Layered Double Hydroxide (MgFeAl-LDH)-Based Polypropylene (PP) Nanocomposite: Mechanical Properties and Thermal Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MgAl and MgFeAl Layered Double Hydroxides (LDHs)
2.2. Preparation of MgAl and MgFeAl-LDH/PP Nanocomposites
2.3. Characterization Methods
3. Results and Discussion
3.1. Color Parameters (L*, a*, b*) and Opacity Measurement
3.2. Limiting Oxygen Index (LOI) Test, UL94 Test, and Burning Behavior of Different LDH/PP Nanocomposites
UL94 Test
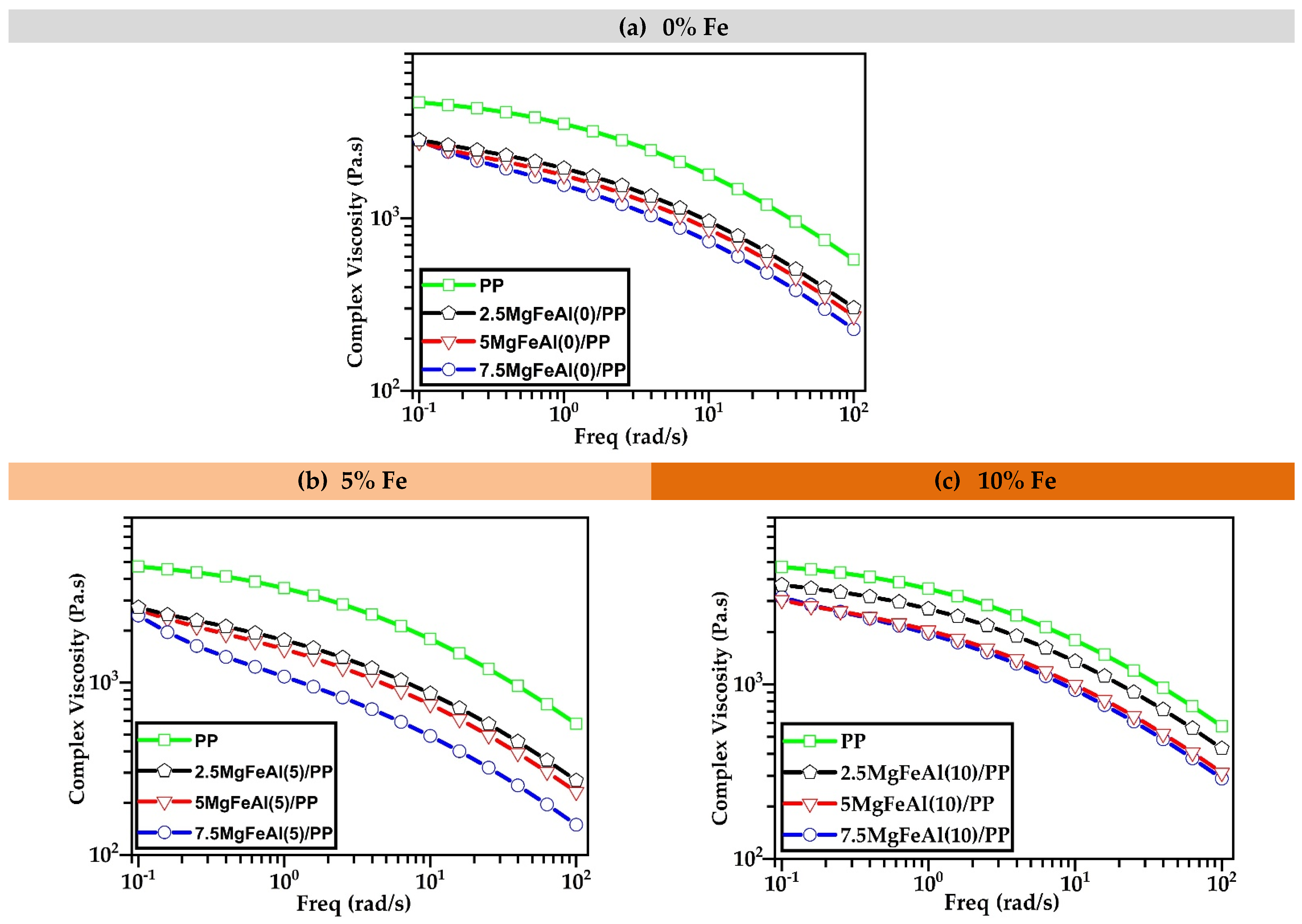
3.3. Rheological Analysis of Different LDH/PP Nanocomposites
3.4. Mechanical Properties (Tensile Testing and Impact Testing)
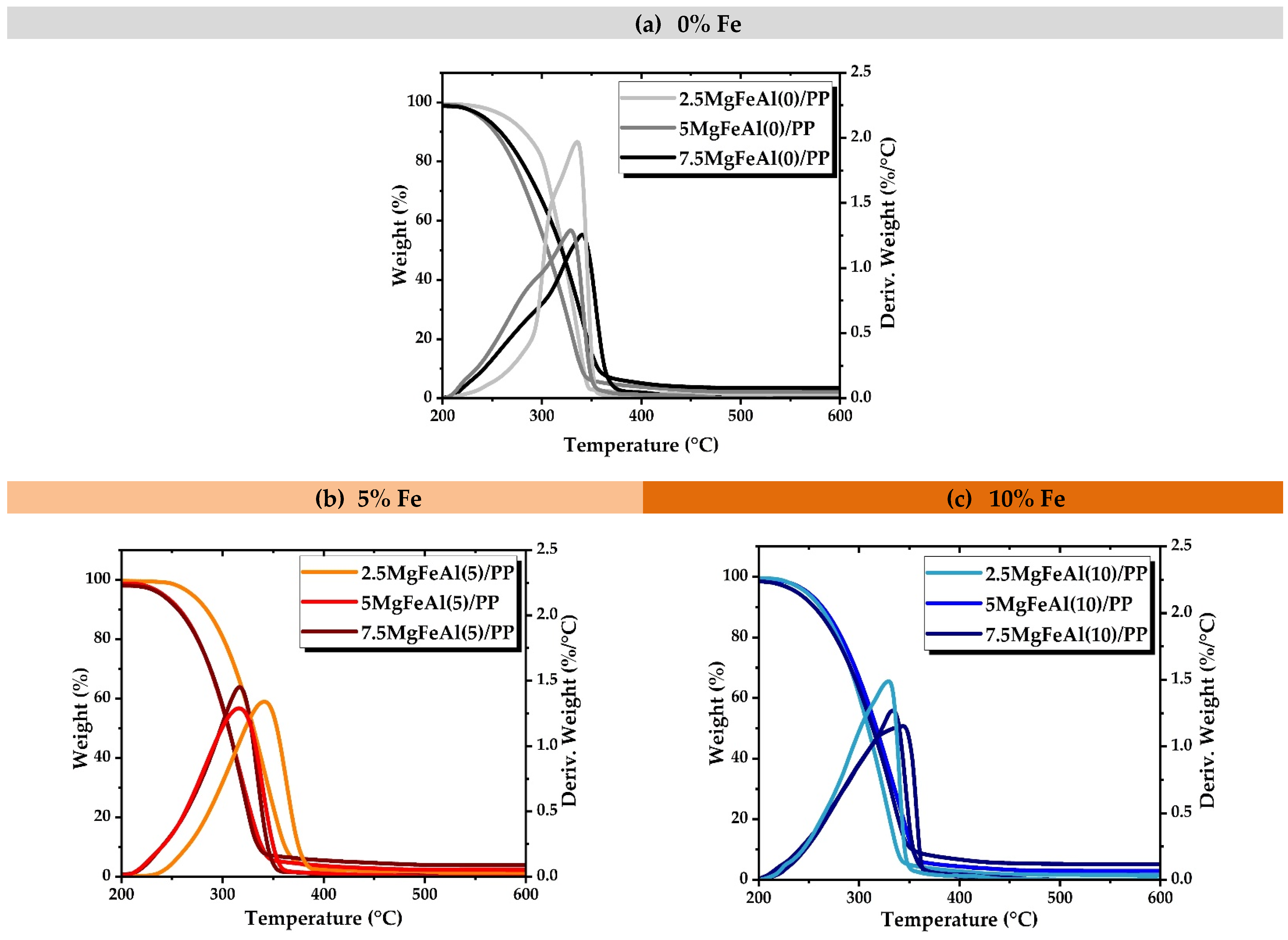
3.5. Thermogravimetric Analysis (TGA) of MgFeAl(0), MgFeAl(5). and MgFeAl(10) PP Nanocomposites
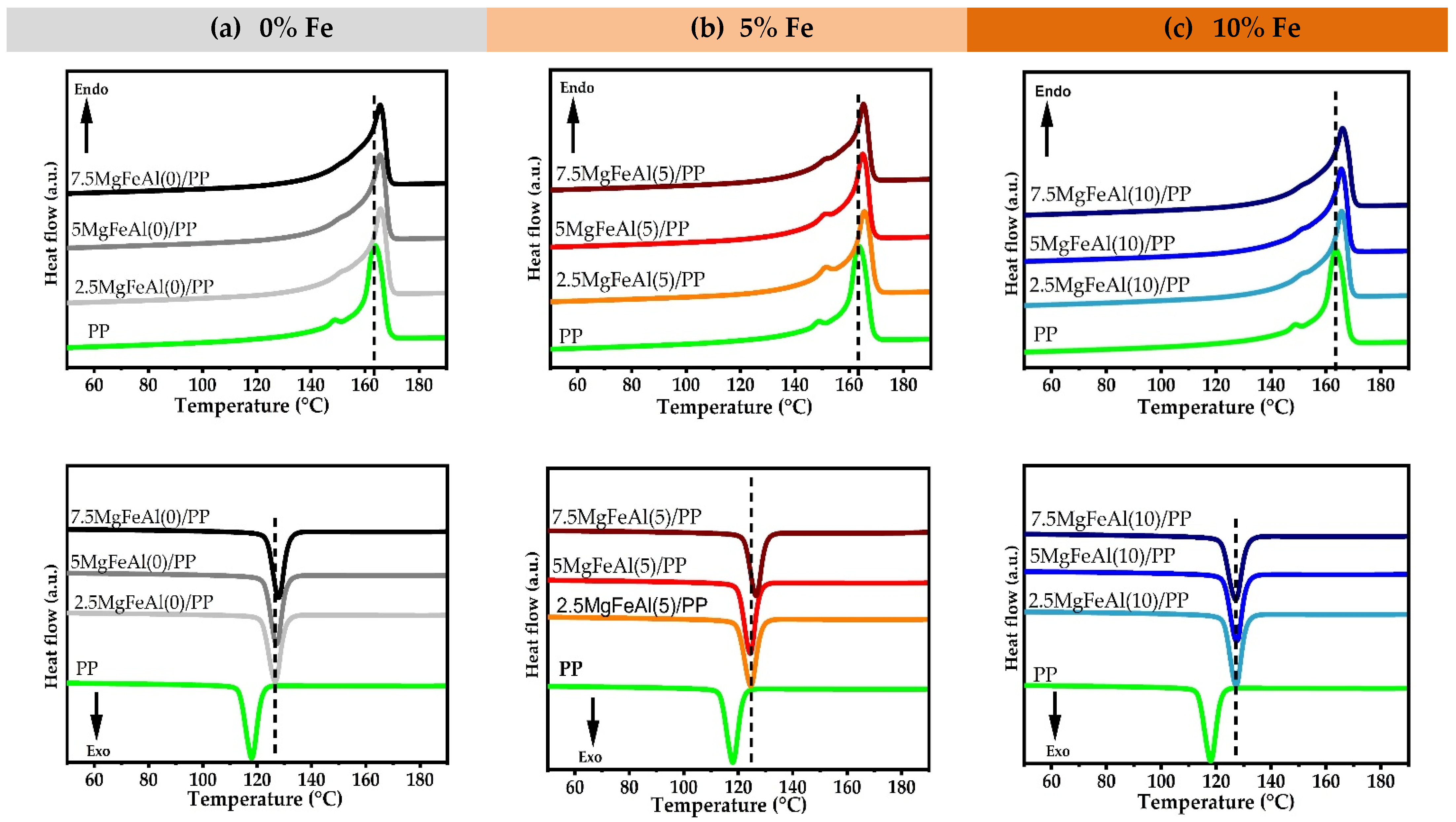
3.6. Differential Scanning Calorimetry (DSC) Analysis of Different LDH/PP Nanocomposites
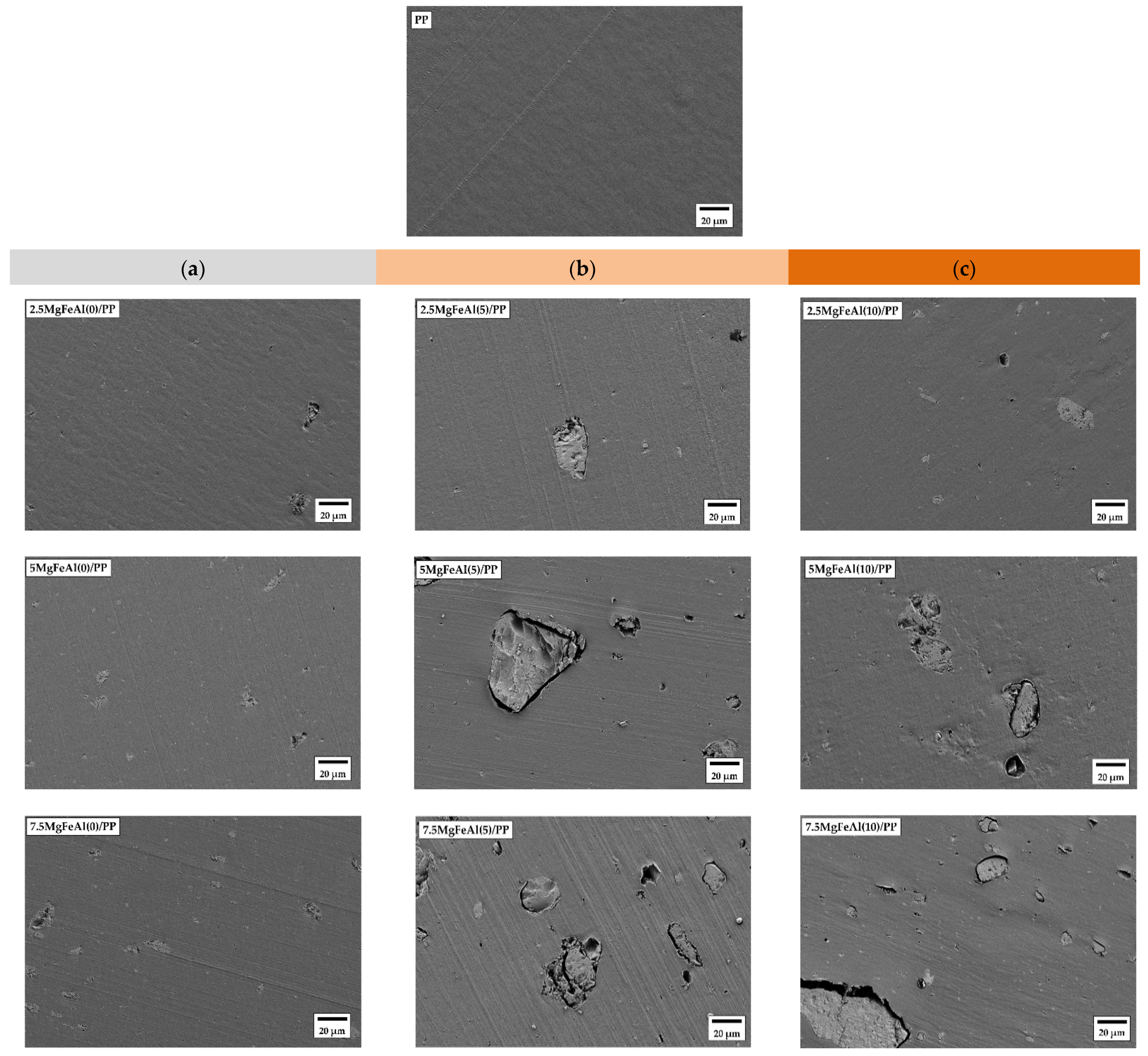
3.7. Scanning Electron Microscopy (SEM) Images and Morphological Analysis of Different LDH/PP Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuang, Y.; Zhao, L.; Zhang, S.; Zhang, F.; Dong, M.; Xu, S. Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials 2010, 3, 5220–5235. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lu, J.; Evans, D.G.; Wei, X.; Chen, J.S. Chapter 18—Functional host-guest materials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 493–543. [Google Scholar] [CrossRef]
- Naseem, S.; Leuteritz, A.; Wageknecht, U. 12—Polymer nanocomposites based on layered double hydroxides (LDHs). In Processing of Polymer Nanocomposites; Kenig, S., Ed.; Hanser: Munich, Germany, 2019; pp. 343–369. [Google Scholar] [CrossRef]
- Allmann, R. The crystal structure of pyroaurite. Acta Crystallograph. Sect. B 1968, 24, 972–977. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Segregation and cation-ordering in sjogrenite and pyroaurite. Mineral. Mag. 1969, 37, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef] [Green Version]
- Gevers, B.R.; Naseem, S.; Leuteritz, A.; Labuschagné, F.J.W.J. Comparison of nano-structured transition metal modified tri-metal MgMAl–LDHs (M = Fe, Zn, Cu, Ni, Co) prepared using co-precipitation. RSC Adv. 2019, 9, 28262–28275. [Google Scholar] [CrossRef] [Green Version]
- Gevers, B.R.; Labuschagné, F.J.W.J. Green Synthesis of Hydrocalumite (CaAl-OH-LDH) from Ca(OH)2 and Al(OH)3 and the Parameters That Influence Its Formation and Speciation. Crystals 2020, 10, 672. [Google Scholar] [CrossRef]
- Cho, D.-K.; Jeon, C.-W.; Park, I.-K. Growth and optical band gap of CdAl-layered double hydroxide thin structures on rigid substrate. J. Alloy. Compd. 2018, 737, 725–730. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Química Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered double hydroxides (LDH). In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Gevers, B.R.; Naseem, S.; Sheppard, C.J.; Leuteritz, A.; Labuschagne, F.J.W.J. Modification of Layered Double Hydroxides Using First-Row Transition Metals for Superior UV-Vis.-NIR Absorption and the Influence of the Synthesis Method Used. [CrossRef]
- Yu, Z.; Li, X.; Peng, Y.; Min, X.; Yin, D.; Shao, L. MgAl-layered-double-hydroxide/sepiolite composite membrane for high-performance water treatment based on layer-by-layer hierarchical architectures. Polymers 2019, 11, 525. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Ganjali, M.R.; Zarghami Dehaghani, M.; Aghazadeh, M.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Mohaddespour, A.; Inamuddin; Formela, K.; et al. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers 2020, 12, 1157. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; di Bartolomeo, A.; Iemmo, L.; Zampino, D.C.; Vittoria, V.; Gorrasi, G. Ionic Liquid as Dispersing Agent of LDH-Carbon Nanotubes into a Biodegradable Vinyl Alcohol Polymer. Polymers 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 89–119. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Li, D.-Q.; Evans, D.G.; Duan, X. Modulating effect of Mg-Al-CO3 layered double hydroxides on the thermal stability of PVC resin. Polym. Degrad. Stab. 2005, 88, 286–293. [Google Scholar] [CrossRef]
- Li, F.; Duan, X. Applications of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 193–223. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Leuteritz, A.; Heinrich, G. Antioxidant intercalated layered double hydroxides: A new multifunctional nanofiller for polymers. RSC Adv. 2013, 3, 1495–1501. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Bujok, S.; Hodan, J.; Beneš, H. Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques. Nanomaterials 2020, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Vasile, E.; Radu, I.-C.; Galateanu, B.; Rapa, M.; Hudita, A.; Jianu, D.; Stanescu, P.-O.; Cioflan, H.; Zaharia, C. Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials 2020, 13, 4488. [Google Scholar] [CrossRef] [PubMed]
- Beneš, H.; Kredatusová, J.; Peter, J.; Livi, S.; Bujok, S.; Pavlova, E.; Hodan, J.; Abbrent, S.; Konefał, M.; Ecorchard, P. Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites. Nanomaterials 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Jiménez Reinosa, J.; Leret, P.; Álvarez-Docio, C.M.; del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO–TiO2 composites. Boletín Soc. Española Cerámica Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Lonkar, S.P.; Leuteritz, A. Study of modified LDHs as UV protecting materials for polypropylene (PP). AIP Conf. Proc. 2019, 2055, 050002. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.R.; Labuschagné, F.J.W.J.; Leuteritz, A. Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells. Materials 2020, 13, 4384. [Google Scholar] [CrossRef]
- Yang, G.; Takei, T.; Yanagida, S.; Kumada, N. Enhanced Supercapacitor Performance Based on CoAl Layered Double Hydroxide-Polyaniline Hybrid Electrodes Manufactured Using Hydrothermal-Electrodeposition Technology. Molecules 2019, 24, 976. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liang, X.; Lu, Y.; Li, H.; Xu, X. Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water. Sensors 2020, 20, 4146. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.-J.; Liu, F.-X.; Ni, Z.-M.; Shi, W.; Xue, J.-L.; Qian, P.-P. Ti-based layered double hydroxides: Efficient photocatalysts for azo dyes degradation under visible light. Appl. Catal. B Environ. 2014, 144, 570–579. [Google Scholar] [CrossRef]
- Djeda, R.; Mailhot, G.; Prevot, V. Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering 2020, 4, 39. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Kutlu, B.; Leuteritz, A.; Häußler, L.; Oertel, U.; Heinrich, G. Stabilization of polypropylene using dye modified layered double hydroxides. Polym. Degrad. Stab. 2014, 102, 9–14. [Google Scholar] [CrossRef]
- Naseem, S.; Lonkar, S.P.; Leuteritz, A.; Labuschagné, F.J.W.J. Different transition metal combinations of LDH systems and their organic modifications as UV protecting materials for polypropylene (PP). RSC Adv. 2018, 8, 29789–29796. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Lin, W. Ammonium polyphosphate intercalated layered double hydroxide and zinc borate as highly efficient flame retardant nanofillers for polypropylene. Polymers 2018, 10, 1114. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-N.; Chen, M.-Y.; Yang, B.-J. Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin. Polymers 2019, 11, 1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, Thermal Stability, and Flammability Properties of Polymer-Layered Double Hydroxide (LDH) Nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]
- Camino, G.; Maffezzoli, A.; Braglia, M.; de Lazzaro, M.; Zammarano, M. Effect of hydroxides and hydroxycarbonate structure on fire retardant effectiveness and mechanical properties in ethylene-vinyl acetate copolymer. Polym. Degrad. Stab. 2001, 74, 457–464. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Effect of hydrotalcite on the thermal stability, mechanical properties, rheology and flame retardance of poly(vinyl chloride). Polym. Int. 2004, 53, 698–707. [Google Scholar] [CrossRef]
- Costa, F.R.; Saphiannikova, M.; Wagenknecht, U.; Heinrich, G. Layered double hydroxide based polymer nanocomposites. In Wax Crystal Control Nanocomposites Stimuli-Responsive Polymers; Springer: Berlin/Heidelberg, Germany, 2008; pp. 101–168. [Google Scholar] [CrossRef]
- Costa, F.R.; Wagenknecht, U.; Heinrich, G. LDPE/Mg-Al layered double hydroxide nanocomposite: Thermal and flammability properties. Polym. Degrad. Stab. 2007, 92, 1813–1823. [Google Scholar] [CrossRef]
- Costa, F.R.; Abdel-Goad, M.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on polyethylene and Mg-Al layered double hydroxide. I. Synthesis and characterization. Polymer 2005, 46, 4447–4453. [Google Scholar] [CrossRef]
- Quispe-Dominguez, R.; Naseem, S.; Leuteritz, A.; Kuehnert, I. Synthesis and characterization of MgAl-DBS LDH/PLA composite by sonication-assisted masterbatch (SAM) melt mixing method. RSC Adv. 2019, 9, 658–667. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Paran, S.M.R.; Vijayan, P.P.; Ganjali, M.R.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.J.; Stadler, F.; Saeb, M.R. A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites. J. Compos. Sci. 2020, 4, 111. [Google Scholar] [CrossRef]
- Balart, R.; Montanes, N.; Dominici, F.; Boronat, T.; Torres-Giner, S. Environmentally Friendly Polymers and Polymer Composites. Materials 2020, 13, 4892. [Google Scholar] [CrossRef]
- Eyenga, I.I.; Focke, W.W.; Prinsloo, L.C.; Tolmay, A.T. Photodegradation: A solution for the shopping bag “visual pollution” problem? Macromol. Symp. 2002, 178, 139–152. [Google Scholar] [CrossRef]
- Magagula, B.; Nhlapo, N.; Focke, W.W. Mn2Al-LDH- and Co2Al-LDH-stearate as photodegradants for LDPE film. Polym. Degrad. Stab. 2009, 94, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Spörer, Y.; Leuteritz, A.; Kuehnert, I.; Wagenknecht, U.; Heinrich, G.; Wang, D.-Y. Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv. 2015, 5, 78979–78985. [Google Scholar] [CrossRef]
- Nagendra, B.; Rosely, C.V.S.; Leuteritz, A.; Reuter, U.; Gowd, E.B. Polypropylene/Layered Double Hydroxide Nanocomposites: Influence of LDH Intralayer Metal Constituents on the Properties of Polypropylene. ACS Omega 2017, 2, 20–31. [Google Scholar] [CrossRef]
- Gomez, N.A.G.; Wypych, F. Nanocomposites of polyethylene and ternary (Mg + Zn/Al) layered double hydroxide modified with an organic UV absorber. J. Polym. Res. 2019, 26, 203. [Google Scholar] [CrossRef]
- Parida, K.; Satpathy, M.; Mohapatra, L. Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: Effects on textural properties and photocatalytic activity for H2 generation. J. Mater. Chem. 2012, 22, 7350–7357. [Google Scholar] [CrossRef]
- Kang, H.T.; Lv, K.; Yuan, S.L. Synthesis, characterization, and SO2 removal capacity of MnMgAlFe mixed oxides derived from hydrotalcite-like compounds. Appl. Clay Sci. 2013, 72, 184–190. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Wang, Y.; Evans, D.G.; Duan, X. Synthesis and characterization of a UV absorbent-intercalated Zn-Al layered double hydroxide. Polym. Degrad. Stab. 2006, 91, 789–794. [Google Scholar] [CrossRef]
- Chai, H.; Lin, Y.; Evans, D.G.; Li, D. Synthesis and UV Absorption Properties of 2-Naphthylamine-1,5-disulfonic Acid Intercalated Zn-Al Layered Double Hydroxides. Ind. Eng. Chem. Res. 2008, 47, 2855–2860. [Google Scholar] [CrossRef]
- Charifou, R.; Gouanvé, F.; Fulchiron, R.; Espuche, E. Polypropylene/layered double hydroxide nanocomposites: Synergistic effect of designed filler modification and compatibilizing agent on the morphology, thermal, and mechanical properties. J. Polym. Sci. Part. B Polym. Phys. 2015, 53, 782–794. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Xia, C.; Yan, D.; Lin, Y.; Wei, M. Fabrication of host-guest UV-blocking materials by intercalation of fluorescent anions into layered double hydroxides. RSC Adv. 2015, 5, 23708–23714. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, W.; Cao, J.; Huang, Y. Synthesis of 5-sulfosalicylic acid-intercalated layered double hydroxide and its effects on wood flour/polypropylene composites during accelerated UV weathering. J. Appl. Polym. Sci. 2017, 134, 44597. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Wagenknecht, U.; Kretzschmar, B.; Reinhardt, G. Investigations of fire retardant properties of polypropylene-clay-nanocomposites. Macromol. Symp. 2003, 194, 207–212. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, J.; Gao, Y.; Zhang, Z.; Wang, J.; Zhang, X.; Yan, X.; Umar, A.; Guo, Z.; O’Hare, D. Polypropylene/Mg3Al-tartrazine LDH nanocomposites with enhanced thermal stability, UV absorption, and rheological properties. RSC Adv. 2013, 3, 26017–26024. [Google Scholar] [CrossRef]
- Ardanuy, M.; Velasco, J.I.; Realinho, V.; Arencón, D.; Martínez, A.B. Non-isothermal crystallization kinetics and activity of filler in polypropylene/Mg-Al layered double hydroxide nanocomposites. Thermochim. Acta 2008, 479, 45–52. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Labuschagné, F.J.W.J.; Leuteritz, A. Catalytic degradation study of iron (Fe) containing LDH-PP composites. AIP Conf. Proc. 2020, 2289, 020041. [Google Scholar] [CrossRef]
- Nagendra, B.; Mohan, K.; Gowd, E.B. Polypropylene/Layered Double Hydroxide (LDH) Nanocomposites: Influence of LDH Particle Size on the Crystallization Behavior of Polypropylene. ACS Appl. Mater. Interfaces 2015, 7, 12399–12410. [Google Scholar] [CrossRef]
- Mittal, V. (Ed.) Crystallization in polymer nanocomposites. In Optimization of Polymer Nanocomposite Properties; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Wu, T.; Tong, Y.; Qiu, F.; Yuan, D.; Zhang, G.Z.; Qu, J.P. Morphology, rheology property, and crystallization behavior of PLLA/OMMT nanocomposites prepared by an innovative eccentric rotor extruder. Polym. Adv. Technol. 2017, 29, 1–11. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y. Preparation of polypropylene/Mg-Al layered double hydroxides nanocomposites through wet pan-milling: Non-isothermal crystallization behaviour. R. Soc. Open Sci. 2018, 5, 171070. [Google Scholar] [CrossRef] [Green Version]
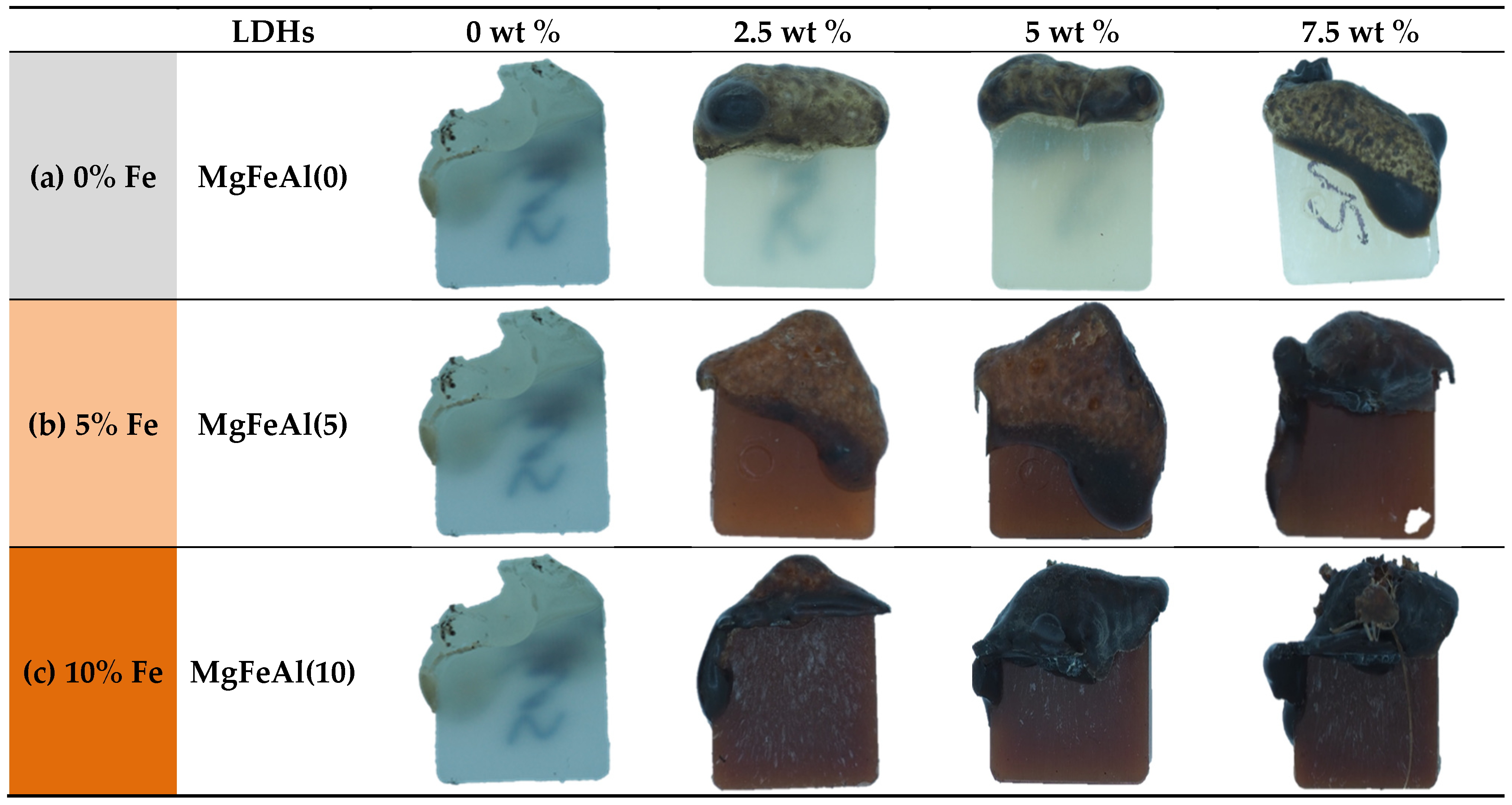

| Sr. No. | LDHs | Amount (wt%) | PP (wt%) | Sample Color |
|---|---|---|---|---|
| 1 | - | 0 | 100 |  |
| 2 | MgFeAl(0) | 2.5 | 97.5 |  |
| 3 | 5 | 95 |  | |
| 4 | 7.5 | 92.5 |  | |
| 5 | MgFeAl(5) | 2.5 | 97.5 |  |
| 6 | 5 | 95 |  | |
| 7 | 7.5 | 92.5 |  | |
| 8 | MgFeAl(10) | 2.5 | 97.5 |  |
| 9 | 5 | 95 |  | |
| 10 | 7.5 | 92.5 |  |
| Sr. No. | LDHs | Amount (wt%) | Color Parameters (L*, a*, b*) | Opacity |
|---|---|---|---|---|
| 1 | - | 0 | 65.3, 0.81, 6.75 | 22.53 |
| 2 | MgFeAl(0) | 2.5 | 55.08, 1.62, 14.08 | 41.98 |
| 3 | 5 | 51.81, 2.14, 13.64 | 48.83 | |
| 4 | 7.5 | 48.59, 2.33, 12.69 | 60.64 | |
| 5 | MgFeAl(5) | 2.5 | 32. 38, 11.16, 9.6 | 88.82 |
| 6 | 5 | 33.16, 8.08, 7.18 | 96.72 | |
| 7 | 7.5 | 35.55, 9.33, 7.55 | 96.06 | |
| 8 | MgFeAl(10) | 2.5 | 30.82, 9.36, 7.64 | 97.59 |
| 9 | 5 | 30.67, 8.86, 7.31 | 98.88 | |
| 10 | 7.5 | 35.92, 7.71, 6.33 | 101.25 |
| LDHs Contents (%) | Limiting Oxygen Index (LOI) | ||
|---|---|---|---|
| MgFeAl(0)/PP | MgFeAl(5)/PP | MgFeAl(10)/PP | |
| 0 | 20 ± 0.08 | 20 ± 0.08 | 20 ± 0.08 |
| 2.5 | 21.6 ± 0.15 | 21.7 ± 0.07 | 21.4 ± 0.11 |
| 5 | 20.8 ± 0.1 | 21.8 ± 0.1 | 21.7 ± 0.11 |
| 7.5 | 21 ± 0.08 | 22.4 ± 0.11 | 21.9 ± 0.15 |
| LDH Contents (%) | Burning Rate (mm/min) | ||
|---|---|---|---|
| MgFeAl(0)/PP | MgFeAl(5)/PP | MgFeAl(10)/PP | |
| 0 | 14.2 ± 0.17 | 14.2 ± 0.17 | 14.2 ± 0.17 |
| 2.5 | 16.3 ± 0.03 | 19.8 ± 0.13 | 15.7 ± 0.28 |
| 5 | 14.9 ± 0.15 | 17.1 ± 0.29 | 13.6 ± 0.35 |
| 7.5 | 14.2 ± 0.03 | 15.3 ± 0.09 | 12.8 ± 0.49 |
| Sr. No. | LDH/PP Nanocomposites | Youngs Modulus (MPa) | Tensile Strength (MPa) | Charpy Impact Strength (kJ/m2) |
|---|---|---|---|---|
| 1 | PP | 1340 ± 32 | 37.3 ± 1.5 | 110 ± 11 |
| 2 | 2.5 MgFeAl(0) | 1630 ± 60 | 33.7 ± 0.4 | 57.6 ± 5.4 |
| 3 | 5 MgFeAl(0) | 1710 ± 100 | 34.3 ± 0.8 | 55.4 ± 4.3 |
| 4 | 7.5 MgFeAl(0) | 1850 ± 100 | 35.1 ± 1.1 | 47.5 ± 4.5 |
| 5 | 2.5 MgFeAl(5) | 1630 ± 120 | 35.1 ± 0.5 | 57.0 ± 3.3 |
| 6 | 5 MgFeAl(5) | 1670 ± 80 | 34.6 ± 0.4 | 47.9 ± 4.8 |
| 7 | 7.5 MgFeAl(5) | 1800 ± 80 | 34.5 ± 0.6 | 35.9 ± 7.6 |
| 8 | 2.5 MgFeAl(10) | 1650 ± 60 | 34.1 ± 0.6 | 69.8 ± 3.9 |
| 9 | 5 MgFeAl(10) | 1670 ± 40 | 33.5 ± 0.6 | 55.3 ± 3.1 |
| 10 | 7.5 MgFeAl(10) | 1700 ± 140 | 33.5 ± 0.3 | 44.4 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseem, S.; Wießner, S.; Kühnert, I.; Leuteritz, A. Layered Double Hydroxide (MgFeAl-LDH)-Based Polypropylene (PP) Nanocomposite: Mechanical Properties and Thermal Degradation. Polymers 2021, 13, 3452. https://doi.org/10.3390/polym13193452
Naseem S, Wießner S, Kühnert I, Leuteritz A. Layered Double Hydroxide (MgFeAl-LDH)-Based Polypropylene (PP) Nanocomposite: Mechanical Properties and Thermal Degradation. Polymers. 2021; 13(19):3452. https://doi.org/10.3390/polym13193452
Chicago/Turabian StyleNaseem, Sajid, Sven Wießner, Ines Kühnert, and Andreas Leuteritz. 2021. "Layered Double Hydroxide (MgFeAl-LDH)-Based Polypropylene (PP) Nanocomposite: Mechanical Properties and Thermal Degradation" Polymers 13, no. 19: 3452. https://doi.org/10.3390/polym13193452
APA StyleNaseem, S., Wießner, S., Kühnert, I., & Leuteritz, A. (2021). Layered Double Hydroxide (MgFeAl-LDH)-Based Polypropylene (PP) Nanocomposite: Mechanical Properties and Thermal Degradation. Polymers, 13(19), 3452. https://doi.org/10.3390/polym13193452






