Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
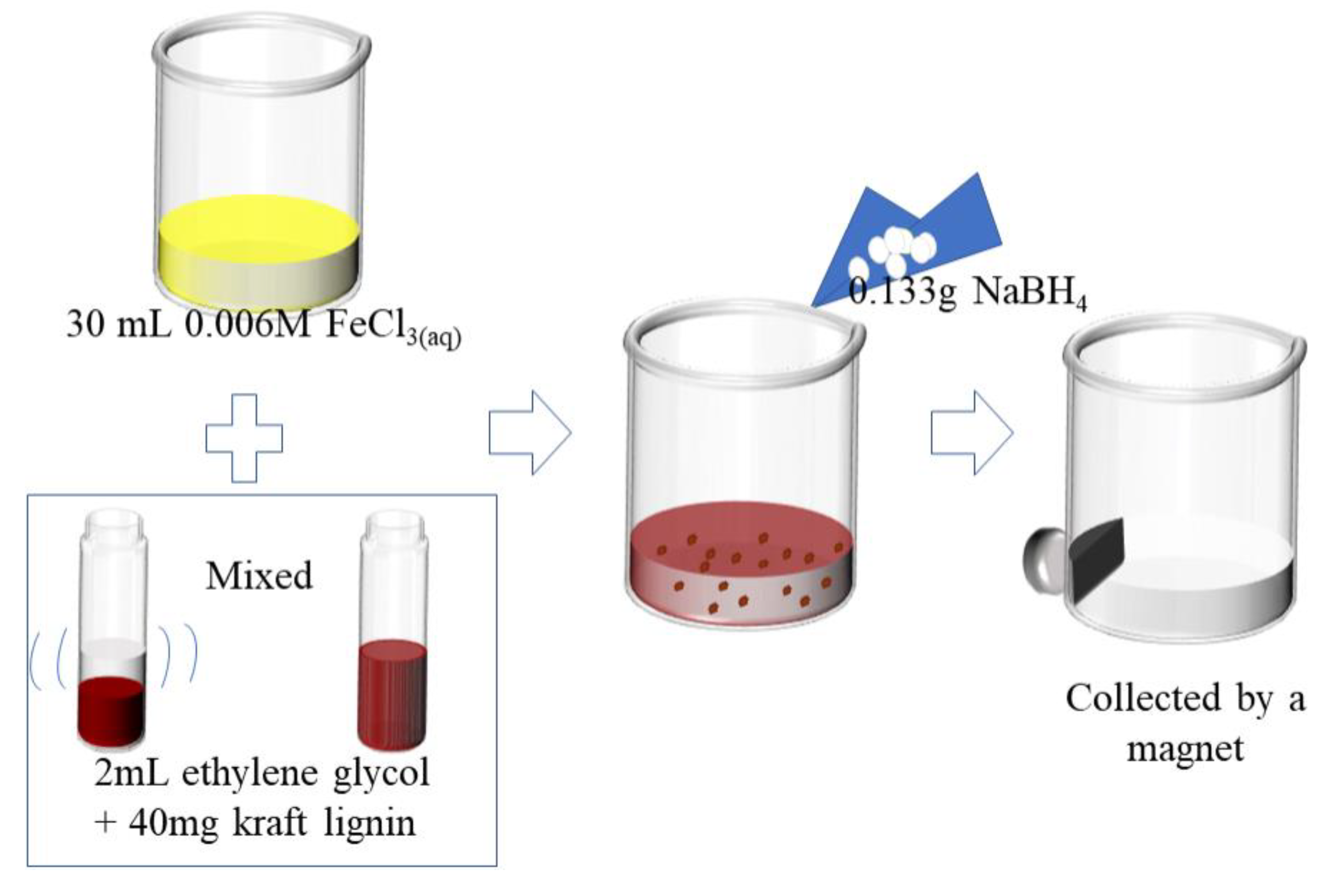
2.2. Preparation of Nanoscale Lignin-Stabilized nZVI
2.3. Dispersibility and Stability against Oxidation of nZVI/n-Lignin
2.4. Removal of Methylene Blue over nZVI/n-Lignin in Aqueous Solutions
3. Results and Discussion
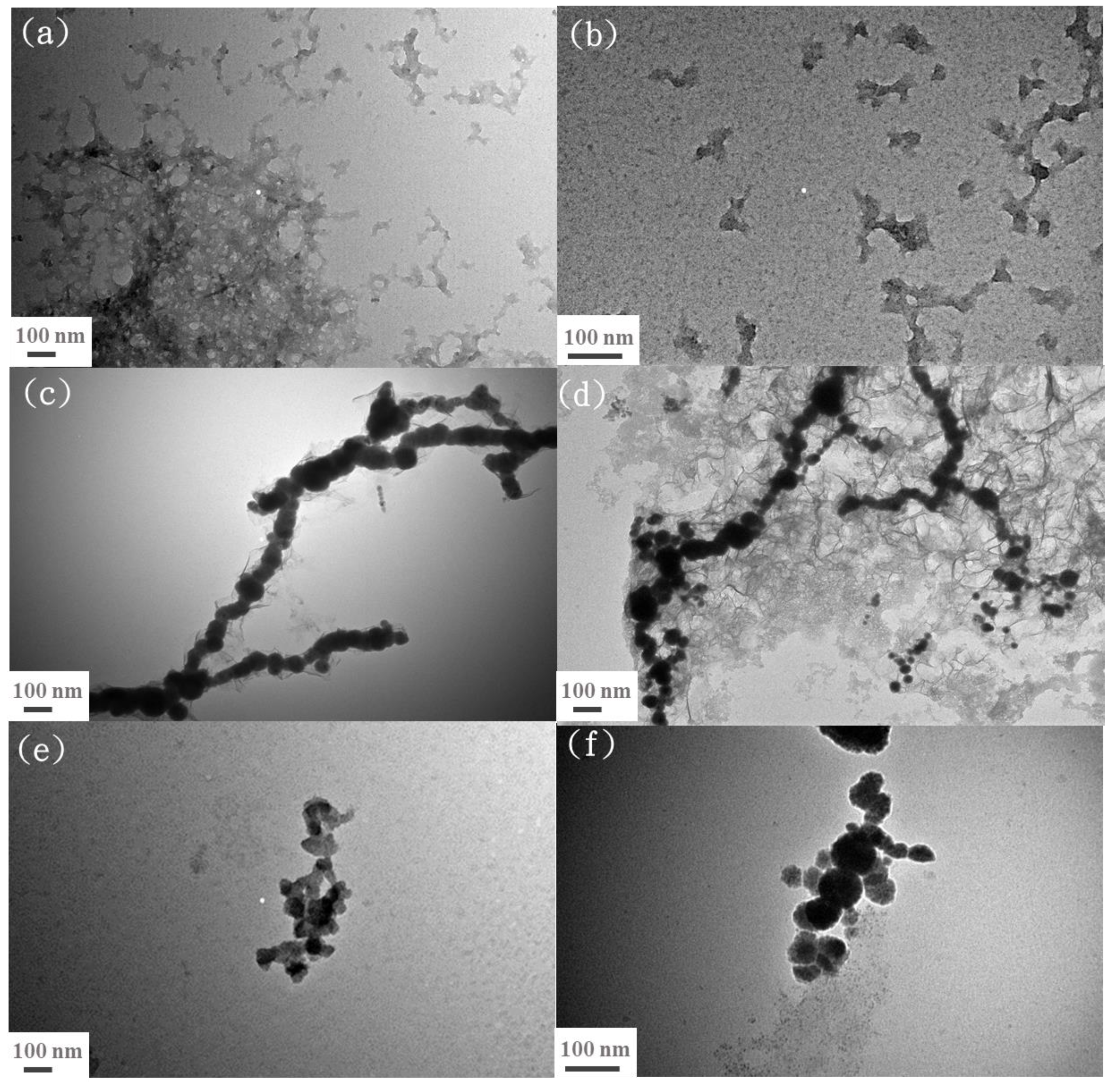
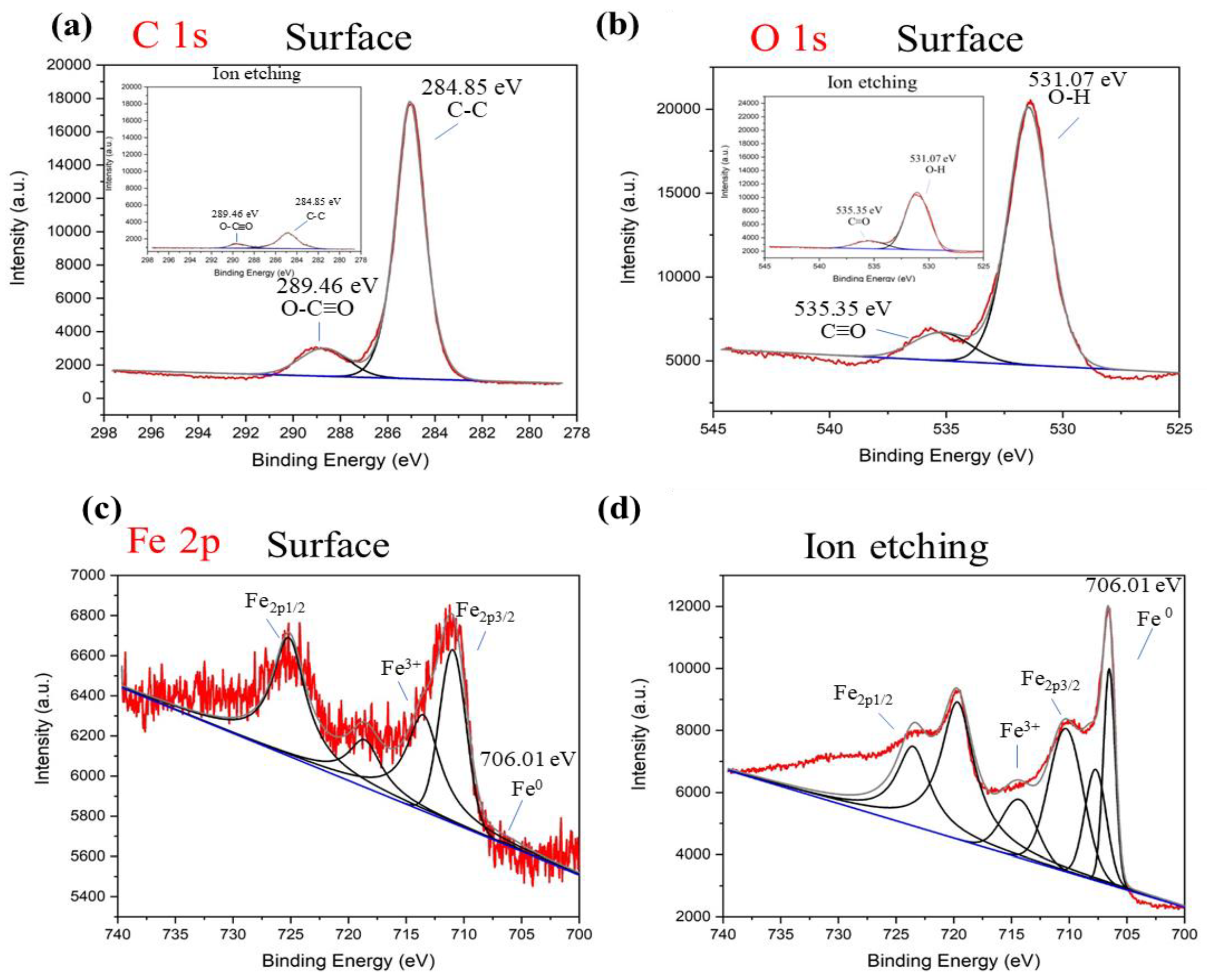
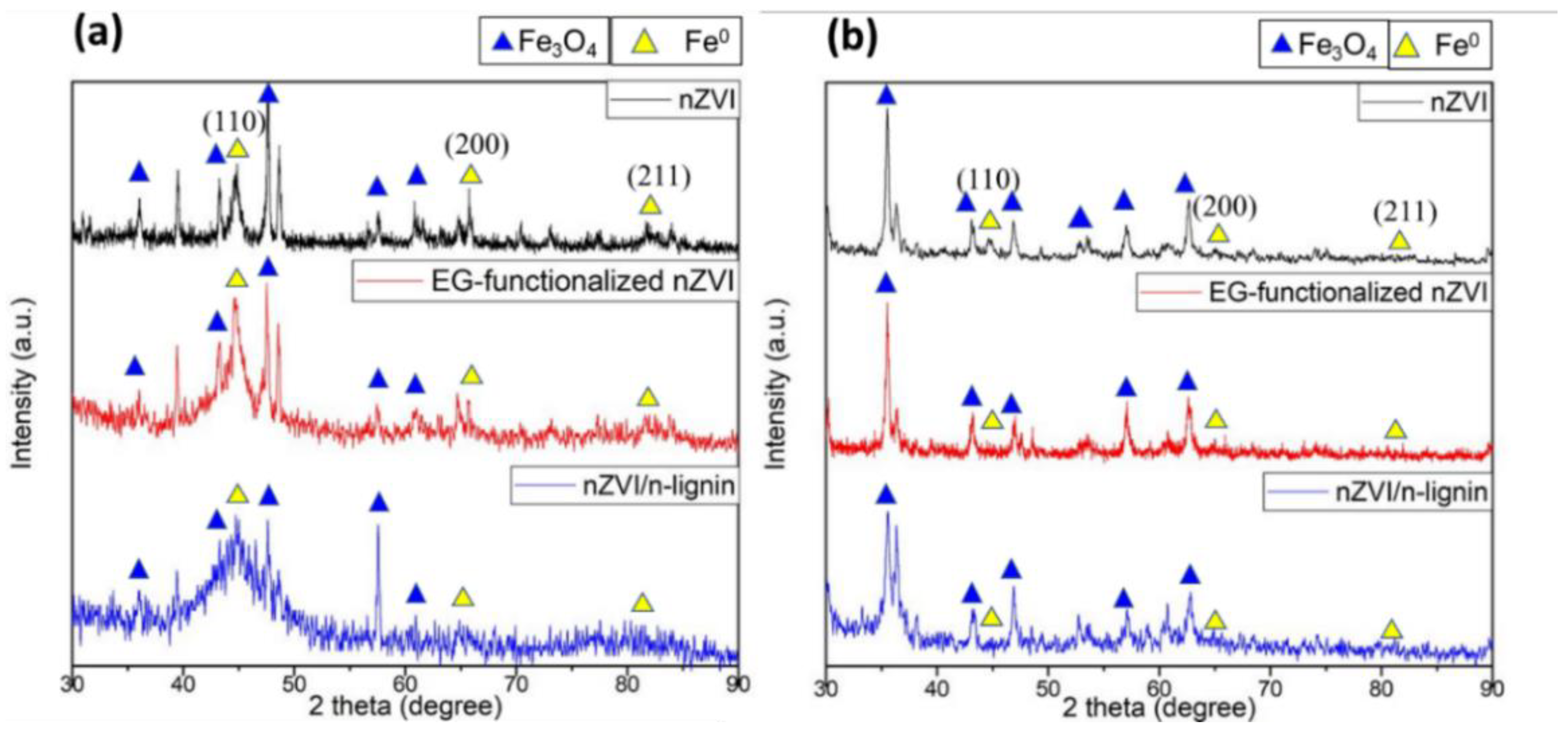
3.1. Characterization of Bare nZVI, EG-Functionalized nZVI, and nZVI/n-Lignin
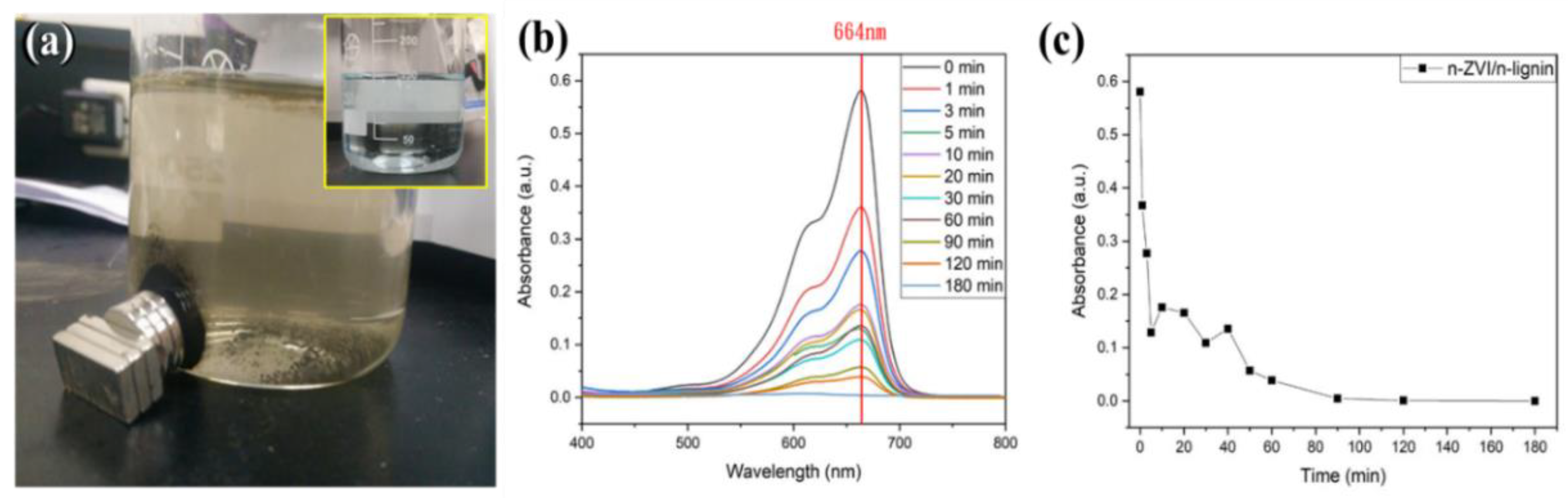
3.2. Dispersibility and Stability against Oxidation of nZVI/n-Lignin
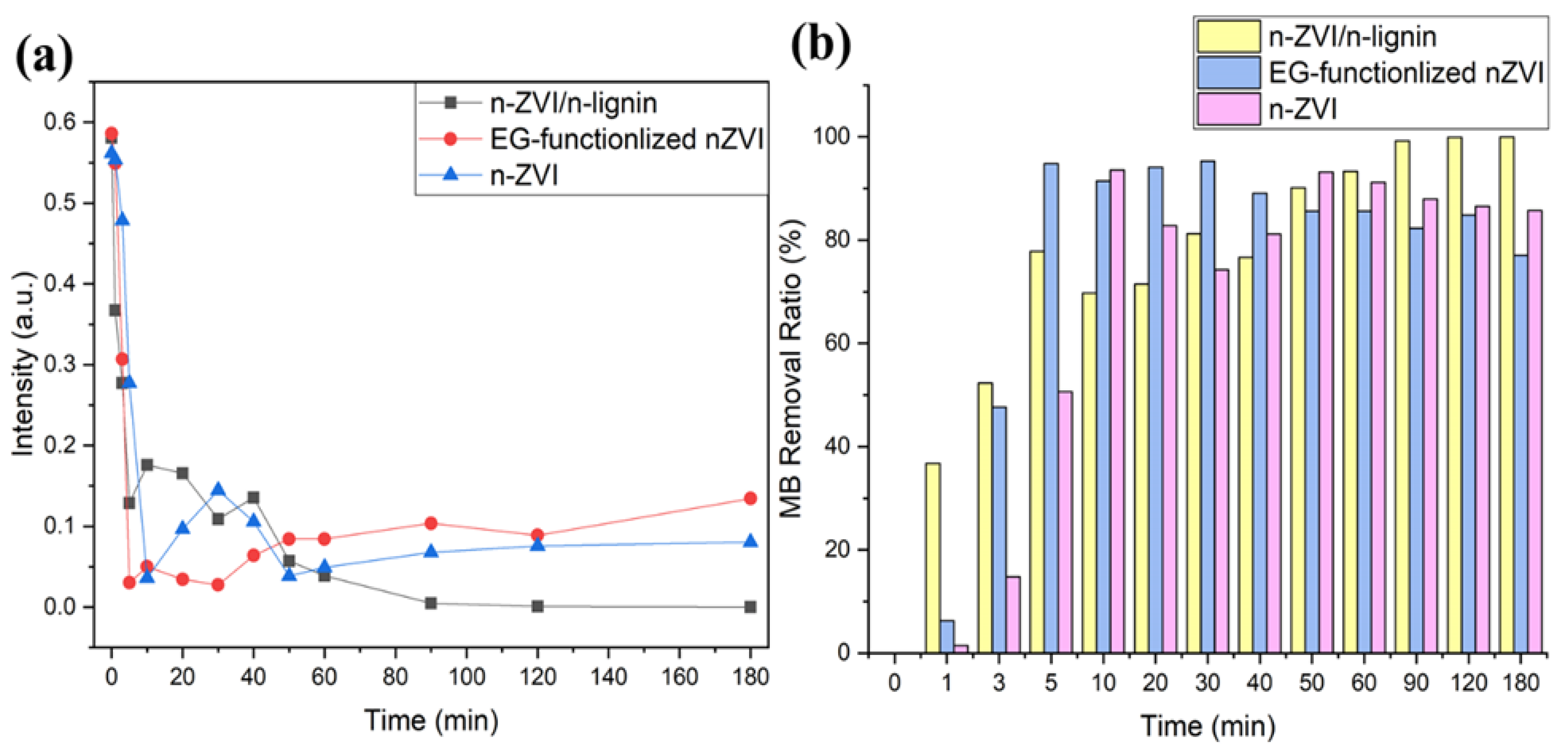
3.3. Removal of Methylene Blue in Aqueous Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nanozerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis and their biological effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Lien, H.L.; Koel, B.E.; Zhang, W. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, C.A.; Araujo-Martinez, R.F.; Martinez-Castanon, G.A.; Morales-Sanchez, J.E.; Guajardo-Pacheco, J.M.; Gonzalez-Hernandez, J.; Lee, T.-J.; Shin, H.-S.; Hwang, Y.; Ruiz, F. Preparation of air stable nanoscale zero valent iron functionalized by ethylene glycol without inert condition. Chem. Eng. J. 2018, 336, 112–122. [Google Scholar] [CrossRef]
- Fan, T.; Li, Y.; Zhang, H. Surfactant-free solvothermal synthesis of 3D flowerlike iron alkoxide (Fe-EG) micro/nanostructures: Structure, formation mechanism, and fenton oxidation of azo dyes. Ind. Eng. Chem. Res. 2017, 56, 11684–11696. [Google Scholar] [CrossRef]
- Xiang, S.; Cheng, W.; Chi, F.; Nie, X.; Hayat, T.; Alharbi, N.S. Photocatalytic removal of U(VI) from wastewater via synergistic carbon-supported zero-valent iron nanoparticles and S. Putrefaciens. ACS Appl. Nano Mater. 2020, 3, 1131–1138. [Google Scholar] [CrossRef]
- Gozdecka, A.; Wiacek, A.E. Effect of UV radiation and chitosan coating on the adsorption-photocatalytic activity of TiO2 particles. Mater. Sci. Eng. C 2018, 93, 582–594. [Google Scholar] [CrossRef]
- Jungcharoen, P.; O’Carroll, D.; Anotai, J.; Phenrat, T. Synthesis of Lignin-modified nanoscale zerovalent iron applied to arsenic removal. In Proceedings of the Full Paper Proceeding of International Conference on Engineering and Technology, Computer, Basics and Applied Sciences, ECBA-2017, Marrakesh, Morocco, 7–8 January 2017; Volume 3, pp. 1–6. [Google Scholar]
- Wang, Z.; Chen, G.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater. J. Clean. Prod. 2020, 267, 122009. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elagovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One-pot synthesis of lignin-stabilized platinum and palladium nanoparticles and their catalytic behavior in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; d’Alessandro, N.; D’ambrosio, P.; Bressan, M. Palladium nanoparticles, stabilized by lignin, as catalyst for cross-coupling reactions in water. Inorg. Chim. Acta 2013, 399, 12–18. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Raghavendra, P.K. Hydrogenation of carbonyl compounds to alcohols catalyzed by lignin supported palladium nanoparticles. Mater. Today Proc. 2019, 9, 295–305. [Google Scholar] [CrossRef]
- Rak, M.J.; Friscic, T.; Moores, A. Mechanochemical synthesis of Au, Pd, Ru, and Re nanoparticles with lignin as a bio-based reducing agent and stabilizing matrix. Faraday Discuss. 2014, 170, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Wang, X.; Hamel, J.; Zhu, H.; Sun, R. Lignin-AuNPs liquid marble for remotely-controllable detection of Pb2+. Sci. Rep. 2016, 6, 38164. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.-K.; Kadam, A.; Kumar, G.; Jeon, B.-H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Kwon, G.-J.; Han, S.-Y.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, N.H.; Alle, M.; Bandi, R.; Lee, S.H. Adsorption characteristic of Ag nanoparticles on cellulos nanofibers with different chemical compositions. Polymers 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvanimoghaddam, K.; Czech, B.; Wiacek, A.E.; Bundyra, W.C.; Naebe, M. Sustainable carbon microtube derived from cotton waste for environmental applications. Chem. Eng. J. 2019, 361, 1605–1616. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129. [Google Scholar] [CrossRef]
- Mu, L.; Shi, Y.; Wang, H.; Zhu, J. Lignin in ethylene glycol and poly(ethylene glycol): Fortified lubricants with internal hydrogen bonding. ACS Sustain. Chem. Eng. 2016, 4, 1840–1849. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, W.; Singh, S.; Simmons, B.; Cheng, G. On the solution structure of kraft lignin in ethylene glycol and its implication for nanoparticle preparation. Nanoscale Adv. 2019, 1, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid isolated high yield lignin nanoparticles as innovative antioxidant/antimicrobial organic materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Li, X.-Q.; Cao, J.; Zhang, W.-X.; Wang, H.P. Characterization of Zero-valent Iron Nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Desalegn, B.; Megharaj, M.; Chen, Z.; Naidu, R. Green synthesis of zero valent iron nanoparticle using mange peel extract and surface characterization using XPS and GC-MS. Heliyon 2019, 5, e01750. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Liu, J.; Han, J.; Zhang, W.-X. Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structure iron oxides. J. Hazard. Mater. 2017, 322, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Pullin, H.; Springell, R.; Parry, S.; Scott, T. The effect of aqueous corrosion on the structure and reactivity of zero-valent iron nanoparticles. Chem. Eng. J. 2017, 308, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci. Technol. 2018, 78, 367. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; Tu, X.; Chen, L.; Liu, X.; Tan, L.; Mao, Y.; Shi, J.; Teng, X.; He, S.; et al. High-yield production of lignin-drived functional carbon nanosheet for dye adsorption. Polymers 2020, 12, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goliszek, M.; Wiacek, A.E.; Wawrzkiewicz, M.; Sevastyanova, O. The impact of lignin addition on the properties of hybrid microspheres based on trimethoxyvinylsiane and divinylbenzene. Eur. Polym. J. 2019, 361, 1605–1616. [Google Scholar] [CrossRef]
- Mao, Z.; Wu, Q.; Wang, M.; Yang, Y.; Long, J.; Chen, X. Tunable synthesis of SiO2-encapsulated zero-valent iron nanoparticles for degradation of organic dyes. Nanoscale Res. Lett. 2014, 9, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.-Y.; Wang, P.-W.; Liao, W.; Yu, I.-S. Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue. Polymers 2021, 13, 3847. https://doi.org/10.3390/polym13213847
Peng F-Y, Wang P-W, Liao W, Yu I-S. Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue. Polymers. 2021; 13(21):3847. https://doi.org/10.3390/polym13213847
Chicago/Turabian StylePeng, Fang-Yi, Pei-Wen Wang, Weisheng Liao, and Ing-Song Yu. 2021. "Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue" Polymers 13, no. 21: 3847. https://doi.org/10.3390/polym13213847
APA StylePeng, F.-Y., Wang, P.-W., Liao, W., & Yu, I.-S. (2021). Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue. Polymers, 13(21), 3847. https://doi.org/10.3390/polym13213847





