove, M.A. Soft nanohand grabs a growing nanoparticle. Mater. Chem. Front. 2019, 3, 1555–1564. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Xuan, S.; Lo, C.-M. Reversible Switching between Hydrophilic and Hydrophobic Superparamagnetic Iron Oxide Microspheres via One-Step Supramolecular Dynamic Dendronization: Exploration of Dynamic Wettability. ACS Appl. Mater. Interfaces 2009, 1, 2005–2012. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Gajek, A.; Kslezak, M.; Dzialak, P.; Klajnert-Maculewicz, B. Molecular Mechanisms of Antitumor Activity of PAMAM Dendrimer Conjugates with Anticancer Drugs and a Monoclonal Antibody. Polymers 2019, 11, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Malinga-Drozd, M.; Uram, Ł.; Wróbel, K.; Wołowlec, S. Chiral Recognition of Homochiral Poly(amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells in Vitro. Polymers 2021, 13, 1049. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, C.-S.; Ho, W.K.-W.; Chen, Y.; Cai, Z.; Leung, K.C.-F. Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer. Polymers 2021, 13, 3909. https://doi.org/10.3390/polym13223909
Kwan C-S, Ho WK-W, Chen Y, Cai Z, Leung KC-F. Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer. Polymers. 2021; 13(22):3909. https://doi.org/10.3390/polym13223909
Chicago/Turabian StyleKwan, Chak-Shing, Watson K.-W. Ho, Yanyan Chen, Zongwei Cai, and Ken Cham-Fai Leung. 2021. "Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer" Polymers 13, no. 22: 3909. https://doi.org/10.3390/polym13223909
APA StyleKwan, C.-S., Ho, W. K.-W., Chen, Y., Cai, Z., & Leung, K. C.-F. (2021). Synthesis of Functional Building Blocks for Type III-B Rotaxane Dendrimer. Polymers, 13(22), 3909. https://doi.org/10.3390/polym13223909