Rhenium Nanostructures Loaded into Amino-Functionalized Resin as a Nanocomposite Catalyst for Hydrogenation of 4-Nitrophenol and 4-Nitroaniline
Abstract
:1. Introduction
2. Experimental
2.1. Materials, Methods of Analyses, and Instrumentation
2.2. Synthesis of Heterogenous NCat
2.3. Catalytic Activity
3. Results and Discussion
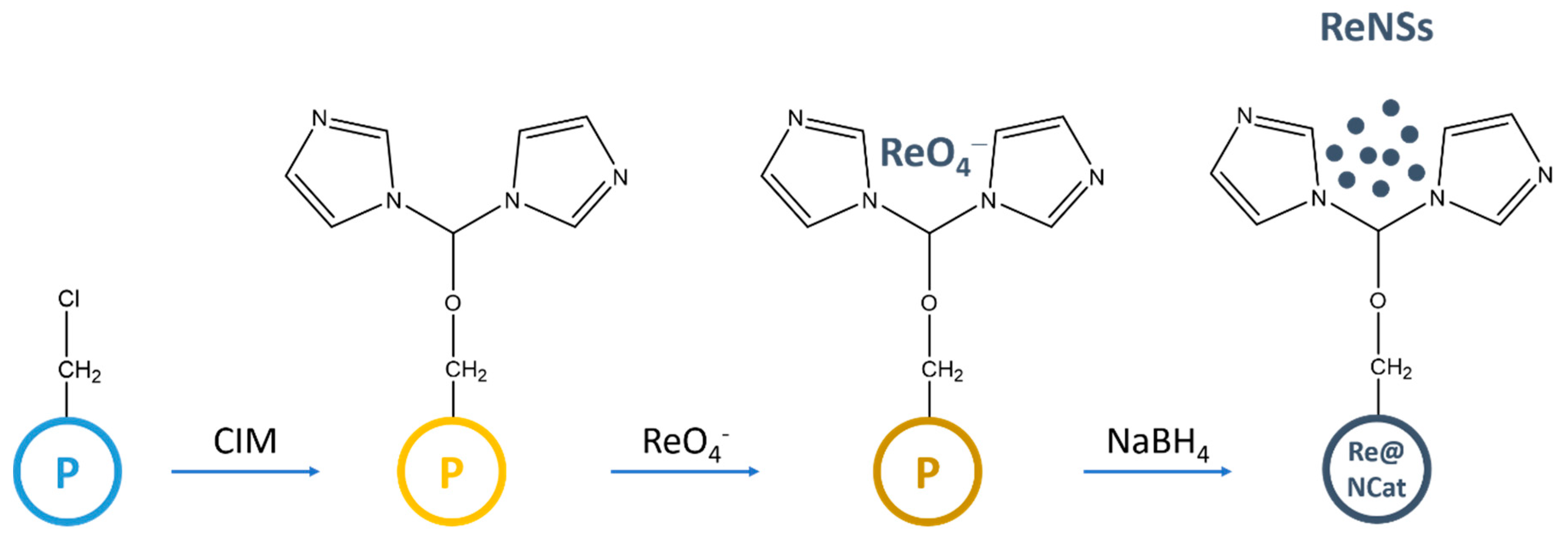
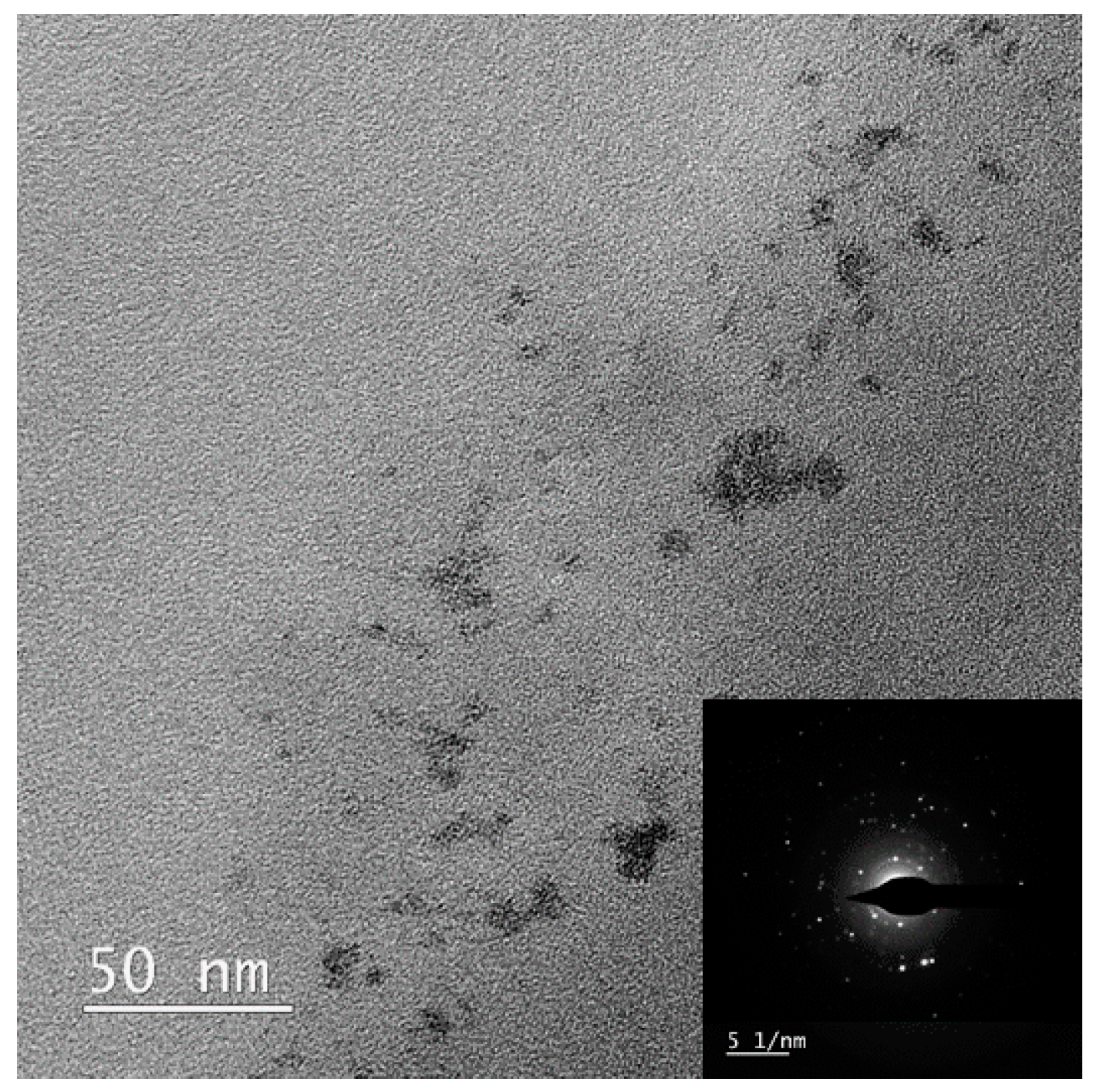
3.1. Synthesis of the Nanocomposite with ReNSs
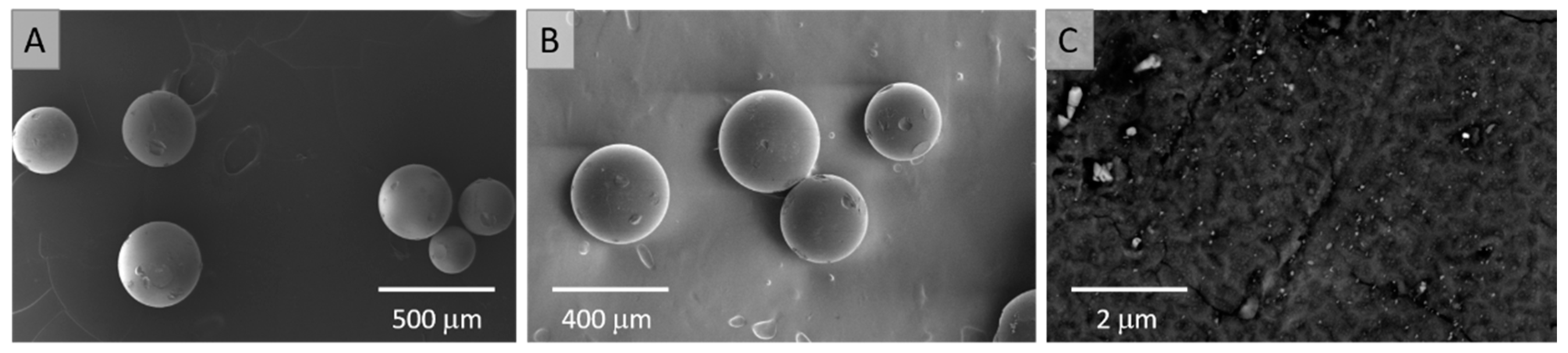
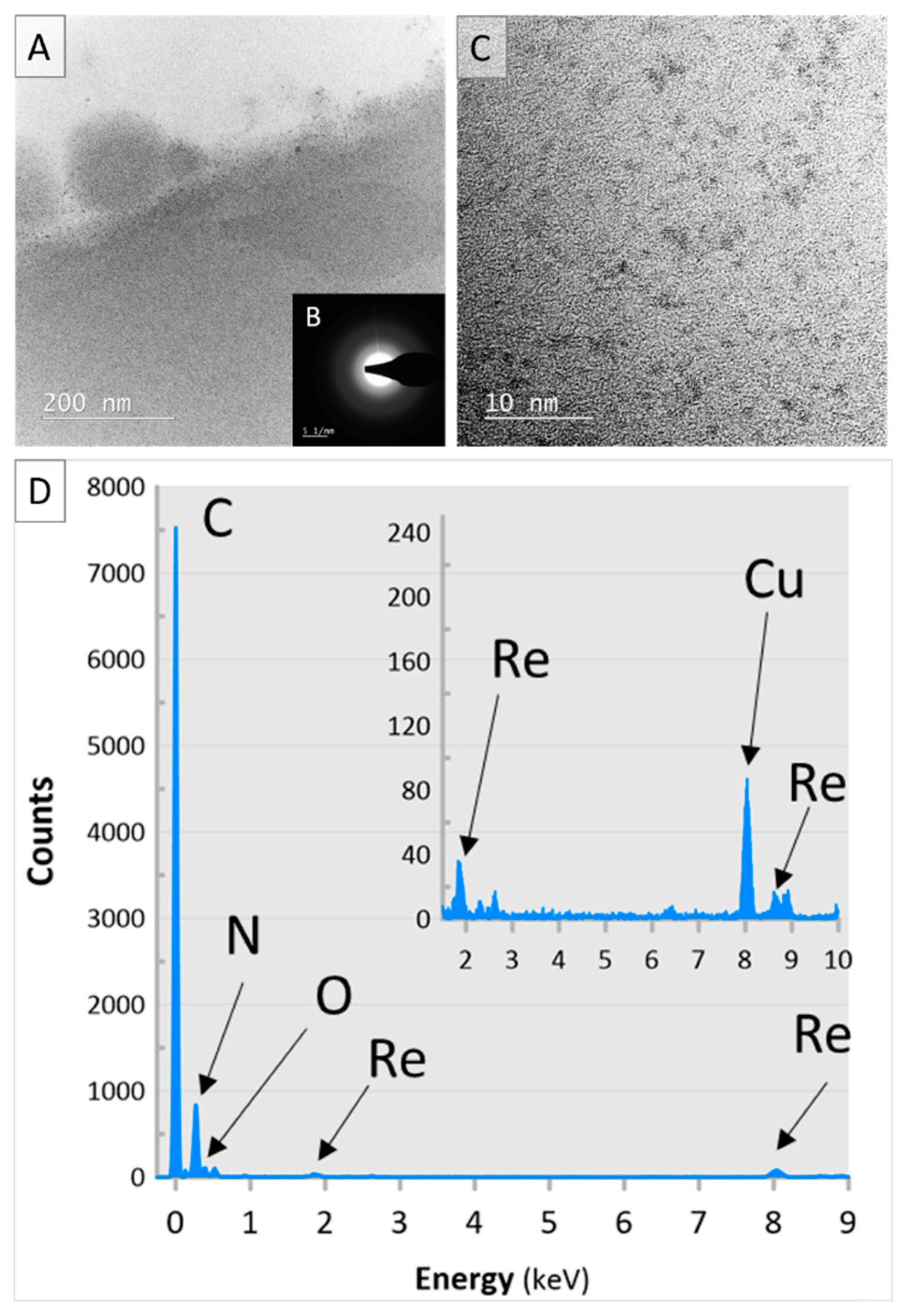
3.2. Loading the Anion Exchange Resin with the ReNSs
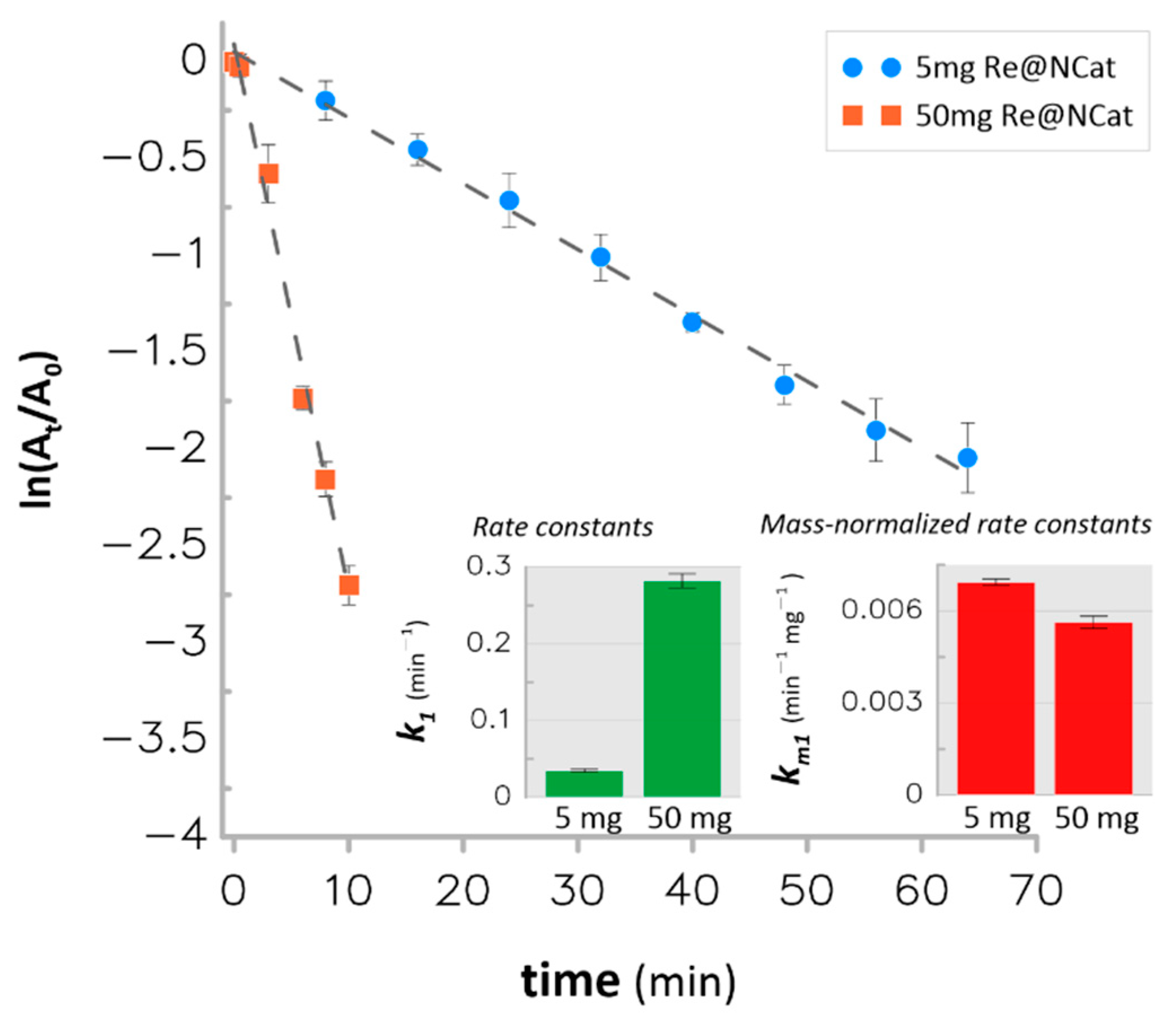
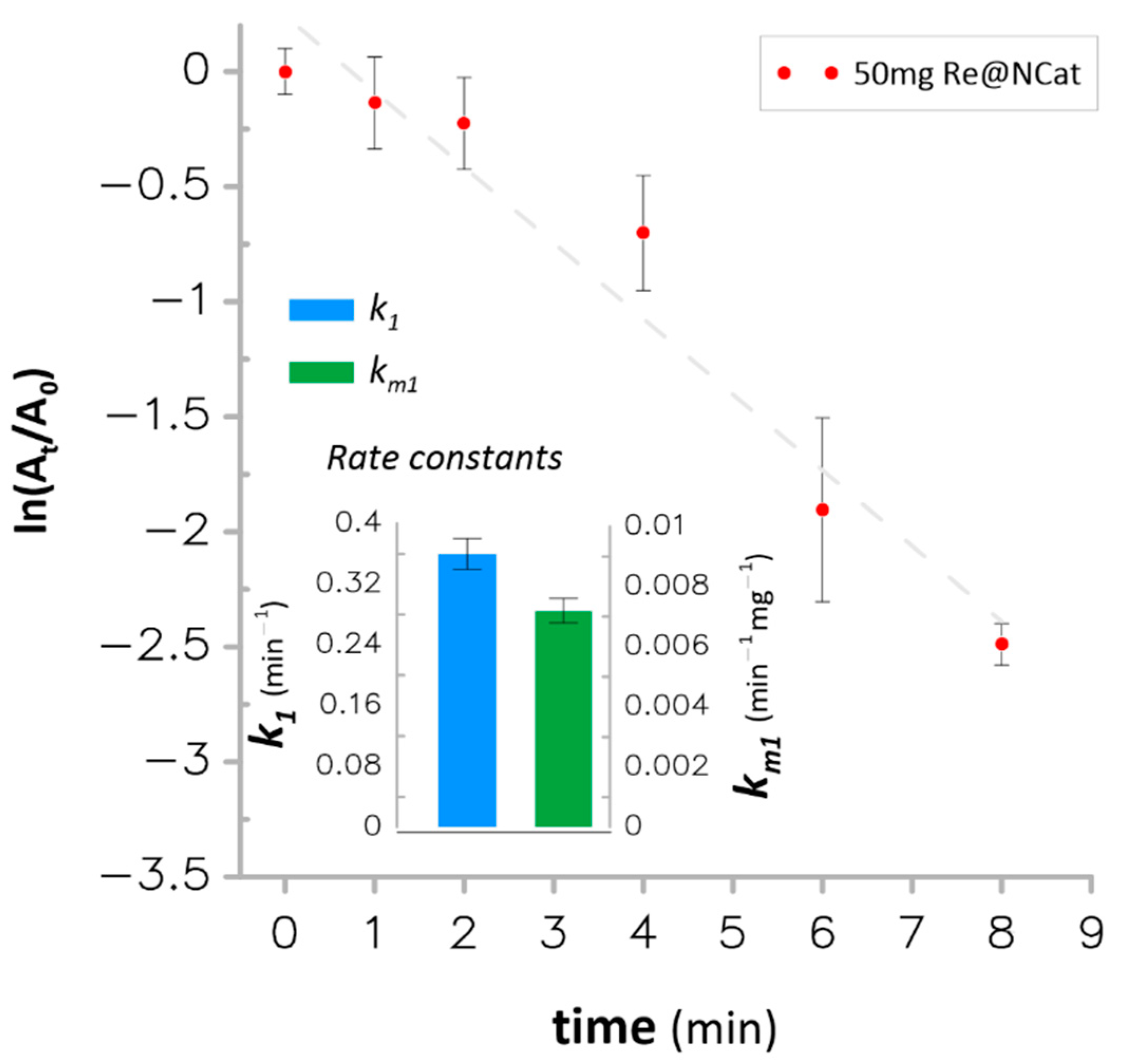
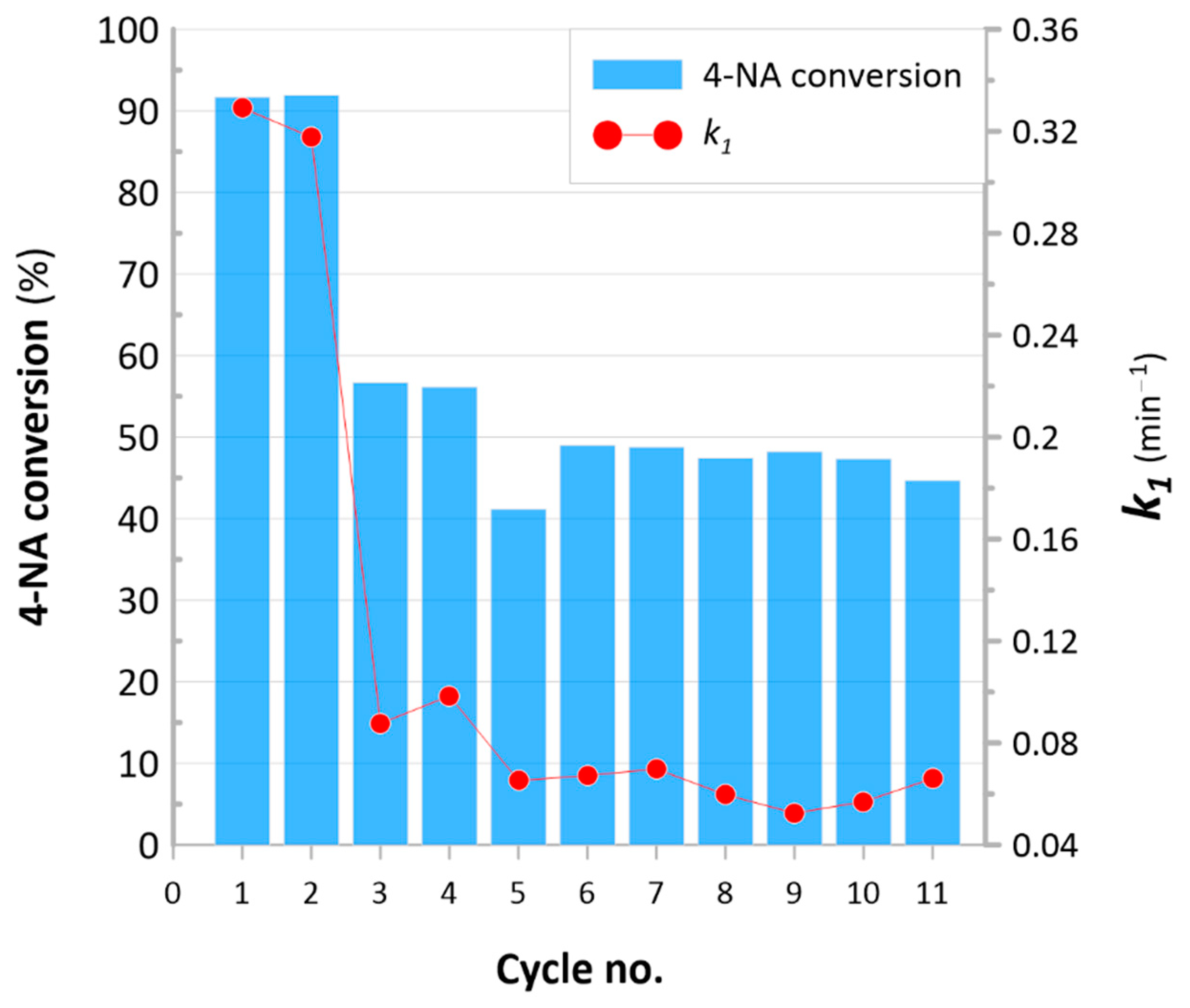
3.3. Catalytic Hydrogenation of Nitroaromatics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benbrahim-Tallaa, L.; Baan, R.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, B.F.; Guha, V.; Loomis, N.; Straif, D. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012, 13, 663–664. [Google Scholar] [CrossRef] [Green Version]
- WHO Elaboration on Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 26 August 2020).
- Wei, H.; Liu, X.; Wang, A.; Zhang, L.; Qiao, B.; Yang, X.; Huang, Y.; Miao, S.; Liu, J.; Zhang, T. FeO x-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Indolese, A.; Schnyder, A. Applied homogeneous catalysis by organometallic complexes. Curr. Sci. 2000, 78, 1336–1344. [Google Scholar]
- Sheldon, R.A.; Van Bekkum, H. Fine Chemicals through Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold-and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Abad, A.; Concepción, P.; Corma, A.; García, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066–4069. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.T.; Lai, D.Y. Aromatic amino and nitro–amino compounds and their halogenated derivatives. In Patty’s Toxicoligy; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 1–96. [Google Scholar]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L.A. Polyvinylpyridine-supported palladium nanoparticles: A valuable catalyst for the synthesis of alkynyl ketones via acyl sonogashira reactions. Catal. Lett. 2020, 150, 652–659. [Google Scholar] [CrossRef]
- Albano, G.; Evangelisti, C.; Aronica, L.A. Hydrogenolysis of benzyl protected phenols and aniline promoted by supported palladium nanoparticles. ChemistrySelect 2017, 2, 384–388. [Google Scholar] [CrossRef]
- Hedayati, K.; Goodarzi, M.; Ghanbari, D. Hydrothermal synthesis of Fe3O4 nanoparticles and flame resistance magnetic poly styrene nanocomposite. J. Nanostruct. 2017, 7, 32–39. [Google Scholar]
- Cyganowski, P. Fully recyclable gold-based nanocomposite catalysts with enhanced reusability for catalytic hydrogenation of p-nitrophenol. Colloids Surf. A 2021, 612, 125995. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Leśniewicz, A.; Pohl, P.; Dzimitrowicz, A. Highly efficient and convenient nanocomposite catalysts produced using in-situ approach for decomposition of 4-nitrophenol. Colloids Surf. A 2020, 590, 124452. [Google Scholar] [CrossRef]
- Zacharaki, E.; Bremmer, G.M.; Vajeeston, P.; Kalyva, M.; Fjellvåg, H.; Kooyman, P.J.; Sjåstad, A.O. One-pot synthesis of cobalt–rhenium nanoparticles taking the unusual β-Mn type structure. Nanoscale Adv. 2020, 2, 1850–1853. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.Y.; Chow, W.Y.; Fan, W.Y. Preparation of rhenium nanoparticles via pulsed-laser decomposition and catalytic studies. J. Colloid Interface Sci. 2012, 369, 164–169. [Google Scholar] [CrossRef]
- Vargas-Uscategui, A.; Mosquera, E.; Cifuentes, L. Analysis of the electrodeposition process of rhenium and rhenium oxides in alkaline aqueous electrolyte. Electrochim. Acta 2013, 109, 283–290. [Google Scholar] [CrossRef]
- Rojas, J.; Castano, C.H. Synthesis of rhenium oxide nanoparticles (RexOy) by gamma irradiation. Radiat. Phys. Chem. 2014, 99, 1–5. [Google Scholar] [CrossRef]
- Tong, Y.; Bai, S.; Zhang, H.; Ye, Y. Rhenium coating prepared on carbon substrate by chemical vapor deposition. Appl. Surf. Sci. 2012, 261, 390–395. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G.A. Rhenium nanochemistry for catalyst preparation. Minerals 2012, 2, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Kirilin, A.V.; Tokarev, A.V.; Manyar, H.; Hardacre, C.; Salmi, T.; Mikkola, J.-P.; Murzin, D.Y. Aqueous phase reforming of xylitol over Pt-Re bimetallic catalyst: Effect of the Re addition. Catal. Today 2014, 223, 97–107. [Google Scholar] [CrossRef]
- Hurley, K.D.; Zhang, Y.; Shapley, J.R. Ligand-enhanced reduction of perchlorate in water with heterogeneous Re− Pd/C catalysts. J. Am. Chem. Soc. 2009, 131, 14172–14173. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ma, L.; Dai, W.; Chen, Y.; Sinyukov, A.M.; Liang, H. Polymer encapsulated self-assemblies of ultrasmall rhenium nanoparticles: Catalysis and SERS applications. ACS Sustain. Chem. Eng. 2017, 5, 10186–10198. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lin, K.-C.; Liu, S.-B. Well-dispersed rhenium nanoparticles on three-dimensional carbon nanostructures: Efficient catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2017, 506, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Cyganowski, P.; Cierlik, A.; Leśniewicz, A.; Pohl, P.; Jermakowicz-Bartkowiak, D. Separation of Re (VII) from Mo (VI) by anion exchange resins synthesized using microwave heat. Hydrometallurgy 2019, 185, 12–22. [Google Scholar] [CrossRef]
- Long, D.A. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons, Ltd.: Chichester, UK, 2004; Volume 35. [Google Scholar]
- Cyganowski, P.; Lesniewicz, A.; Dzimitrowicz, A.; Wolska, J.; Pohl, P.; Jermakowicz-Bartkowiak, D. Molecular reactors for synthesis of polymeric nanocomposites with noble metal nanoparticles for catalytic decomposition of 4-nitrophenol. J. Colloid Interface Sci. 2019, 541, 226–233. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.-H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawby, R.J.; Morris, D.; Thorsteinson, E.M.; Basolo, F. Chelate and Bridge Complexes of Metal Carbonyl Compounds with a Ditertiary Phosphine: A Study of Their Formation and Interconversion. Inorg. Chem. 1966, 5, 27–33. [Google Scholar] [CrossRef]
- Bartulín, J.; Zunza, H.; Parra, M.L.; Rivas, B.L. Chelating polymers. Polym. Bull. 1986, 16, 293–298. [Google Scholar] [CrossRef]
- Wojaczynska, M.; Kolarz, B.N. Structure of some styrene–divinylbenzene copolymers. J. Appl. Polym. Sci. 1995, 56, 433–439. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D. Piperazine functionalized resins for Au(III), Pt(IV), and Pd(II) sorption. Sep. Sci. Technol. 2014, 49, 1689–1699. [Google Scholar] [CrossRef]
- Sakkas, P.M.; Argirusi, M.; Sourkouni, G.; Argirusis, C. Rhenium oxide nanoparticles–Sonochemical synthesis and integration on anode powders for solid oxide fuel cells. Ultrason. Sonochem. 2020, 69, 105250. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, G.; Li, B.; Zhao, X.; Ji, T.; Song, G.; Xiao, Z.; Cao, Q.; Xiao, J.; Huang, X. Degradable rhenium trioxide nanocubes with high localized surface plasmon resonance absorbance like gold for photothermal theranostics. Biomaterials 2018, 159, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, E.; Gruzeł, G.; Pajor-Świerzy, A.; Depciuch, J.; Socha, R.; Kowal, A.; Warszyński, P.; Parlinska-Wojtan, M. Design and assembly of ternary Pt/Re/SnO 2 NPs by controlling the zeta potential of individual Pt, Re, and SnO 2 NPs. J. Nanopart. Res. 2018, 20, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, A.K.; Pramanik, H. Addition of rhenium (Re) to Pt-Ru/f-MWCNT anode electrocatalysts for enhancement of ethanol electrooxidation in half cell and single direct ethanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 13300–13321. [Google Scholar] [CrossRef]
- Al-Dulaimi, N.; Lewis, D.J.; Zhong, X.L.; Malik, M.A.; O’Brien, P. Chemical vapour deposition of rhenium disulfide and rhenium-doped molybdenum disulfide thin films using single-source precursors. J. Mater. Chem. C 2016, 4, 2312–2318. [Google Scholar] [CrossRef] [Green Version]
- Chairam, S.; Konkamdee, W.; Parakhun, R. Starch-supported gold nanoparticles and their use in 4-nitrophenol reduction. J. Saudi Chem. Soc. 2017, 21, 656–663. [Google Scholar] [CrossRef]
- Islam, M.T.; Saenz-Arana, R.; Wang, H.; Bernal, R.; Noveron, J.C. Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4-nitrophenol and methyl orange. New J. Chem. 2018, 42, 6472–6478. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4-nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Najeeb, J.; Sahrif, A.; Intisar, A.; Ahmed, E. Critical review on the chemical reduction of nitroaniline. RSC Adv. 2020, 10, 19041–19058. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Zhang, C.; Cheng, M.; Yi, H.; Liu, X.; Zhou, C.; Xiong, W. Synthetic strategies and application of gold-based nanocatalysts for nitroaromatics reduction. Sci. Total Environ. 2019, 652, 93–116. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mishra, K.; Lee, Y.R. Trimetallic FeAgPt alloy as a nanocatalyst for the reduction of 4-nitroaniline and decolorization of rhodamine B: A comparative study. J. Alloy. Compd. 2017, 701, 456–464. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khalid, R.; Begum, R.; Farooq, U.; Wu, Q.; Wu, W.; Ajmal, M.; Irfan, A.; Naseem, K. Facile synthesis of silver nanoparticles in a crosslinked polymeric system by in situ reduction method for catalytic reduction of 4-nitroaniline. Environ. Technol. 2019, 40, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, J.; Jin, X.; Li, P.; Jiang, X.; Yuan, J. Polyurethane/Keratin/AgNPs nanofibrous mats as catalyst support for 4-nitroaniline reduction. Mater. Lett. 2019, 237, 9–13. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14, 715. [Google Scholar] [CrossRef]
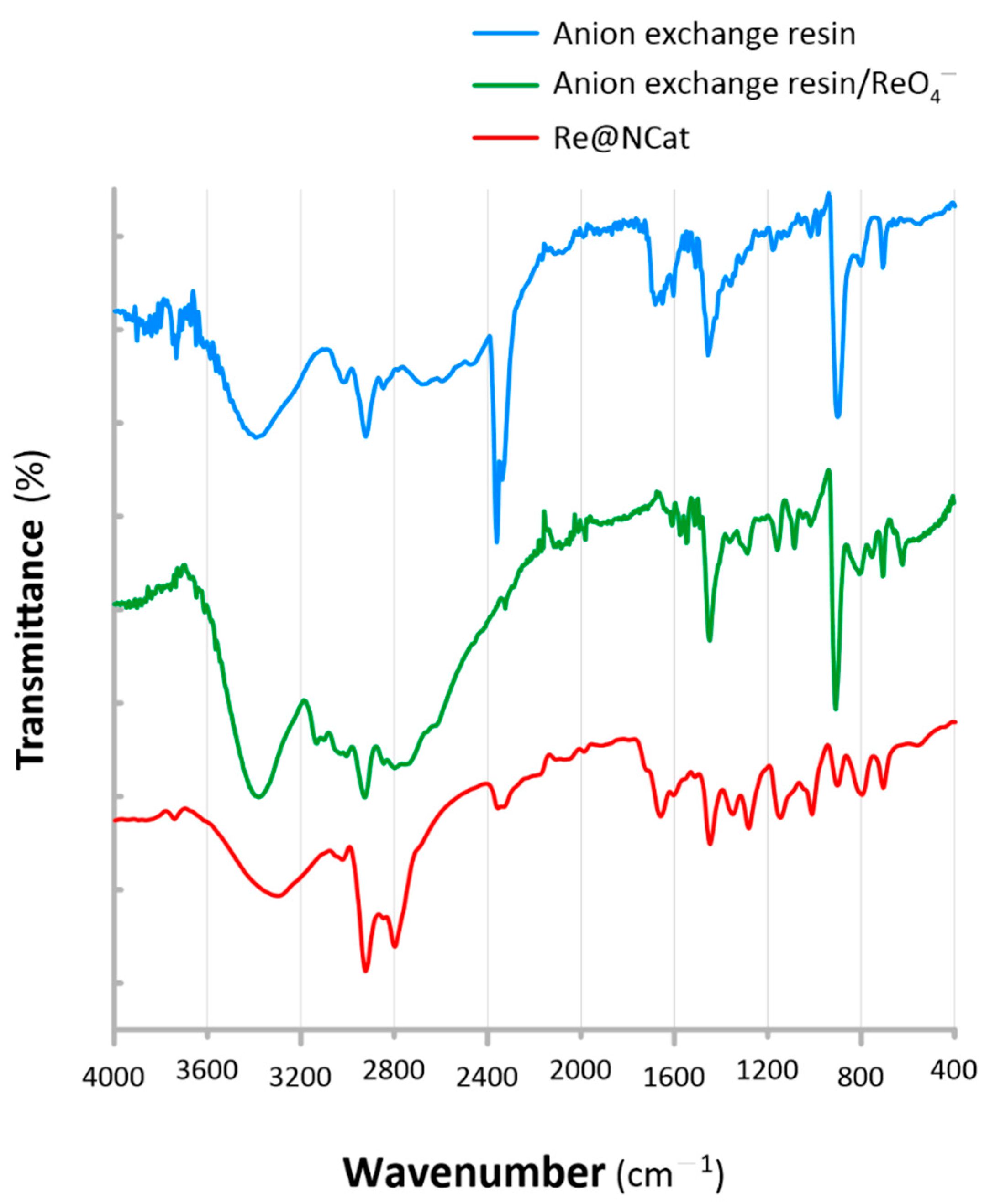
| Interactions | Characteristic Band Location/Shift (cm−1) | ||||
|---|---|---|---|---|---|
| A-X Resin | A-X Resin + ReO4− | Re@NCat | |||
| N–H stretching | 3394 | 3369 |  | 3297 | |
| C=N aromatic deformation | 1540 |  | 1544 |  | 1546 |
| R–N–CO–N–R stretching | 1648 |  | 1649 |  | 1658 |
| N–C–N stretching | 1357 |  | 1359 |  | 1349 |
| N+–H aromatic stretching | 2472 |  | 2454 |  | - |
| H–bridge | - |  | 2794 |  | 2796 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyganowski, P.; Dzimitrowicz, A.; Jamroz, P.; Jermakowicz-Bartkowiak, D.; Pohl, P. Rhenium Nanostructures Loaded into Amino-Functionalized Resin as a Nanocomposite Catalyst for Hydrogenation of 4-Nitrophenol and 4-Nitroaniline. Polymers 2021, 13, 3796. https://doi.org/10.3390/polym13213796
Cyganowski P, Dzimitrowicz A, Jamroz P, Jermakowicz-Bartkowiak D, Pohl P. Rhenium Nanostructures Loaded into Amino-Functionalized Resin as a Nanocomposite Catalyst for Hydrogenation of 4-Nitrophenol and 4-Nitroaniline. Polymers. 2021; 13(21):3796. https://doi.org/10.3390/polym13213796
Chicago/Turabian StyleCyganowski, Piotr, Anna Dzimitrowicz, Piotr Jamroz, Dorota Jermakowicz-Bartkowiak, and Pawel Pohl. 2021. "Rhenium Nanostructures Loaded into Amino-Functionalized Resin as a Nanocomposite Catalyst for Hydrogenation of 4-Nitrophenol and 4-Nitroaniline" Polymers 13, no. 21: 3796. https://doi.org/10.3390/polym13213796
APA StyleCyganowski, P., Dzimitrowicz, A., Jamroz, P., Jermakowicz-Bartkowiak, D., & Pohl, P. (2021). Rhenium Nanostructures Loaded into Amino-Functionalized Resin as a Nanocomposite Catalyst for Hydrogenation of 4-Nitrophenol and 4-Nitroaniline. Polymers, 13(21), 3796. https://doi.org/10.3390/polym13213796











