Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Extraction of Nano-Fibrillated Cellulose (NFC)
2.2.1. Bran Preparation
2.2.2. Chemical Treatment
2.3. Synthesis of Silver Nanoparticles (Ag-NP)
2.4. Sorption Studies
Adsorption Isotherms
2.5. Characterisation of Nano-Fibrillated Cellulose and Silver Nanoparticles
2.5.1. Ultraviolet Spectroscopy (UV)
2.5.2. Field Emission Scanning Electron Microscopy (FESEM)
2.5.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.4. Transmission Electron Microscopy (TEM)
2.5.5. X-ray Diffraction (XRD)
2.5.6. Atomic Absorption Spectroscopy (AAS)
3. Results and Discussion
3.1. UV
3.2. XRD
3.3. FTIR
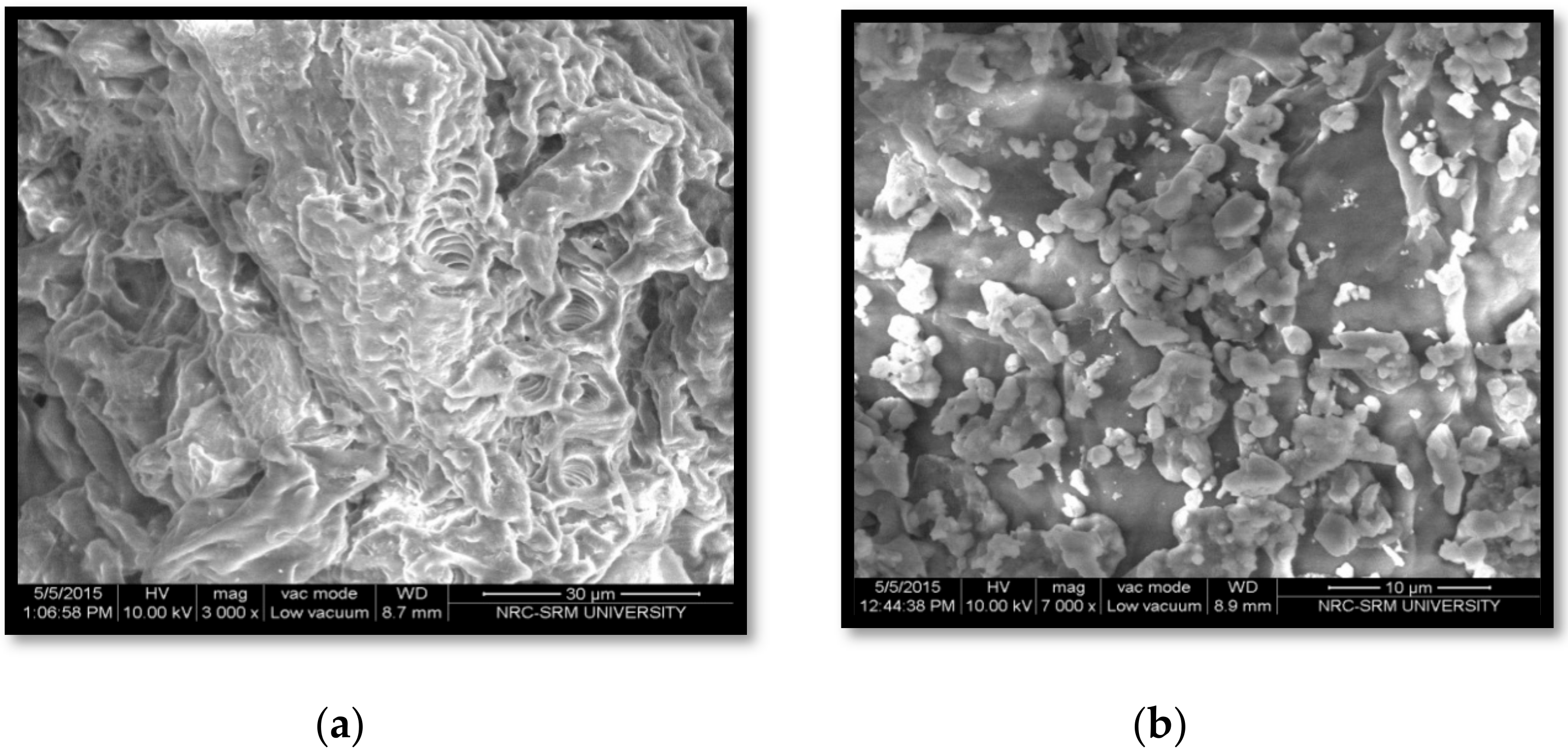
3.4. FESEM
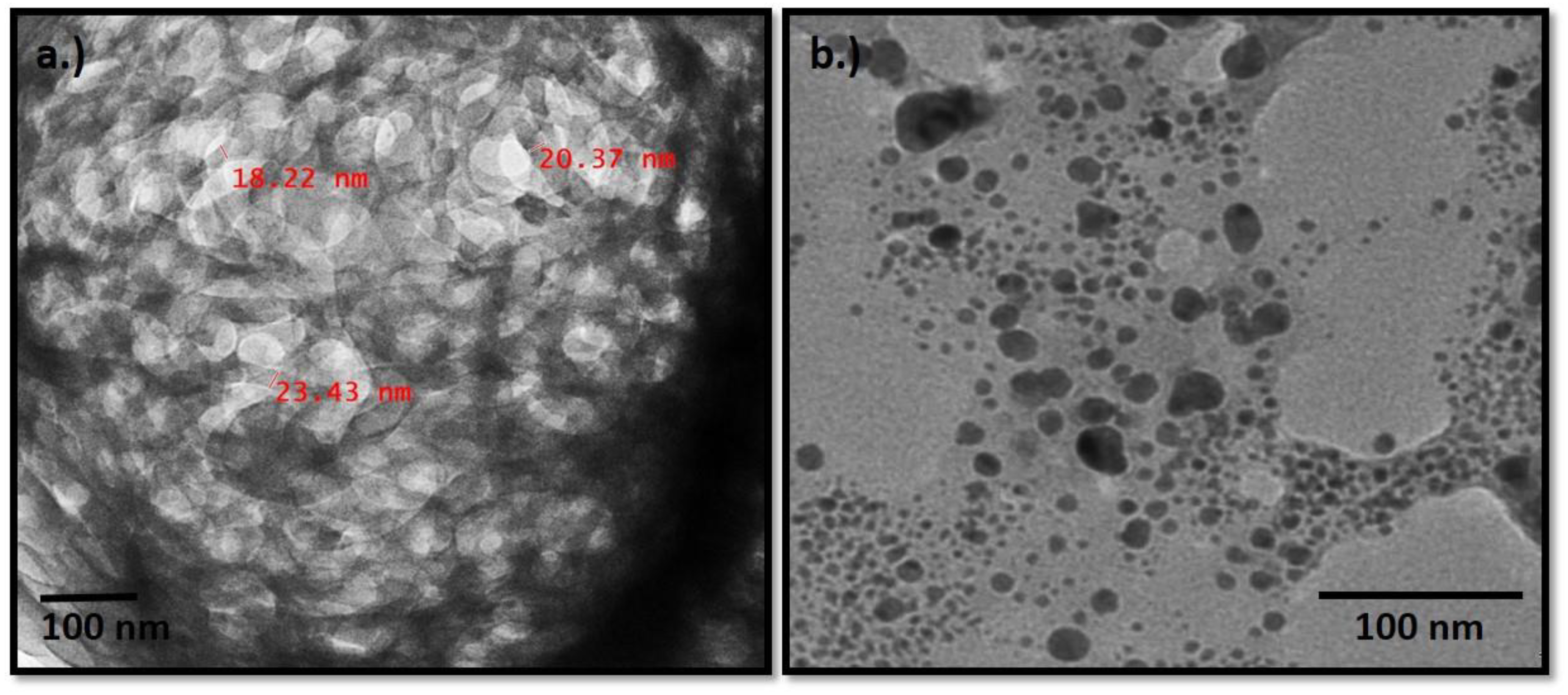
3.5. TEM
3.5.1. Nano-Fibrillated Cellulose
3.5.2. Ag Nanoparticles
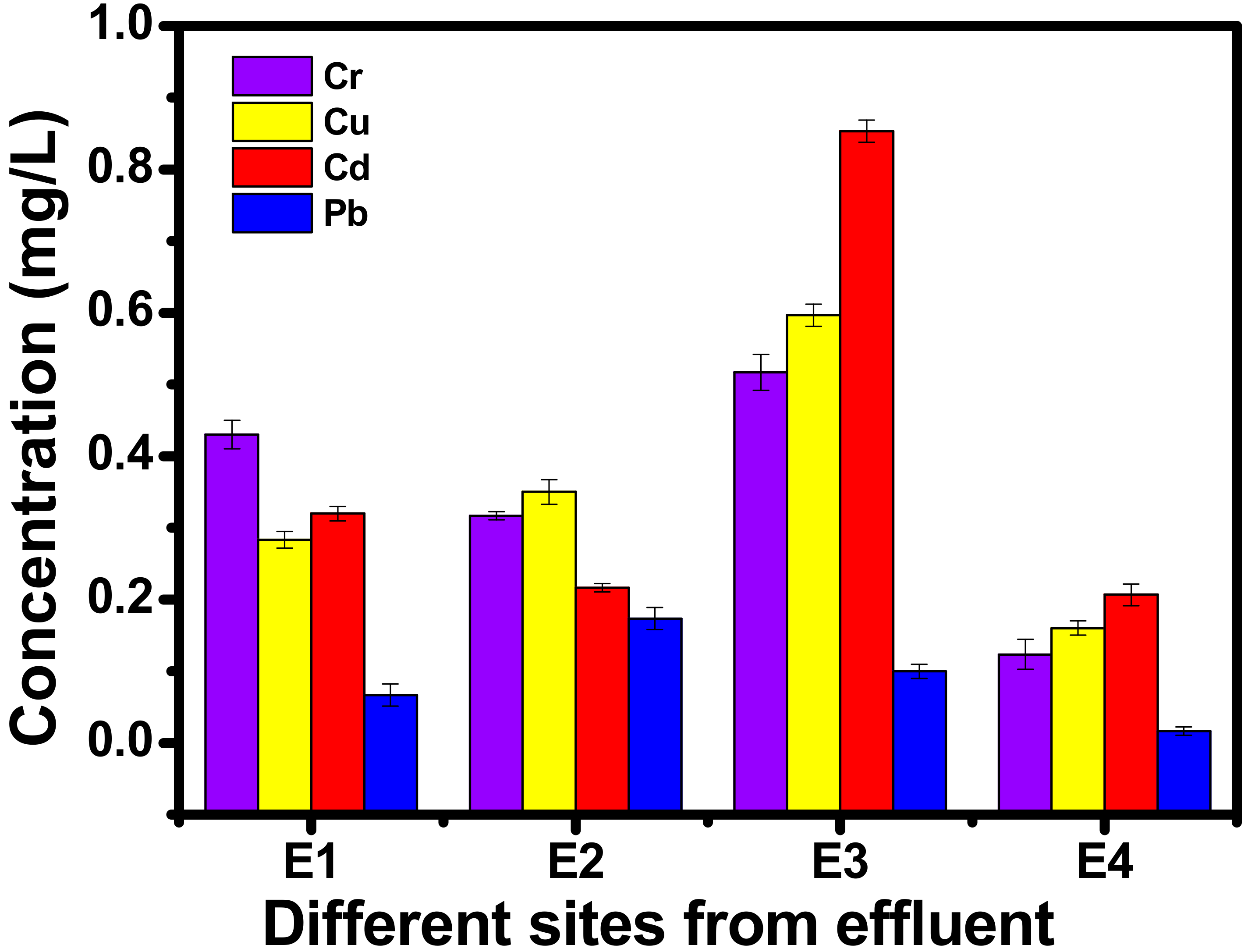
3.6. AAS
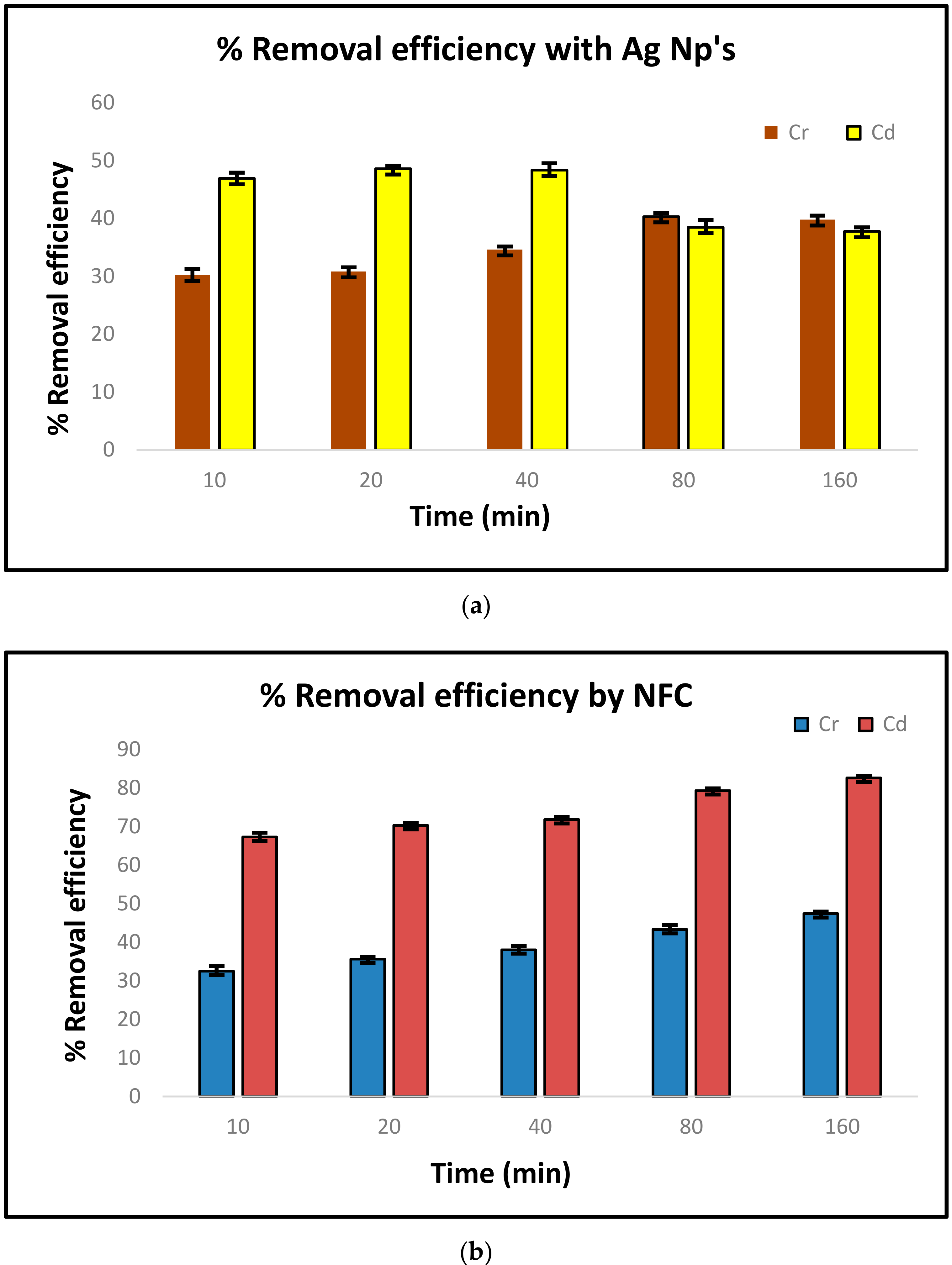
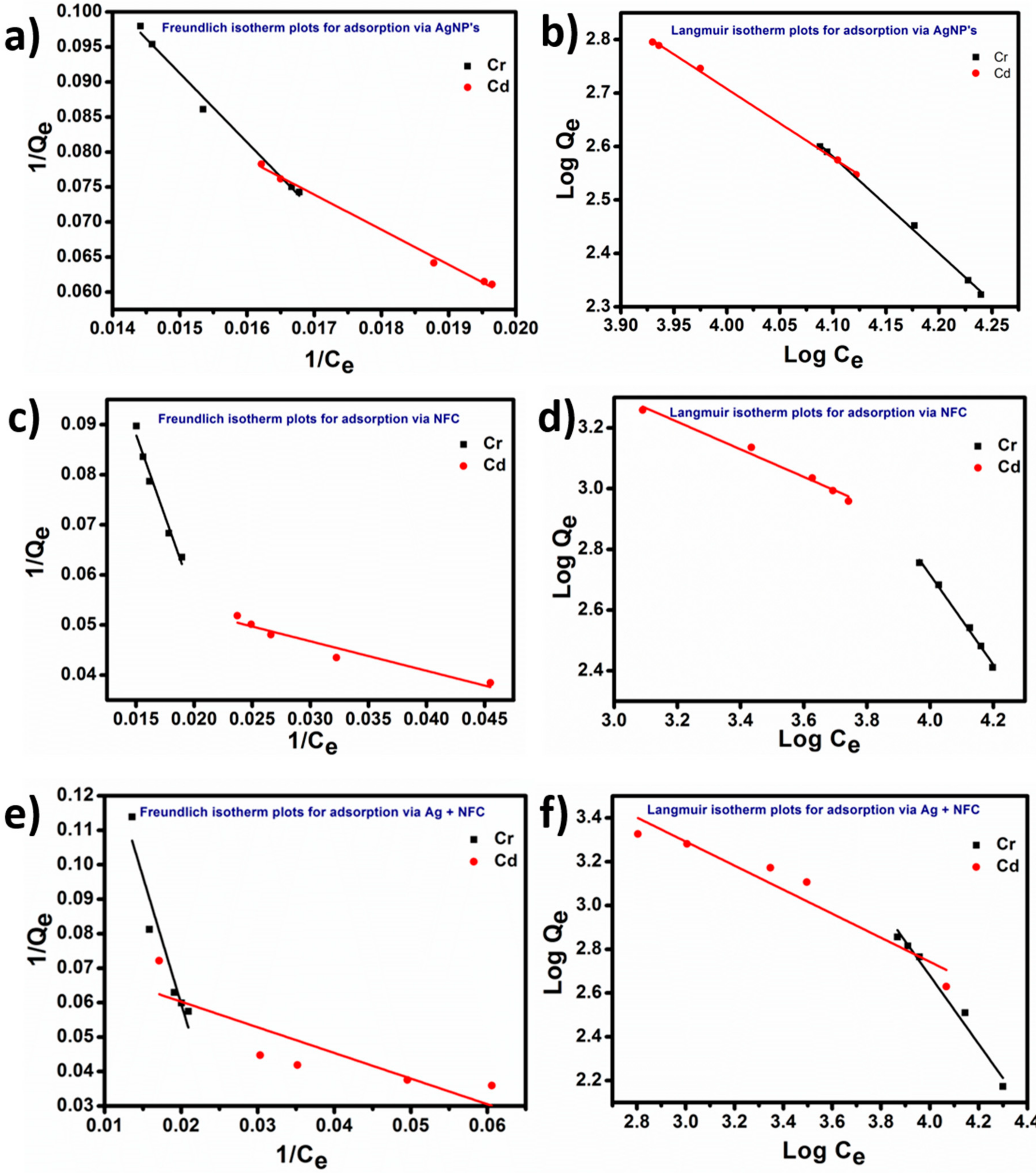
3.7. Sorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio- and nanocomposites: A review. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Keramat, J.; Nasirpour, A.; Hamdami, N.; Behzad, T.; Aranda, L.; Vilasi, M.; Desobry, S. Structural and mechanical properties of clay nanocomposite foams based on cellulose for the food-packaging industry. J. Appl. Polym. Sci. 2016, 133, 2079. [Google Scholar] [CrossRef]
- Da Silva, A.E.; Rodrigues, H.; Salgado Gomes, M.C.; Eleamen, E.; Nagashima, T.; Tabosa Egito, E.S. Xylan, a Promising Hemicellulose for Pharmaceutical Use. In Products and Applications of Biopolymers; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. Handb. Nanocellulose Cellul. Nanocomposites 2017, 1–49. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Purkait, B.S.; Ray, D.; Sengupta, S.; Kar, T.; Mohanty, A. Isolation of Cellulose Nanoparticles from Sesame Husk. Ind. Eng. Chem. Res. 2011, 50, 871–876. [Google Scholar] [CrossRef]
- Tavker, N.; Gaur, U.K.; Sharma, M. Agro-waste extracted cellulose supported silver phosphate nanostructures as a green photocatalyst for improved photodegradation of RhB dye and industrial fertilizer effluents. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Tavker, N.; Sharma, M. Designing of waste fruit peels extracted cellulose supported molybdenum sulfide nanostructures for photocatalytic degradation of RhB dye and industrial effluent. J. Environ. Manag. 2020, 255. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.; Saini, A.; Maji, P.K. Energy efficient facile extraction process of cellulose nanofibres and their dimensional characterization using light scattering techniques. Carbohydr. Polym. 2017, 165, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, D.; de MoraisTeixeira, E.; da Silva Curvelo, A.A.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crop. Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Anne Christma, F.L.; Toyin, A.J.; Gopinath, S.C.B.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Isotopic composition of cellulose from aquatic organisms. Geochim. Cosmochim. Acta 1981, 45, 1885–1894. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Li, D.; Cheng, Y.-L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Pontaza-Licona, Y.S.; Ramos-Jacques, A.L.; Cervantes-Chavez, J.A.; López-Miranda, J.L.; de JesúsRuíz-Baltazar, Á.; Maya-Cornejo, J.; Rodríguez-Morales, A.L.; Esparza, R.; Estevez, M.; Pérez, R.; et al. Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Results Phys. 2019, 12, 1670–1679. [Google Scholar] [CrossRef]
- Aghdam, S.Z.; Karimi, M.S.; Tabatabaee, A.; Minaeian, S. The antibacterial effects of the mixture of silver nanoparticles with the shallot and nettle alcoholic extracts. J. Appl. Biotechnol. Rep. 2019. [Google Scholar] [CrossRef]
- Cabral Pinto, M.; da Silva, E.A.F. Heavy Metals of Santiago Island (Cape Verde) Alluvial Deposits: Baseline Value Maps and Human Health Risk Assessment. Int. J. Environ. Res. Public Health 2018, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave carbonized cellulose for trace pharmaceutical adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Cabral-Pinto, M.M.S.; Inácio, M.; Neves, O.; Almeida, A.A.; Pinto, E.; Oliveiros, B.; da Silva, E.A.F. Human Health Risk Assessment Due to Agricultural Activities and Crop Consumption in the Surroundings of an Industrial Area. Expo. Health 2020, 12, 629–640. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Tavker, N.; Gaur, U.K.; Sharma, M. Highly Active Agro-Waste-Extracted Cellulose-Supported CuInS2 Nanocomposite for Visible-Light-Induced Photocatalysis. ACS Omega 2019, 4, 11777–11784. [Google Scholar] [CrossRef]
- Tavker, N.; Sharma, M. Enhanced photocatalytic activity of nanocellulose supported zinc oxide composite for RhB dye as well as ciprofloxacin drug under sunlight/visible light. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1961, p. 030013. [Google Scholar]
- Desta, M.B. Batch sorption experiments: Langmuir and freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (eragrostis tef) agricultural waste. J. Thermodyn. 2013, 1. [Google Scholar] [CrossRef]
- Ahmed, R.; Yamin, T.; Ansari, M.S.; Hasany, S.M. Sorption Behaviour of Lead(II) Ions from Aqueous Solution onto Haro River Sand. Adsorpt. Sci. Technol. 2006, 24, 475–486. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewskaa, E.; Małeka, K.; Celichowski, G.; Orlowski, P.; Krzyzowska, M.; Grobelny, J. The Synthesis of Monodisperse Silver Nanoparticles with Plant Extracts; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Lefatshe, K.; Muiva, C.M.; Kebaabetswe, L.P. Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydr. Polym. 2017, 164, 301–308. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Bai, H.; Liu, X. Cellulose-Nanowhisker-Templated Synthesis of Titanium Dioxide/Cellulose Nanomaterials with Promising Photocatalytic Abilities. J. Appl. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Mosaviniya, M.; Kikhavani, T.; Tanzifi, M.; Yarakibc, M.T.; Tajbakhsh, P.; Lajevardi, A. Facile Green Synthesis of Silver Nanoparticles Using Crocus Haussknechtii Bois Bulb Extract: Catalytic Activity and Antibacterial Properties; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Silvestro, I.; Vocciante, M. Chromium(III) Removal from Wastewater by Chitosan Flakes. Appl. Sci. 2020, 10, 1925. [Google Scholar] [CrossRef]
- Vikrant, K.; Kumar, V.; Vellingiri, K.; Kim, K.H. Nanomaterials for the abatement of cadmium (II) ions from water/wastewater. Nano Res. 2019. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavker, N.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers 2021, 13, 234. https://doi.org/10.3390/polym13020234
Tavker N, Yadav VK, Yadav KK, Cabral-Pinto MM, Alam J, Shukla AK, Ali FAA, Alhoshan M. Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers. 2021; 13(2):234. https://doi.org/10.3390/polym13020234
Chicago/Turabian StyleTavker, Neha, Virendra Kumar Yadav, Krishna Kumar Yadav, Marina MS Cabral-Pinto, Javed Alam, Arun Kumar Shukla, Fekri Abdulraqeb Ahmed Ali, and Mansour Alhoshan. 2021. "Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis" Polymers 13, no. 2: 234. https://doi.org/10.3390/polym13020234
APA StyleTavker, N., Yadav, V. K., Yadav, K. K., Cabral-Pinto, M. M., Alam, J., Shukla, A. K., Ali, F. A. A., & Alhoshan, M. (2021). Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers, 13(2), 234. https://doi.org/10.3390/polym13020234







