Heterometal Grafted Metalla-ynes and Poly(metalla-ynes): A Review on Structure–Property Relationships and Applications
Abstract
1. Introduction
2. Impact on Properties
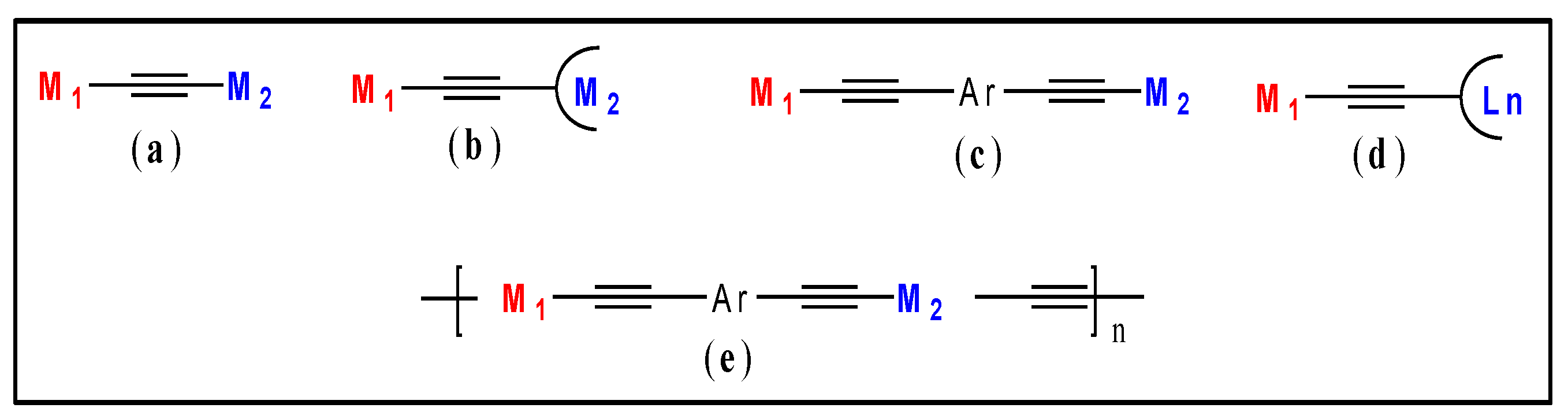
2.1. Main-Chain Systems
2.2. Side-Chain Systems
2.2.1. d-d Metal-Containing Systems
| Code | Metals | λabs(nm) | λems(nm) | Lifetime of S1(τa, µs) | Lifetime of T1(τb, µs) | Φ (%) | Ref. |
|---|---|---|---|---|---|---|---|
| O1 (R = Me) | Pd/Re | 236, 284, 336sh, 416 | 628 a, 589 b | ˂0.1 | 0.73 | - | [50] |
| O1 (R = H) | Pd/Re | 238, 284, 334sh, 426 | 639 a, 594 b | ˂0.1 | 0.62 | - | [50] |
| O2 (dppm) | Pd/Ru | 386 | - | - | - | - | [51] |
| O2 (dppe) | Pd/Ru | 389 | - | - | - | - | [51] |
| O3 | Ru/Cu | 308, 494 | - | - | - | - | [52] |
| O3 | Ru/Mn | 308, 468 | - | - | - | - | [52] |
| O4 | Ru/Cu | 308, 494 | - | - | - | - | [52] |
| O4 | Ru/Mn | 306, 468 | - | - | - | - | [52] |
| O8 | Pt/Re | 271, 382, 427 | 595 | 1.5, 0.22 | - | 0.0018 | [26] |
| O9 | Pt/Ru | 243, 291, 360, 504 | 658 | <0.1 | - | 0.045 | [26] |
| O10 | Pt/Ir | 255, 315, 415, 435 | 560 a, 613 a, 663 a,550 b, 595 b, 651 b | 2.3 | 2.5, 1.9 | 3.3 | [54] |
| Code | Metals | Molecular Weight (× 104) | λabs (nm) | λems (nm) | Lifetime of S1 (τa, ns) | Lifetime of T1, (τb, µs) | Φ (%) | Eg (eV) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mw | Mn | |||||||||
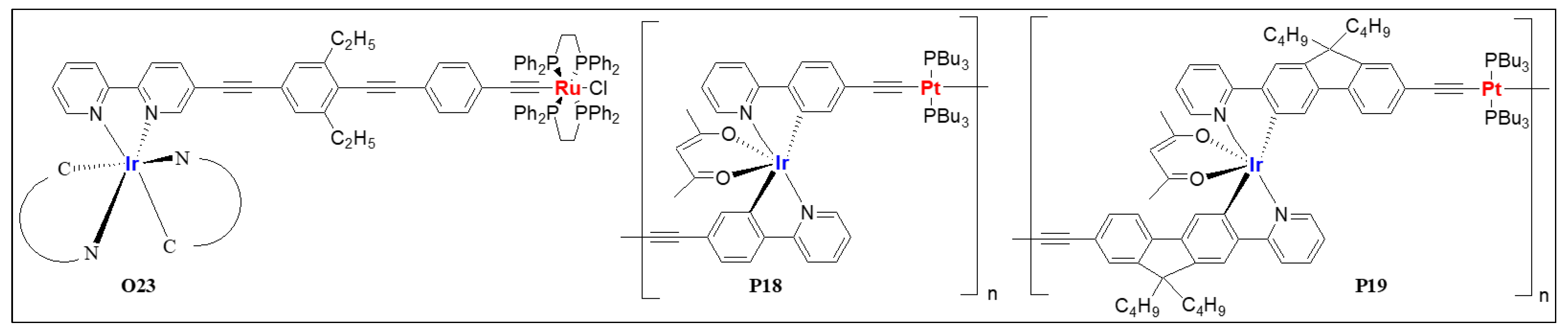
| P12 | Pt/Ir | 1.33 | 1.18 | 250, 280, 340, 435 | 617 a, 563 b | 1220 | 5.67 | 2.60 | - | [55] |
| P13 | Pt | 1.22 | 2.48 | 253, 270, 295, 390 | 561 a, 562 b | 9200 | 70 | 12.80 | - | [55] |
| P14a | Pt/Ir | - | - | 250, 260, 315, 415, 485 | 628 a, 547 b, 592 b, 640 b | 800 | 4.81 | 1.0 | - | [56] |
| P14b | Pt/Ir | - | - | 250, 280, 340, 435 | 555 a, 617 a, 655 a, 558 b, 596 b, 654 b | 2500, 1900 | 5.7, 3.3 | 2.6 | - | [56] |
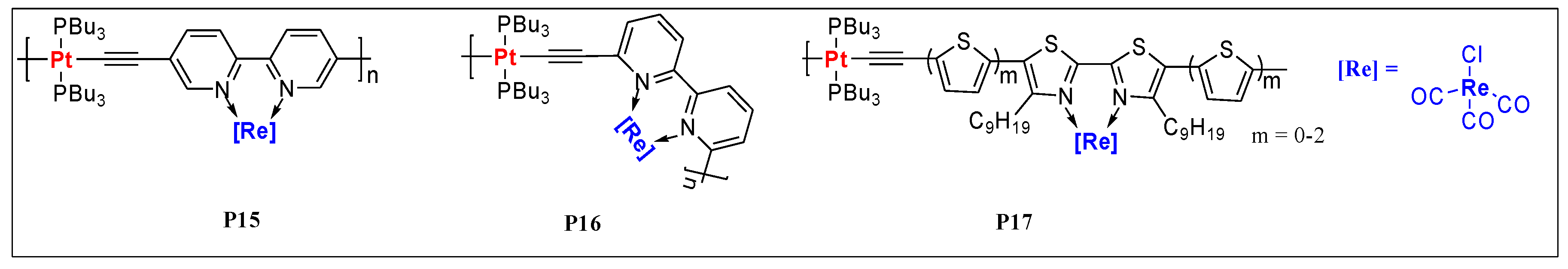
| P15 | Pt/Re | 6.1 | 7.7 | 343, 419, 448 | - | - | - | - | - | [33] |
| P16 | Pt/Re | 5.5 | 8.3 | 276, 300, 324, 388, 402 | - | - | - | - | - | [33] |
| P17 (m = 0) | Pt/Re | 1.3 | 3.8 | 230, 306, 539 | 582 a | 0.22 | - | 0.05 | 2.18 | [58] |
| P17 (m = 1) | Pt/Re | 1.0 | 1.9 | 229, 362, 548 | 659 a | 0.44 | - | 0.19 | 1.95 | [58] |
| P17 (m = 2) | Pt/Re | 0.9 | 1.9 | 229, 400, 552 | 729 a | 0.34 | - | 0.10 | 1.85 | [58] |
| P18 | Pt/Ir | 1.3 | - | 229, 260, 287, 379, 400, 446, 465, 487 | 549 a, 588 a, 552 b, 599 b | 110 | 10.82 | 12.3 | 2.44 | [49] |
| P19 | Pt/Ir | 1.1 × 104 | - | 229, 251, 283, 383, 400, 474, 502 | 577 a, 625 a 577 b, 631 b | 510 | 12.12 | 4.80 | 2.39 | [49] |
2.2.2. d-f Metal-Containing Systems
| Code | Metals | λabs (nm) | λems (nm) | Lifetime of S1 (τa, µs) | Φ (%) | Ref. |
|---|---|---|---|---|---|---|
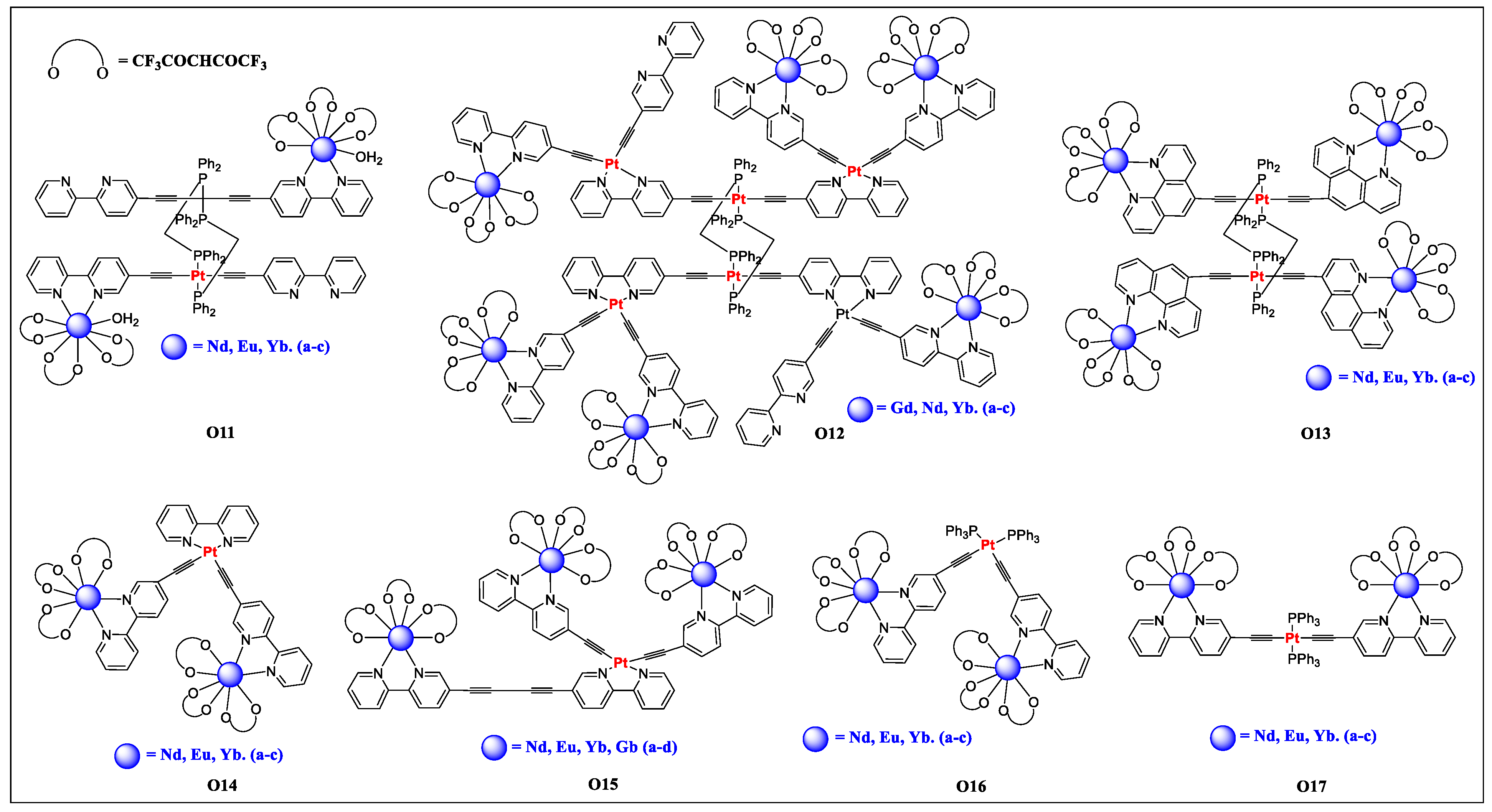
| O11a | Pt/Nd | - | 1060a | - | 0.84 | [28] |
| O11b | Pt/Eu | - | 615 a | 250.60 | 24 | [28] |
| O11c | Pt/Yb | - | 980 | 12.20 | 5.65 | [28] |
| O12a | Pt/Gd | - | 563 | 0.84 | 0.07 | [71] |
| O12b | Pt/Nd | - | 1060 | weak | - | [71] |
| O12c | Pt/Yb | - | 980 | 12.10 | 0.061 | [71] |
| O13a | Pt/Nd | - | 1060 | - | 0.2 | [28] |
| O13b | Pt/Eu | - | 615 | 33.60 | 0.5 | [28] |
| O13c | Pt/Yb | - | 980 | 12.90 | 0.635 | [28] |
| O14a | Pt/Nd | 304, 320, 363, 401 | 1061 | - | - | [72] |
| O14b | Pt/Eu | 301, 323, 365, 400 | 613 | 105 | 5.4 | [72] |
| O14c | Pt/Yb | 295, 322, 365, 400 | 980 | 11.8 | 6.3 | [72] |
| O15a | Pt/Nd | 304, 329, 358, 381, 430 | 1060 | - | - | [72] |
| O15b | Pt/Eu | 299, 329, 357, 382, 430 | 613 | 164 | 1.7 | [72] |
| O15c | Pt/Yb | 294, 328, 357, 382, 430 | 980 | 10.9 | 0.54 | [72] |
| O15d | Pt/Gd | 303, 329, 358, 383, 430 | 572 | 0.40 | 2.4 | [72] |
| O16a | Pt/Nd | 228, 297, 371 | 423, 1061 | <0.01 | - | [73] |
| O16b | Pt/Eu | 228, 303, 375 | 420, 613 | <0.01, 109.5 | 1.4 | [73] |
| O16c | Pt/Yb | 228, 297, 371 | 428, 980 | <0.01, 12.6 | 0.63 | [73] |
| O17a | Pt/Nd | 229, 305, 372 | 421, 1060 | <0.01 | - | [73] |
| O17b | Pt/Eu | 230, 303, 375 | 415, 613 | <0.01, 216.3 | 1.0 | [73] |
| O17c | Pt/Yb | 228, 293, 384 | 448, 980 | <0.01, 12.7 | 0.64 | [73] |
| O18 | Au/Eu | 271, 339 | 460, 580, 592, 611, 654, 700 | 0.0022, 280.00 | 15 | [74] |
| O19 | Au/Eu | 280, 337, 404 | 525, 611 | 0.0017, 434.00 | 21 | [74] |
| O20 | Au/Eu | 276, 336, 495 | 611, 635, 705 | 0.0041, 404.10 | 16 | [74] |
| O21 | Pt/Eu | 325 | 550–725 | 590 | 54 | [32] |
| O21 | Pt/Eu | 325 | 550–725 | 410 | 29 | [32] |
| O22 | Pt/Eu | 416 | 425–531, 550–725 | 39.52, 519.86 | 39 | [34] |
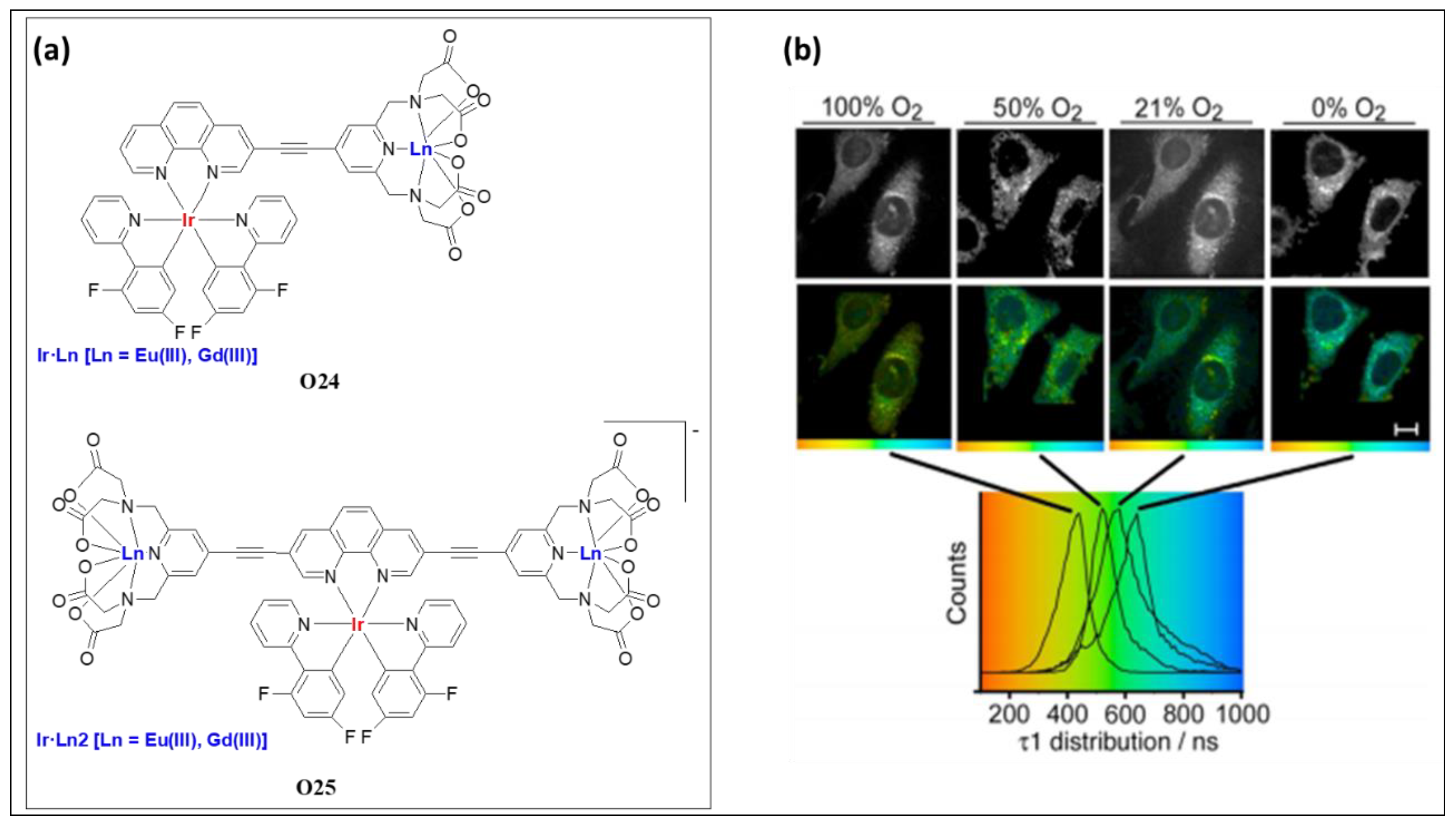
| O24 | Ir/Eu | 242, 283, 343 | 578, 590, 615, 684, 697 | 0.780, 0.116, 0.51, 0.095 | - | [76] |
| O24 | Ir/Gd | 242, 285, 338 | 560 | 1.1, 0.45 0.64, 0.22 | 4.8 | [76] |
| O25 | Ir/Eu | 241, 292, 357 | 616 | 1.24, 0.168 | - | [76] |
| O25 | Ir/Gd | 242, 292, 358 | 595 | 1.26, 0.233 | 2.6 | [76] |
| O26 | Au/Re | 243, 265, 305, 366 | 592 | - | - | [77] |
| O27 | Au/Re | 240, 278, 3.19, 357 | 576 | - | - | [77] |
| O28 | Au/Re | 237, 271, 318, 352 | 607 | - | - | [77] |
| O29 | Au/Re | 368 | 614 | 1060 | 30.4 | [69] |
3. Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| acac | Acetylacetone |
| Bipy | Bipyridine |
| D-A | Donor-acceptor |
| EET | Electronic energy transfer |
| EQE | External quantum efficiency |
| Eg | Energy gap |
| GPC | Gel permission chromatography |
| HOMO | Highest occupied molecule orbital |
| ISC | Inter system crossing |
| Ln(III) | Trivalent lanthanide |
| LUMO | Lowest unoccupied molecular orbital |
| MLCT | Metal to ligand charge transfer |
| Mn | Number average molecular weight |
| Mw | Weight average molecular weight |
| NLO | Non-linear optical |
| NMR | Nuclear magnetic resonance |
| O-E | Optoelectronic |
| OLEDs | Organic light-emitting diodes |
| OPL | Optical power limiting |
| phen | Phenyl |
| PHOLEDs | Phosphorescent organic light-emitting diodes |
| PL | Photo-luminescence |
| PLQY | Photo-luminescence quantum yields |
| RT | Room-temperature |
| SOC | Spin-orbit coupling |
| THF | Tetrahydrofuran |
| UV | Ultraviolet |
| Vis | Visible |
| τ | lifetime |
| ϕ | Quantum yield |
| ε | Absorption coefficient |
| Wavelength of maximum emission | |
| Wavelength of maximum absorption |
References
- Haque, A.; Al-Balushi, R.A.; Al-Busaidi, I.J.; Khan, M.S.; Raithby, P.R. Rise of Conjugated poly-ynes and poly(metalla-ynes): From design through synthesis to structure–property relationships and applications. Chem. Rev. 2018, 118, 8474–8597. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-L.; Yu, Z.-Q.; Wong, W.-Y. Multifunctional polymetallaynes: Properties, functions and applications. Chem. Soc. Rev. 2016, 45, 5264–5295. [Google Scholar] [CrossRef]
- Dong, Q.; Meng, Z.; Ho, C.-L.; Guo, H.; Yang, W.; Manners, I.; Xu, L.; Wong, W.-Y. A molecular approach to magnetic metallic nanostructures from metallopolymer precursors. Chem. Soc. Rev. 2018, 47, 4934–4953. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Al Rasbi, N.K.; Khan, M.S. Phenothiazine-based derivatives for optoelectronic applications: A review. Synth. Met. 2019, 257, 116189. [Google Scholar] [CrossRef]
- Rausch, A.F.; Homeier, H.H.H.; Yersin, H. Organometallic Pt(II) and Ir(III) triplet emitters for oled applications and the role of spin–orbit coupling: A study based on high-resolution optical spectroscopy. In Photophysics of Organometallics; Lees, A.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–235. [Google Scholar]
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH Verlag: Weinheim, Germany; p. 2008.
- Wilson, J.S.; Köhler, A.; Friend, R.H.; Al-Suti, M.K.; Al-Mandhary, M.R.A.; Khan, M.S.; Raithby, P.R. Triplet states in a series of Pt-containing ethynylenes. J. Chem. Phys. 2000, 113, 7627–7634. [Google Scholar] [CrossRef]
- Wilson, J.S.; Chawdhury, N.; Al-Mandhary, M.R.A.; Younus, M.; Khan, M.S.; Raithby, P.R.; Köhler, A.; Friend, R.H. The energy gap law for triplet states in Pt-containing conjugated polymers and monomers. J. Am. Chem. Soc. 2001, 123, 9412–9417. [Google Scholar] [CrossRef] [PubMed]
- Stefko, M.; Tzirakis, M.D.; Breiten, B.; Ebert, M.O.; Dumele, O.; Schweizer, W.B.; Gisselbrecht, J.P.; Boudon, C.; Beels, M.T.; Biaggio, I.; et al. Donor-acceptor (D-A)-substituted polyyne chromophores: Modulation of their optoelectronic properties by varying the length of the acetylene spacer. Chemistry 2013, 19, 12693–12704. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Younus, M.; Al-Mandhary, M.R.A.; Raithby, P.R.; Khan, M.S.; Friend, R.H. Donor-acceptor interactions in organometallic and organic poly-ynes. Syn. Metals 1999, 101, 246–247. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Sekita, M.; Caballero, E.; Luisa Marcos, M.; Salome Rodriguez-Morgade, M.; Guldi, D.M.; Torres, T. Assembling a phthalocyanine and perylenediimide donor-acceptor hybrid through a Platinum(II) diacetylide linker. Chem. -Eur. J. 2013, 19, 14506–14514. [Google Scholar] [CrossRef]
- Shizu, K.; Lee, J.; Tanaka, H.; Nomura, H.; Yasuda, T.; Kaji, H.; Adachi, C. Highly efficient electroluminescence from purely organic donor-acceptor systems. Pure Appl. Chem. 2015, 87, 627–638. [Google Scholar] [CrossRef]
- Cekli, S.; Winkel, R.W.; Schanze, K.S. Effect of Oligomer Length on Photophysical Properties of Platinum Acetylide Donor-Acceptor-Donor Oligomers. J. Phys. Chem. A 2016, 120, 5512–5521. [Google Scholar] [CrossRef]
- Al-Balushi, R.A.; Haque, A.; Jayapal, M.; Al-Suti, M.K.; Husband, J.; Khan, M.S.; Skelton, J.M.; Molloy, K.C.; Raithby, P.R. Impact of the alkyne substitution pattern and metalation on the photoisomerization of azobenzene-based Platinum(II) diynes and polyynes. Inorg. Chem. 2016, 55, 10955–10967. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Husband, J.; Al Rasbi, N.K.; Abou-Zied, O.K.; Al Balushi, R.; Khan, M.S.; Raithby, P.R. Electronic and steric effects of platinum (ii) di-yne and poly-yne substituents on the photo-switching behaviour of stilbene: Experimental and theoretical insights. Dalton Trans. 2021, 50, 2555–2569. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Ho, C.-L. Di-, oligo-and polymetallaynes: Syntheses, photophysics, structures and applications. Coord. Chem. Rev. 2006, 250, 2627–2690. [Google Scholar] [CrossRef]
- Haque, A.; Al-Balushi, R.A.; Khan, M.S. σ-Acetylide complexes for biomedical applications: Features, challenges and future directions. J. Organomet. Chem. 2019, 897, 95–106. [Google Scholar] [CrossRef]
- Al-Rasbi, N.K.; Derossi, S.; Sykes, D.; Faulkner, S.; Ward, M.D. Bimetallic Pt (II)-bipyridyl-diacetylide/Ln (III) tris-diketonate adducts based on a combination of coordinate bonding and hydrogen bonding between the metal fragments: Syntheses, structures and photophysical properties. Polyhedron 2009, 28, 227–232. [Google Scholar] [CrossRef][Green Version]
- Lazarides, T.; Sykes, D.; Faulkner, S.; Barbieri, A.; Ward, M.D. On the mechanism of d–f energy transfer in RuII/LnIII and OsII/LnIII dyads: Dexter-type energy transfer over a distance of 20 Å. Chem. Eur. J. 2008, 14, 9389–9399. [Google Scholar] [CrossRef] [PubMed]
- Judkins, E.C.; Zeller, M.; Ren, T. Synthesis and characterizations of macrocyclic Cr (III) and Co (III) 1-ethynyl naphthalene and 9-ethynyl anthracene complexes: An investigation of structural and spectroscopic properties. Inorg. Chem. 2018, 57, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yang, X.; Liu, B.; Zhong, D.; Zhou, G.; Wong, W.-Y. New heterobimetallic Au (I)–Pt (II) polyynes achieving a good trade-off between transparency and optical power limiting performance. J. Mater. Chem. C 2018, 6, 11416–11426. [Google Scholar] [CrossRef]
- Xu, L.; Ho, C.-L.; Liu, L.; Wong, W.-Y. Molecular/polymeric metallaynes and related molecules: Solar cell materials and devices. Coord. Chem. Rev. 2017, 373, 233–257. [Google Scholar] [CrossRef]
- Li, H.; Xie, C.; Lan, R.; Zha, S.; Chan, C.-F.; Wong, W.-Y.; Ho, K.-L.; Chan, B.D.; Luo, Y.; Zhang, J.-X.; et al. A Smart Europium–Ruthenium complex as anticancer prodrug: Controllable drug release and real-time monitoring under different light excitations. J. Med. Chem. 2017, 60, 8923–8932. [Google Scholar] [CrossRef]
- Xu, H.-B.; Deng, J.-G.; Kang, B. Designed synthesis and photophysical properties of multifunctional hybrid lanthanide complexes. RSC Adv. 2013, 3, 11367–11384. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zhang, L.-Y.; Chen, Z.-N. Syntheses, characterization and luminescence of Pt–M (M=Re, Ru, Gd) heteronuclear complexes with 5-ethynyl-2,2′-bipyridine. Inorg. Chim. Acta 2007, 360, 163–169. [Google Scholar] [CrossRef]
- Koutsantonis, G.A.; Low, P.J.; Mackenzie, C.F.; Skelton, B.W.; Yufit, D.S. Coordinating Tectons: Bimetallic Complexes from bipyridyl terminated group 8 alkynyl complexes. Organometallics 2014, 33, 4911–4922. [Google Scholar] [CrossRef]
- Xu, H.-B.; Shi, L.-X.; Ma, E.; Zhang, L.-Y.; Wei, Q.-H.; Chen, Z.-N. Diplatinum alkynyl chromophores as sensitisers for lanthanide luminescence in Pt2Ln2 and Pt2Ln4 (Ln=Eu, Nd, Yb) arrays with acetylide-functionalized bipyridine/phenanthroline. Chem. Commun. 2006, 1601–1603. [Google Scholar] [CrossRef]
- Torelli, S.; Imbert, D.; Cantuel, M.; Bernardinelli, G.H.; Delahaye, S.; Hauser, A.; Bünzli, J.-C.G.; Piguet, C. Tuning the decay time of lanthanide-based near infrared luminescence from micro-to milliseconds through d-> f energy transfer in discrete heterobimetallic complexes. Chem. -Eur. J. 2005, 11, 3228–3242. [Google Scholar] [CrossRef]
- Haque, A.; El Moll, H.; Alenezi, K.M.; Khan, M.S.; Wong, W.-Y. Functional materials based on cyclometalated platinum (II) β-diketonate complexes: A Review of structure–property relationships and applications. Materials 2021, 14, 4236. [Google Scholar] [CrossRef] [PubMed]
- Ronson, T.K.; Lazarides, T.; Adams, H.; Pope, S.J.; Sykes, D.; Faulkner, S.; Coles, S.J.; Hursthouse, M.B.; Clegg, W.; Harrington, R.W.; et al. Luminescent PtII (bipyridyl)(diacetylide) chromophores with pendant binding sites as energy donors for sensitised near-infrared emission from lanthanides: Structures and photophysics of PtII/LnIII assemblies. Chem.–A Euro. J. 2006, 12, 9299–9313. [Google Scholar] [CrossRef]
- Ilmi, R.; Haque, A.; Al-Busaidi, I.J.; Al Rasbi, N.K.; Khan, M.S. Synthesis and photophysical properties of hetero trinuclear complexes of tris β-diketonate Europium with organoplatinum chromophore. Dye. Pigment. 2019, 162, 59–66. [Google Scholar] [CrossRef]
- Haque, A.; Al-Balushi, R.; Al-Busaidi, I.J.; Al-Rasbi, N.K.; Al-Bahri, S.; Al-Suti, M.K.; Abou-Zied, O.K.; Khan, M.S.; Skelton, J.M.; Raithby, P.R. Two is better than one? Investigating the effect of incorporating Re(CO)3Cl Side-chains into Pt(II) di-ynes and poly-ynes. Inorg. Chem. 2021, 60, 745–759. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Ilmi, R.; Dutra, J.D.; Oliveira, W.F.; Haque, A.; Al Rasbi, N.K.; Marken, F.; Raithby, P.R.; Khan, M.S. Utilization of a Pt(II) di-yne Chromophore incorporating a 2,2′-bipyridine-5,5′-diyl spacer as a chelate to synthesize a green and red emitting d-f-d heterotrinuclear complex. Dalton Trans. 2021, 50, 1465–1477. [Google Scholar] [CrossRef]
- Beer, P.D.; Szemes, F.; Passaniti, P.; Maestri, M. Luminescent Ruthenium (II) bipyridine− Calix [4] arene complexes as receptors for lanthanide cations. Inorg. Chem. 2004, 43, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Glover, P.B.; Ashton, P.R.; Childs, L.J.; Rodger, A.; Kercher, M.; Williams, R.M.; De Cola, L.; Pikramenou, Z. Hairpin-shaped heterometallic luminescent lanthanide complexes for DNA intercalative recognition. J. Am. Chem. Soc. 2003, 125, 9918–9919. [Google Scholar] [CrossRef] [PubMed]
- Klink, S.I.; Keizer, H.; van Veggel, F.C. Transition metal complexes as photosensitizers for near-infrared lanthanide luminescence. Angew. Chem. 2000, 39, 4319–4321. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Xiao, X.; Chen, X.; Hu, M.; Geng, X.; Wang, Z.; Zhao, J. Recent development of the transition metal complexes showing strong absorption of visible light and long-lived triplet excited state: From molecular structure design to photophysical properties and applications. Coord. Chem. Rev. 2020, 417, 213371. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Wang, J.; Yang, C. Diverse emission properties of transition metal complexes beyond exclusive single phosphorescence and their wide applications. Coord. Chem. Rev. 2021, 433, 213755. [Google Scholar] [CrossRef]
- Long, N.J.; Williams, C.K. Metal alkynyl sigma complexes: Synthesis and materials. Angew Chem. 2003, 42, 2586–2617. [Google Scholar] [CrossRef]
- Packheiser, R.; Ecorchard, P.; Rüffer, T.; Lang, H. Heteromultimetallic transition metal complexes based on unsymmetrical platinum (II) bis-acetylides. Organometallics 2008, 27, 3534–3546. [Google Scholar] [CrossRef]
- Sonogashira, K.; Ohga, K.; Takahashi, S.; Hagihara, N. Studies of poly-yne polymers containing transition metals in the main chain. J. Organomet. Chem. 1980, 188, 237–243. [Google Scholar] [CrossRef]
- Sonogashira, K.; Kataoka, S.; Takahashi, S.; Hagihara, N. Studies of poly-yne polymers containing transition metals in the main chain: III. Synthesis and characterization of a poly-yne polymer containing mixed metals in the main chain. J. Organomet. Chem. 1978, 160, 319–327. [Google Scholar] [CrossRef]
- Lang, H.; Packheiser, R. Mixed transition metal acetylides connected by carbon-rich bridging units: On the way to heterohexametallic organometallics. Collect. Czech. Chem. Commun. 2007, 72, 435–452. [Google Scholar] [CrossRef]
- Lavastre, O.; Even, M.; Dixneuf, P.H.; Pacreau, A.; Vairon, J.-P. Novel ruthenium-or iron-containing tetraynes as precursors of mixed-metal oligomers. Organometallics 1996, 15, 1530–1531. [Google Scholar] [CrossRef]
- Zhou, G.J.; Wong, W.Y.; Lin, Z.; Ye, C. White Metallopolyynes for optical limiting/transparency trade-off optimization. Angew. Chem. Int. Ed. 2006, 45, 6189–6193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.J.; Wong, W.Y.; Ye, C.; Lin, Z. Optical power limiters based on colorless di-, oligo-, and polymetallaynes: Highly Transparent materials for eye protection devices. Adv. Funct. Mater. 2007, 17, 963–975. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.-T.; Alvarez-Falcón, M.M.; Jones, P.G. Platinum (II) and mixed platinum (II)/gold (I) σ-alkynyl complexes. The first anionic σ-alkynyl metal polymers. Organometallics 2005, 24, 2764–2772. [Google Scholar] [CrossRef]
- Zhou, G.; He, Y.; Yao, B.; Dang, J.; Wong, W.Y.; Xie, Z.; Zhao, X.; Wang, L. Electrophosphorescent Heterobimetallic oligometallaynes and their applications in solution-processed organic light-emitting devices. Chem.–Asian J. 2010, 5, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.H.-F.; Lam, S.C.-F.; Yam, V.W.-W.; Zhu, N.; Cheung, K.-K.; Fathallah, S.; Costuas, K.; Halet, J.-F. Luminescent heterometallic branched alkynyl complexes of Rhenium (I)−Palladium (II): Potential building blocks for heterometallic metallodendrimers. Organometallics 2004, 23, 4924–4933. [Google Scholar] [CrossRef]
- Koutsantonis, G.A.; Jenkins, G.I.; Schauer, P.A.; Szczepaniak, B.; Skelton, B.W.; Tan, C.; White, A.H. Coordinating tectons: Bipyridyl-terminated group 8 alkynyl complexes. Organometallics 2009, 28, 2195–2205. [Google Scholar] [CrossRef]
- Di Piazza, E.; Boilleau, C.; Vacher, A.; Merahi, K.; Norel, L.; Costuas, K.; Roisnel, T.; Choua, S.; Turek, P.; Rigaut, S. Ruthenium carbon-rich group as a redox-switchable metal coupling unit in linear trinuclear complexes. Inorg. Chem. 2017, 56, 14540–14555. [Google Scholar] [CrossRef]
- Soliman, A.M.; Fortin, D.; Harvey, P.D.; Zysman-Colman, E. Hybrid charged heterometallic Pt–Ir complexes: Tailoring excited states by taking the best of both worlds. Chem. Commun. 2012, 48, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Fortin, D.; Zysman-Colman, E.; Harvey, P.D. Unexpected evolution of optical properties in Ir–Pt complexes upon repeat unit increase: Towards an understanding of the photophysical behaviour of organometallic polymers. Chem. Commun. 2012, 48, 6271–6273. [Google Scholar] [CrossRef]
- Soliman, A.M.; Fortin, D.; Zysman-Colman, E.; Harvey, P.D. Photonics of a conjugated organometallic Pt–Ir polymer and its model compounds exhibiting hybrid CT excited states. Macromol. Rapid Commun. 2012, 33, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Zysman-Colman, E.; Harvey, P.D. Strategic modulation of the photonic properties of conjugated organometallic Pt–Ir polymers exhibiting hybrid ct-excited states. Macromol. Rapid Commun. 2015, 36, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Al-Mandhary, M.R.A.; Al-Suti, M.K.; Hisahm, A.K.; Raithby, P.R.; Ahrens, B.; Mahon, M.F.; Male, L.; Marseglia, E.A.; Tedesco, E.; et al. Structural characterisation of a series of acetylide-functionalised oligopyridines and the synthesis, characterisation and optical spectroscopy of platinum di-ynes and poly-ynes containing oligopyridyl linker groups in the backbone. J. Chem. Soc. Dalton Trans. 2002, 1358–1368. [Google Scholar] [CrossRef]
- Li, L.; Ho, C.-L.; Wong, W.-Y. Versatile control of the optical bandgap in heterobimetallic polymers through complexation of bithiazole-containing polyplatinynes with ReCl(CO)5. J. Organomet. Chem. 2012, 703, 43–50. [Google Scholar] [CrossRef]
- Al-Khalili, A.N.; Al-Busaidi, I.J.; Ilmi, R.; Al-Mandhary, M.; Khan, M.S.; Al-Rasbi, N.K. Investigation of binding tendency of Eu(III) and La(III)-Schiff base complexes to selected oxy-anions and amino acids. Inorg. Chim. Acta 2020, 501, 119226. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Buenzli, J.C.G. Lanthanide Probes in Life, Chemical and Earth Sciences; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Ilmi, R.; Khan, M.S.; Li, Z.; Zhou, L.; Wong, W.-Y.; Marken, F.; Raithby, P.R. Utilization of Ternary Europium Complex for Organic Electroluminescent Devices and as a Sensitizer to Improve Electroluminescence of Red-Emitting Iridium Complex. Inorg. Chem. 2019, 58, 8316–8331. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.-Y.; Chen, Z.-N. Sensitized EuIII luminescence through energy transfer from PtM2 (M=Ag or Au) alkynyl chromophores in PtM2Eu2 heteropentanuclear complexes. J. Mater. Chem. C 2013, 1, 3661–3668. [Google Scholar] [CrossRef]
- Ward, M.D. Transition-metal sensitised near-infrared luminescence from lanthanides in d–f heteronuclear arrays. Coord. Chem. Rev. 2007, 251, 1663–1677. [Google Scholar] [CrossRef]
- Lis, S.; Elbanowski, M.; Mąkowska, B.; Hnatejko, Z. Energy transfer in solution of lanthanide complexes. J. Photochem. Photobiol. A Chem. 2002, 150, 233–247. [Google Scholar] [CrossRef]
- Etchells, I.M.; Pfrunder, M.C.; Williams, J.A.G.; Moore, E.G. Quantification of energy transfer in bimetallic Pt(ii)–Ln(iii) complexes featuring an N^C^N-cyclometallating ligand. Dalton Trans. 2019, 48, 2142–2149. [Google Scholar] [CrossRef]
- Wong, W.Y.; Ho, C.L. Organometallic photovoltaics: A new and versatile approach for harvesting solar energy using conjugated polymetallaynes. Acc. Chem. Res. 2010, 43, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, M.; Haque, A.; Al-Busaidi, I.J.; Al-Balushi, R.; Al-Suti, M.K.; Islam, S.M.; Khan, M.S. Synthesis, characterization and photocell studies of a Pt(II) poly-yne covalently attached to a fullerene. J. Organomet. Chem. 2017, 842, 32–38. [Google Scholar] [CrossRef]
- Chawdhury, N.; Kohler, A.; Friend, R.H.; Wong, W.Y.; Lewis, J.; Younus, M.; Raithby, P.R.; Corcoran, T.C.; Al-Mandhary, M.R.A.; Khan, M.S. Evolution of lowest singlet and triplet excited states with number of thienyl rings in platinum poly-ynes. J. Chem. Phy. 1999, 110, 4963–4970. [Google Scholar] [CrossRef]
- Ziessel, R.; Diring, S.; Kadjane, P.; Charbonnière, L.; Retailleau, P.; Philouze, C. Highly Efficient Blue Photoexcitation of Europium in a Bimetallic Pt–Eu Complex. Chem.-Asian J. 2007, 2, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-B.; Zhang, L.-Y.; Xie, Z.-L.; Ma, E.; Chen, Z.-N. Heterododecanuclear Pt6Ln6 (Ln = Nd, Yb) arrays of 4-ethynyl-2,2′-bipyridine with sensitized near-IR lanthanide luminescence by Pt → Ln energy transfer. Chem. Commun. 2007, 2744–2746. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zhang, L.-Y.; Chen, Z.-H.; Shi, L.-X.; Chen, Z.-N. Sensitization of lanthanide luminescence by two different Pt → Ln energy transfer pathways in PtLn3 heterotetranuclear complexes with 5-ethynyl-2,2′-bipyridine. Dalton Trans. 2008, 4664–4670. [Google Scholar] [CrossRef]
- Xu, H.-B.; Ni, J.; Chen, K.-J.; Zhang, L.-Y.; Chen, Z.-N. Preparation, Characterization, and Photophysical Properties of cis- or trans-PtLn2 (Ln = Nd, Eu, Yb) Arrays with 5-Ethynyl-2,2′-bipyridine. Organometallics 2008, 27, 5665–5671. [Google Scholar] [CrossRef]
- Belyaev, A.; Slavova, S.O.; Solovyev, I.V.; Sizov, V.V.; Jänis, J.; Grachova, E.V.; Koshevoy, I.O. Solvatochromic dual luminescence of Eu–Au dyads decorated with chromophore phosphines. Inorg. Chem. Front. 2020, 7, 140–149. [Google Scholar] [CrossRef]
- Li, X.-L.; Dai, F.-R.; Zhang, L.-Y.; Zhu, Y.-M.; Peng, Q.; Chen, Z.-N. Sensitization of lanthanide luminescence in heterotrinuclear PtLn2 (Ln= Eu, Nd, Yb) complexes with terpyridyl-functionalized alkynyl by energy transfer from a platinum (II) alkynyl chromophore. Organometallics 2007, 26, 4483–4490. [Google Scholar] [CrossRef]
- Jana, A.; Crowston, B.J.; Shewring, J.R.; McKenzie, L.K.; Bryant, H.E.; Botchway, S.W.; Ward, A.D.; Amoroso, A.J.; Baggaley, E.; Ward, M.D. Heteronuclear Ir (III)–Ln (III) luminescent complexes: Small-molecule probes for dual modal imaging and oxygen sensing. Inorg. Chem. 2016, 55, 5623–5633. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Luminescent Re (I) and Re (I)/Au (I) complexes as cooperative partners in cell imaging and cancer therapy. Chem. Sci. 2014, 5, 4434–4446. [Google Scholar] [CrossRef]
- Zhao, H.; Simpson, P.V.; Barlow, A.; Moxey, G.J.; Morshedi, M.; Roy, N.; Philip, R.; Zhang, C.; Cifuentes, M.P.; Humphrey, M.G. Syntheses, spectroscopic, electrochemical, and third-order nonlinear optical studies of a hybrid tris {ruthenium (alkynyl)/(2-phenylpyridine)} iridium complex. Chem. Eur. J. 2015, 21, 11843–11854. [Google Scholar] [CrossRef]
- Haque, A.; Faizi, M.S.H.; Rather, J.A.; Khan, M.S. Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: A review. Biorg. Med. Chem. 2017, 25, 2017–2034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-Y.; Zhou, L.-P.; Sun, Q.-F. Strongly luminescent 5d/4f heterometal–organic macrocycles with open metal sites: Post-assembly modification and sensing. Dalton Trans. 2019, 48, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-Y.; Zhou, L.-P.; Cai, L.-X.; Li, X.-Z.; Zhou, J.; Sun, Q.-F. Chiral auxiliary and induced chiroptical sensing with 5d/4f lanthanide–organic macrocycles. Chem. Commun. 2020, 56, 2861–2864. [Google Scholar] [CrossRef]









| Code | Metals | Molecular Weight (×104) | λabs (nm) | λems (nm) | Lifetime of S1 (τa, ns) | Lifetime of T1 (τb, µs) | Φ (%) | Eg (eV) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mw | Mn | |||||||||
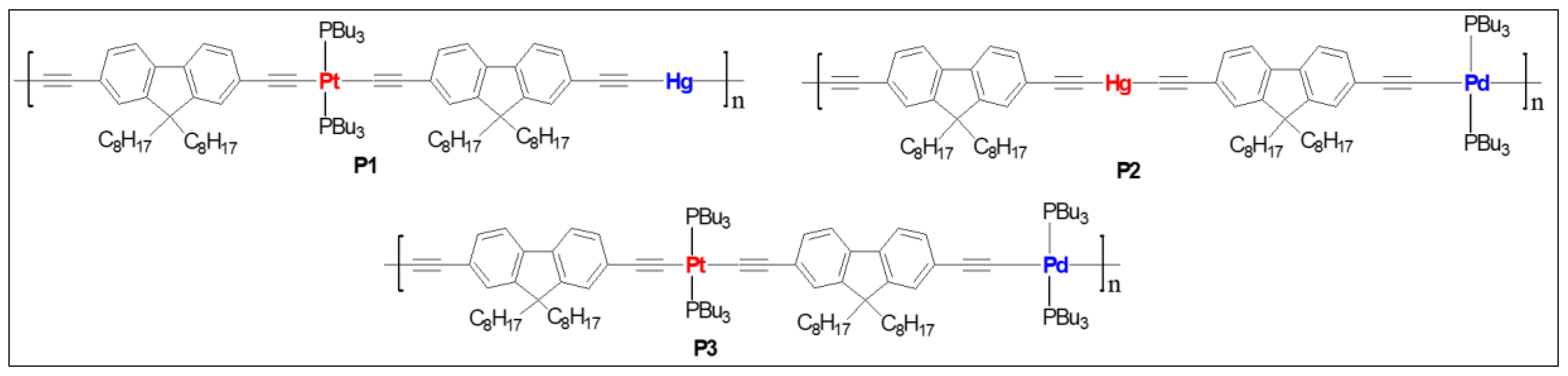
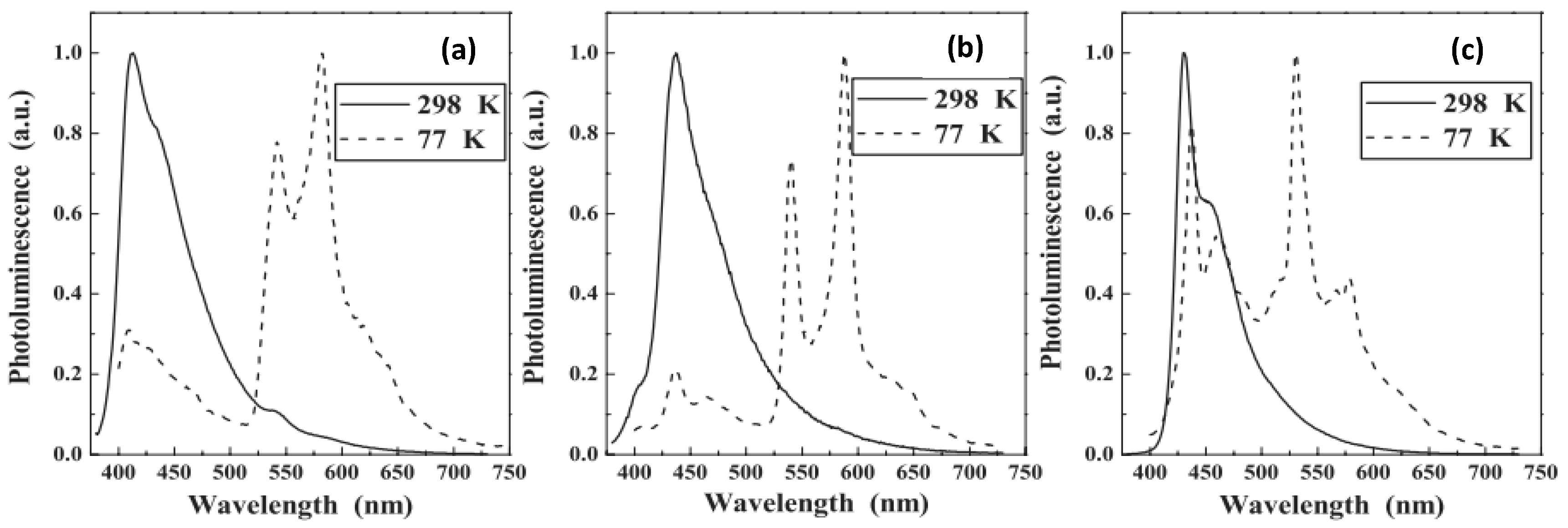
| P1 | Pt/Hg | 2.9 | 1.5 | 386 | 409 a, 542 b,582 b | 1.23 | 194.38, 202.84 | 0.52 | 3.01 | [46] |
| P2 | Pd/Hg | 0.45 | 0.40 | 411 | 430 a, 544 b, 578 b | 0.92 | 12.19, 22.71 | 12.60 | 2.85 | [47] |
| P3 | Pt/Pd | 2.5 | 1.3 | 415 | 437 a, 540 b, 588 b | 0.89 | 193.7, 188.2 | 0.60 | 2.83 | [47] |
| P6 | Pt/Au | - | 2.4 | 270, 276, 305, 319, 345, 362, 387 | 417 a, 438a, 450a, 539 a, 548 b, 587 b, 625 b, 642 b | 0.71 | 181.63 | 1.03 | 2.98 | [22] |
| P7 | Pt/Au | - | 2.7 | 264, 276, 316sh, 377 | 504a, 504 b, 545 b, 563 b | 10370 | 130.80 | 0.62 | 3.00 | [22] |
| P8 | Pt/Au | - | 2.9 | 253, 262, 276sh, 317, 335 | 405 a, 425 a, 455 a, 495 a, 534 a, 456 b, 492 b, 507 b, 527 b | 0.99 | 44.31 | 0.27 | 3.19 | [22] |
| P9 | Pt/Au | - | 2.1 | 275, 305, 319, 344, 361, 384 | 412 a, 432 a, 449sh a, 542 a, 584 a, 548 b, 586 b, 619 b | 0.63 | 137.84 | 1.66 | 3.00 | [22] |
| P10 | Pt/Au | - | 2.4 | 268, 275, 314sh, 373 | 504 a, 503 b, 529 b, 603 b | 6460 | 165.23 | 1.69 | 3.01 | [22] |
| P11 | Pt/Au | - | 3.1 | 253, 261, 316, 334 | 402 a, 421 a, 438 a, 455 a, 503 a, 457 b, 493 b, 507 b | 0.72 | 40.14 | 0.30 | 3.12 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Balushi, R.A.; Haque, A.; Al-Busaidi, I.J.; Al-Sharji, H.; Khan, M.S. Heterometal Grafted Metalla-ynes and Poly(metalla-ynes): A Review on Structure–Property Relationships and Applications. Polymers 2021, 13, 3654. https://doi.org/10.3390/polym13213654
Al-Balushi RA, Haque A, Al-Busaidi IJ, Al-Sharji H, Khan MS. Heterometal Grafted Metalla-ynes and Poly(metalla-ynes): A Review on Structure–Property Relationships and Applications. Polymers. 2021; 13(21):3654. https://doi.org/10.3390/polym13213654
Chicago/Turabian StyleAl-Balushi, Rayya A., Ashanul Haque, Idris J. Al-Busaidi, Houda Al-Sharji, and Muhammad S. Khan. 2021. "Heterometal Grafted Metalla-ynes and Poly(metalla-ynes): A Review on Structure–Property Relationships and Applications" Polymers 13, no. 21: 3654. https://doi.org/10.3390/polym13213654
APA StyleAl-Balushi, R. A., Haque, A., Al-Busaidi, I. J., Al-Sharji, H., & Khan, M. S. (2021). Heterometal Grafted Metalla-ynes and Poly(metalla-ynes): A Review on Structure–Property Relationships and Applications. Polymers, 13(21), 3654. https://doi.org/10.3390/polym13213654









