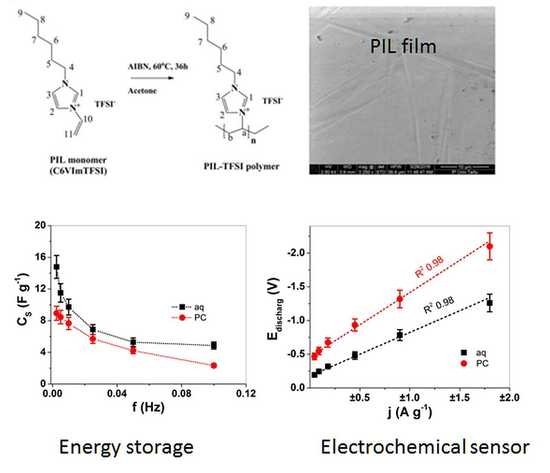
Solvent Effect in Imidazole-Based Poly(Ionic liquid) Membranes: Energy Storage and Sensing
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
2.2. Synthesis and Polymerization of Polymerizable Ionic Liquid (PIL) Monomer
2.3. Electrochemical Characterization of PIL Polymers
2.4. Material Characterizations
3. Results and Discussion
3.1. Characterization of PIL Polymer Films
3.1.1. Structure of PIL Monomer and Polymer by SEM, ssNMR and FTIR Spectroscopy
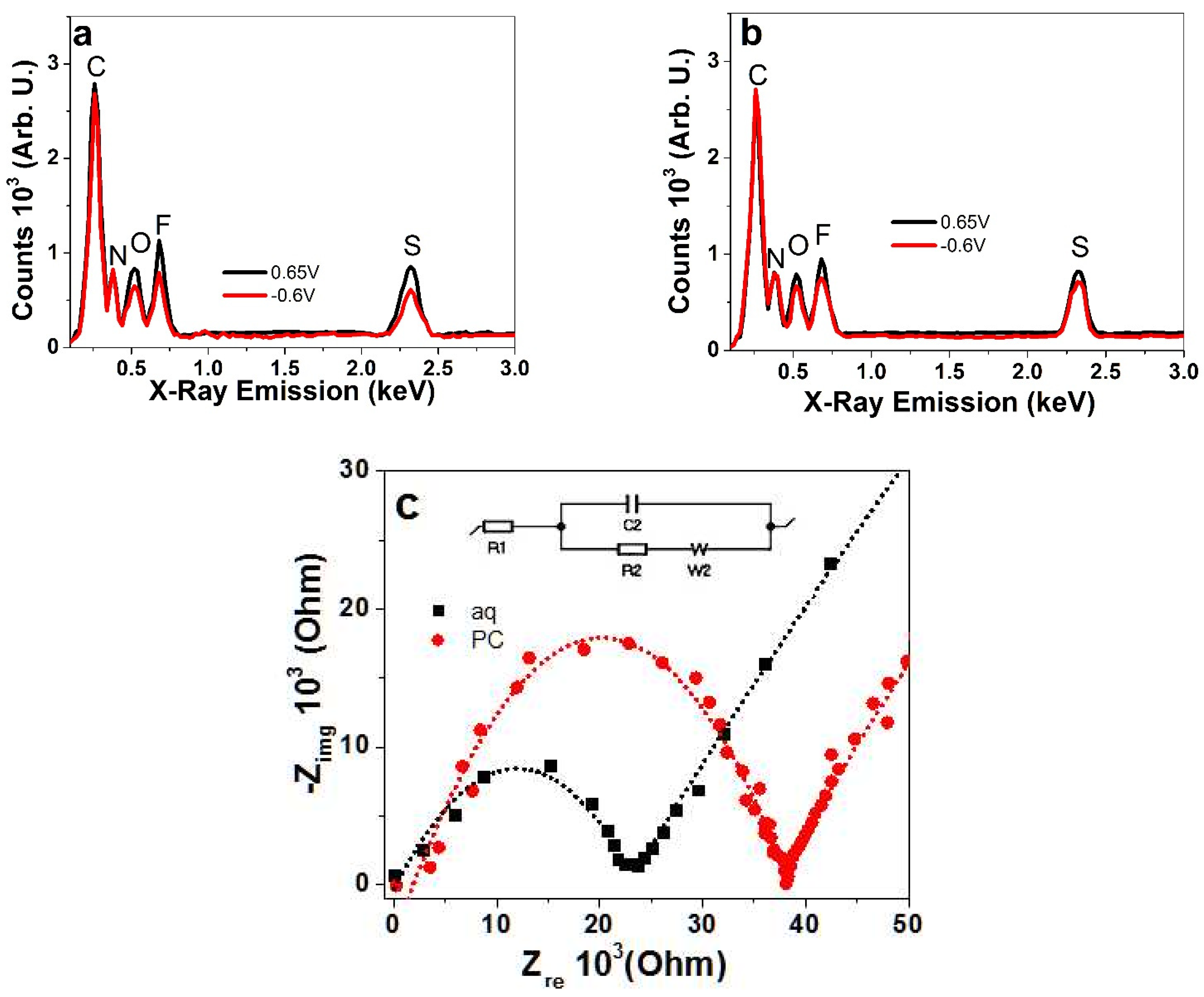
3.1.2. EDX Spectroscopy and EIS Measurements of PIL Polymer Films
3.2. Electrochemical Characterization of PIL Films
3.2.1. Cyclic Voltammetry of PIL Samples in mSICM on Surface and Films
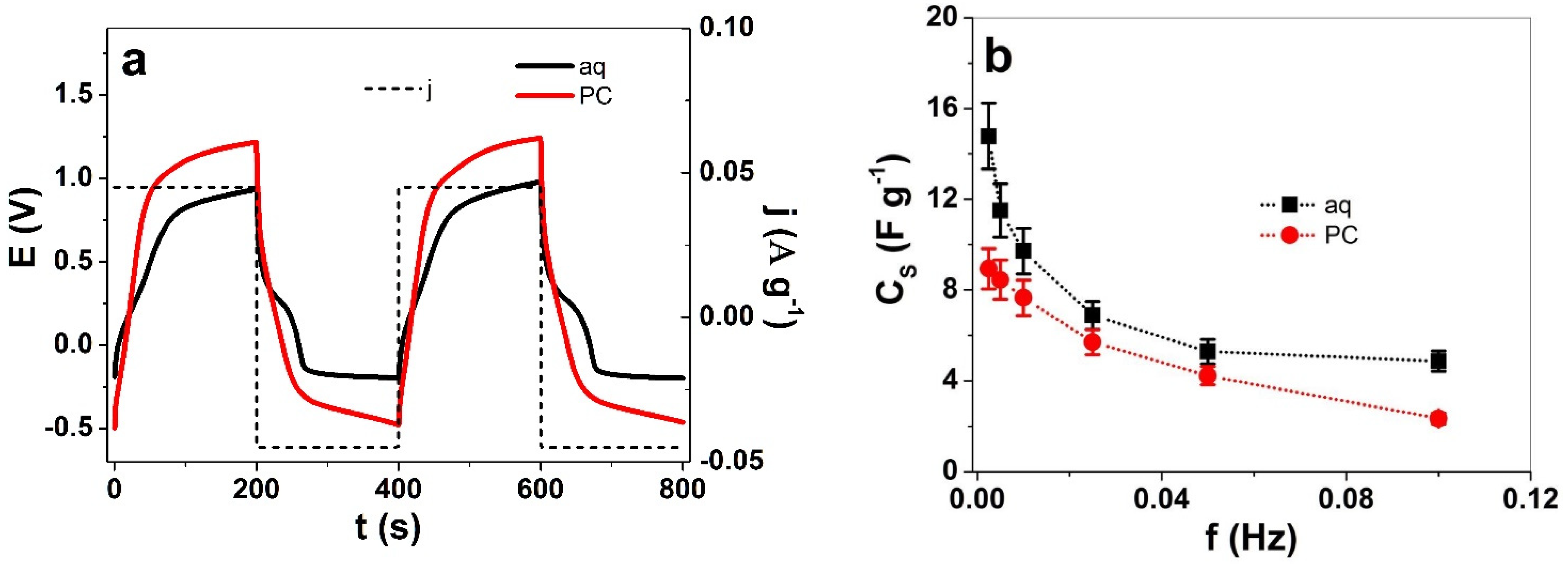
3.2.2. Square Potential Steps Measurements
3.3. Specific Capacitance of PIL Films Applied at Electrolytes with Different Solvents
3.4. Electrochemical Sensor Characteristics of PIL Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaplov, A.; Ponkratov, D.O.; Aubert, P.-H.; Lozinskaya, E.; Plesse, C.; Maziz, A.; Vlasov, P.; Vidal, F.; Vygodskii, Y.S. Truly solid state electrochromic devices constructed from polymeric ionic liquids as solid electrolytes and electrodes formulated by vapor phase polymerization of 3,4-ethylenedioxythiophene. Polymer 2014, 55, 3385–3396. [Google Scholar] [CrossRef]
- Löwe, R.; Hanemann, T.; Zinkevich, T.; Hofmann, A. Structure-property relationship of polymerized ionic liquids for solid-state electrolyte membranes. Polymers 2021, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zhang, Z.; Yang, L.; Hirano, S.-I. An imidazolium-based polymerized ionic liquid via novel synthetic strategy as polymer electrolytes for lithium ion batteries. J. Power Sources 2014, 258, 150–154. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Khan, M.A.; Rossi, F.; Rossetti, A.; Zare, E.N.; et al. Antimicrobial ionic liquid-based materials for biomedical applications. Adv. Funct. Mater. 2021, 2104148, 1–27. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Choi, J.-H.; Xie, W.; Gu, Y.; Frisbie, C.D.; Lodge, T.P. Single ion conducting, polymerized ionic liquid triblock copolymer films: High capacitance electrolyte gates for N-type transistors. ACS Appl. Mater. Interfaces 2015, 7, 7294–7302. [Google Scholar] [CrossRef]
- Marcilla, R.; Mecerreyes, D.; Winroth, G.; Brovelli, S.; Yebra, M.D.M.R.; Cacialli, F. Light-emitting electrochemical cells using polymeric ionic liquid/polyfluorene blends as luminescent material. Appl. Phys. Lett. 2010, 96, 43308. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Ogihara, W. Development of new class of ion conductive polymers based on ionic liquids. Electrochim. Acta 2004, 50, 255–261. [Google Scholar] [CrossRef]
- Nishimura, N.; Ohno, H. 15th anniversary of polymerised ionic liquids. Polymer 2014, 55, 3289–3297. [Google Scholar] [CrossRef]
- Rahman, T.; Barikbin, Z.; Badruddoza, A.Z.M.; Doyle, P.S.; Khan, S.A. Monodisperse polymeric ionic liquid microgel beads with multiple chemically switchable functionalities. Langmuir 2013, 29, 9535–9543. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Soll, S.; Drechsler, M.; Mueller, A.H.E.; Antonietti, M. Mesostructure formation and directional alignment in one step Self-Assembly of poly(ionic liquid)s: Polymerization, mesostructure formation and directional alignment in one step. J. Am. Chem. Soc. 2011, 133, 17556–17559. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Katakabe, T.; Shimosawa, H.; Nazeeruddin, M.K.; Grätzel, M.; Matsui, H.; Kitamura, T.; Tanabe, N.; Watanabe, M. Solid-state dye-sensitized solar cells using polymerized ionic liquid electrolyte with platinum-free counter electrode. Phys. Chem. Chem. Phys. 2010, 12, 1648. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors and biofuel cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Devasurendra, A.M.; Zhang, C.; Young, J.A.; Tillekeratne, L.M.V.; Anderson, J.L.; Kirchhoff, J.R. Electropolymerized pyrrole-based conductive polymeric ionic liquids and their application for solid-phase microextraction. ACS Appl. Mater. Interfaces 2017, 9, 24955–24963. [Google Scholar] [CrossRef] [Green Version]
- Kesküla, A.; Peikolainen, A.-L.; Kiefer, R.; Tamm, T. Consistent response from conducting polymer actuators: Potential window and embedded charges to avoid mixed ion transport. Synth. Met. 2020, 268, 116502. [Google Scholar] [CrossRef]
- Evans, C.M.; Sanoja, G.; Popere, B.C.; Segalman, R.A. Anhydrous proton transport in polymerized ionic liquid block copolymers: Roles of block length, ionic content, and confinement. Macromolecules 2016, 49, 395–404. [Google Scholar] [CrossRef]
- Wang, X.; Xing, L.; Shu, Y.; Chen, X.; Wang, J. Novel polymeric ionic liquid microspheres with high exchange capacity for fast extraction of plasmid DNA. Anal. Chim. Acta 2014, 837, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kesküla, A.; Heinmaa, I.; Tamm, T.; Aydemir, N.; Travas-Sejdic, J.; Peikolainen, A.-L.; Kiefer, R. Improving the electrochemical performance and stability of polypyrrole by polymerizing ionic liquids. Polymers 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and software development for isotonic strain and isometric stress measurements of linear ionic actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, I.J.; Otero, T.; Márquez, M. Diffusion coefficients in swelling polypyrrole: ESCR and cottrell models †. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.; Martínez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2010, 15, 1169–1178. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Elageed, E.H.M.; Qin, L.; Ni, B.; Liu, X.; Gao, G. Swelling poly(ionic liquid)s: Synthesis and application as quasi-homogeneous catalysts in the reaction of ethylene carbonate with aniline. ACS Macro Lett. 2016, 5, 435–438. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G.; Wang, X.; Li, J.; Zhou, Y.; Wang, J. A hierarchical meso-macroporous poly(ionic liquid) monolith derived from a single soft template. Chem. Commun. 2015, 51, 4969–4972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shen, X.; Yan, F.; Qiu, L.; Lee, S.; Sun, B. Solvent-free ionic liquid/poly(ionic liquid) electrolytes for quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 7326–7330. [Google Scholar] [CrossRef]
- Zhao, Q.; Nuli, Y.; Nasiman, T.; Yang, J.; Wang, J. Reversible deposition and dissolution of magnesium from imidazolium-based ionic liquids. Int. J. Electrochem. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, L.; Yang, B.; Du, T.; Zhang, Y. Facile preparation of polymer electrolytes based on the polymerized ionic liquid poly((4-vinylbenzyl)trimethylammonium bis(trifluoromethanesulfonylimide)) for lithium secondary batteries. Electrochim. Acta 2014, 123, 296–302. [Google Scholar] [CrossRef]
- Ohno, H. Design of ion conductive polymers based on ionic liquids. Macromol. Symp. 2007, 249-250, 551–556. [Google Scholar] [CrossRef]
- Pires, J.; Timperman, L.; Jacquemin, J.; Balducci, A.; Anouti, M. Density, conductivity, viscosity, and excess properties of (pyrrolidinium nitrate-based protic ionic liquid + propylene carbonate) binary mixture. J. Chem. Thermodyn. 2013, 59, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Anouti, M.; Jacquemin, J.; Porion, P. Transport properties investigation of aqueous protic ionic liquid solutions through conductivity, viscosity, and NMR self-diffusion measurements. J. Phys. Chem. B 2012, 116, 4228–4238. [Google Scholar] [CrossRef]
- Lee, S.; Becht, G.A.; Lee, B.; Burns, C.T.; Firestone, M.A. Electropolymerization of a bifunctional ionic liquid monomer yields an electroactive liquid-crystalline polymer. Adv. Funct. Mater. 2010, 20, 2063–2070. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z.; Carroll, B. Highly conductive PEO-like polymer electrolytes. Chem. Mater. 1997, 9, 1978–1988. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Zheng, L.; Feng, W.; Zhou, Z.; Nie, J. Solid polymer electrolyte comprised of lithium salt/ether functionalized ammonium-based polymeric ionic liquid with bis(fluorosulfonyl)imide. Electrochim. Acta 2015, 159, 93–101. [Google Scholar] [CrossRef]
- Wang, Z.; Si, Y.; Zhao, C.; Yu, D.; Wang, W.; Sun, G. Flexible and washable poly(ionic liquid) nanofibrous membrane with moisture proof pressure sensing for real-life wearable electronics. ACS Appl. Mater. Interfaces 2019, 11, 27200–27209. [Google Scholar] [CrossRef]
- Zhang, P.; Li, M.; Yang, B.; Fang, Y.; Jiang, X.; Veith, G.; Sun, X.-G.; Dai, S. Polymerized ionic networks with high charge density: Quasi-solid electrolytes in lithium-metal batteries. Adv. Mater. 2015, 27, 8088–8094. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, D.; Li, Z.; Su, G. Gel polymer electrolytes prepared by In Situ polymerization of vinyl monomers in room-temperature ionic liquids. React. Funct. Polym. 2006, 66, 1141–1148. [Google Scholar] [CrossRef]
- Mapesa, E.U.; Chen, M.; Heres, M.F.; Harris, M.A.; Kinsey, T.; Wang, Y.; Long, T.E.; Lokitz, B.S.; Sangoro, J.R. Charge transport in imidazolium-based homo- and triblock poly(ionic liquid)s. Macromolecules 2019, 52, 620–628. [Google Scholar] [CrossRef]
- Kumar, R.; Bocharova, V.; Strelcov, E.; Tselev, A.; Kravchenko, I.I.; Berdzinski, S.; Strehmel, V.; Ovchinnikova, O.S.; Minutolo, J.A.; Sangoro, J.R.; et al. Ion transport and softening in a polymerized ionic liquid. Nanoscale 2015, 7, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Texter, J. Solvent-reversible poration in ionic liquid copolymers. Angew. Chem. Int. Ed. 2007, 46, 2440–2443. [Google Scholar] [CrossRef] [PubMed]
- Oradd, G. Diffusion: A comparison between liquid and solid polymer LiTFSI electrolytes. Solid State Ion. 2002, 152-153, 131–136. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Huang, X.; Mo, Y.; Chen, L. Spectroscopic studies on interactions and microstructures in propylene carbonate? LiTFSI electrolytes. J. Raman Spectrosc. 2001, 32, 900–905. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; de Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Stettner, T.; Lingua, G.; Falco, M.; Balducci, A.; Gerbaldi, C. Protic ionic liquids-based crosslinked polymer electrolytes: A new class of solid electrolytes for energy storage devices. Energy Technol. 2020, 8, 2000742. [Google Scholar] [CrossRef]
- Stettner, T.; Gehrke, S.; Ray, P.; Kirchner, B.; Balducci, A. Water in protic ionic liquids: Properties and use of a new class of electrolytes for energy-storage devices. Chem. Sus. Chem. 2019, 12, 3827–3836. [Google Scholar] [CrossRef]
- Ponkratov, D.O.; Lozinskaya, E.I.; Vlasov, P.S.; Aubert, P.-H.; Plesse, C.; Vidal, F.; Vygodskii, Y.S.; Shaplov, A.S. Synthesis of novel families of conductive cationic poly(ionic liquid)s and their application in all-polymer flexible pseudo-supercapacitors. Electrochim. Acta 2018, 281, 777–788. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic conducting polymers: Synthesis, materials, properties, functions, and devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Otero, T.; Martinez, J.G. Physical and chemical awareness from sensing polymeric artificial muscles. Experiments and modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Smith, E.B. Basic Chemical Thermodynamics; Imperial College Press: London, UK, 1973; ISBN 1-86094-445-0. [Google Scholar]
- Tang, J.; Tang, H.; Sun, W.; Radosz, M.; Shen, Y. Poly(ionic liquid)s as new materials for CO2 absorption. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5477–5489. [Google Scholar] [CrossRef]
- Willa, C.; Yuan, J.; Niederberger, M.; Koziej, D. When nanoparticles meet poly(ionic liquid)s: Chemoresistive CO2 sensing at room temperature. Adv. Funct. Mater. 2015, 25, 2537–2542. [Google Scholar] [CrossRef] [Green Version]





| Solvents with LiTFSI | Linear Equation of Electrical Energy Ue [J g−1] | Linear Equation of Potential at Charging Echarg [V] | Linear Equation of Potential at Discharging Edischarg [V] |
|---|---|---|---|
| aq | |||
| PC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesküla, A.; Peikolainen, A.-L.; Kilmartin, P.A.; Kiefer, R. Solvent Effect in Imidazole-Based Poly(Ionic liquid) Membranes: Energy Storage and Sensing. Polymers 2021, 13, 3466. https://doi.org/10.3390/polym13203466
Kesküla A, Peikolainen A-L, Kilmartin PA, Kiefer R. Solvent Effect in Imidazole-Based Poly(Ionic liquid) Membranes: Energy Storage and Sensing. Polymers. 2021; 13(20):3466. https://doi.org/10.3390/polym13203466
Chicago/Turabian StyleKesküla, Arko, Anna-Liisa Peikolainen, Paul A. Kilmartin, and Rudolf Kiefer. 2021. "Solvent Effect in Imidazole-Based Poly(Ionic liquid) Membranes: Energy Storage and Sensing" Polymers 13, no. 20: 3466. https://doi.org/10.3390/polym13203466
APA StyleKesküla, A., Peikolainen, A.-L., Kilmartin, P. A., & Kiefer, R. (2021). Solvent Effect in Imidazole-Based Poly(Ionic liquid) Membranes: Energy Storage and Sensing. Polymers, 13(20), 3466. https://doi.org/10.3390/polym13203466