Effect of Active Packaging Material Fortified with Clove Essential Oil on Fungal Growth and Post-Harvest Quality Changes in Table Grape during Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Effect of Different Levels of EOs on Aspergillus sp.
2.2.1. Radial Growth and Disk Diffusion Method
2.2.2. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
2.3. Procurement and Preparation of TG Samples
2.4. Evaluation of Odor Intensity in TG Treated with Different EOs
2.5. Preparation of Antifungal Active Packaging for Quality Preservation of TG
Application of Antifungal Active Packaging on TG during Post-Harvest Cold Storage
2.6. Effect of Antifungal Active Packaging on Post-Harvest Quality Losses of TG
2.6.1. Disease Severity and Grape Berry Drop
2.6.2. Weight Loss (%)
2.6.3. Acceptance Score
2.7. Statistical Analysis
3. Results and Discussion
3.1. Impact of EOs at Different Levels on the Radial Growth, Disk Diffusion Method, MIC, and MFC of Aspergillus sp.
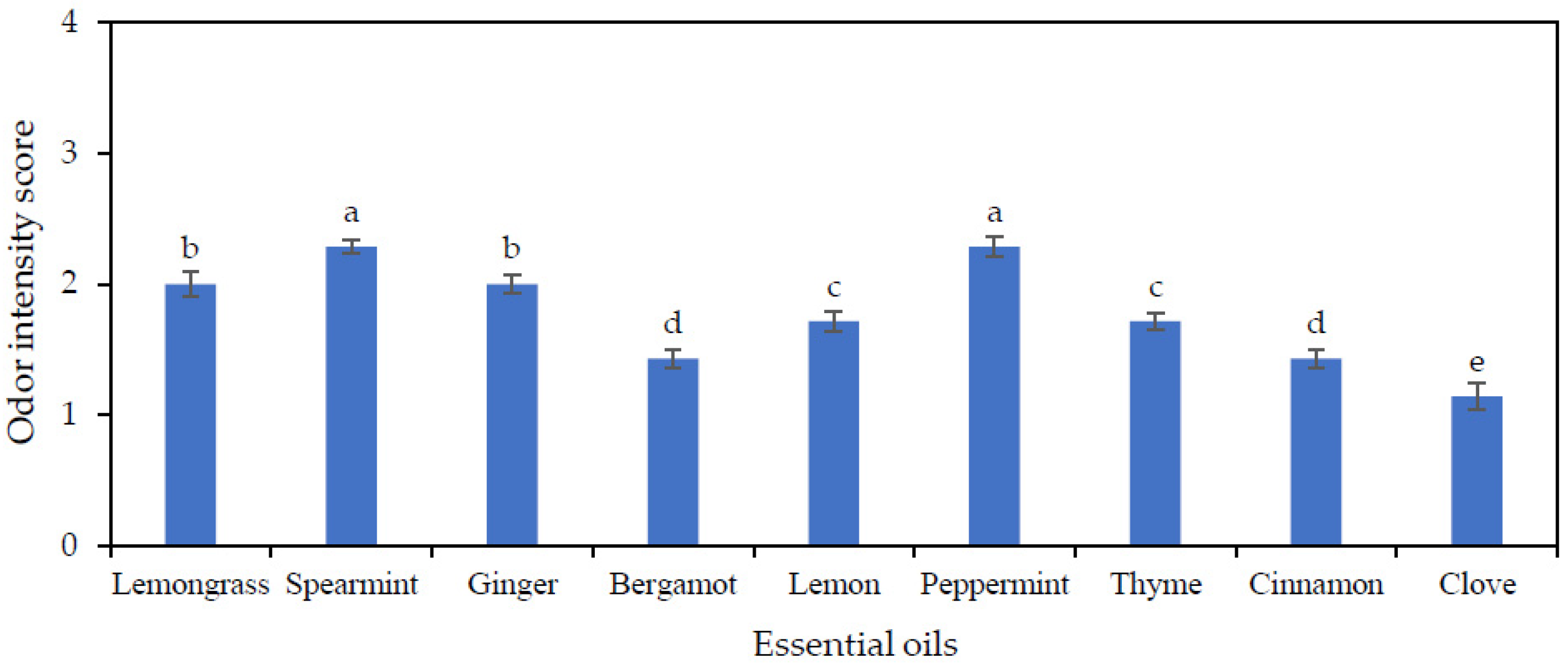
3.2. Evaluation of Odor Intensity of EOs in TG
3.3. Effect of Antifungal Packaging Materials Supplemented with CEO on Quality Changes in TG during Storage
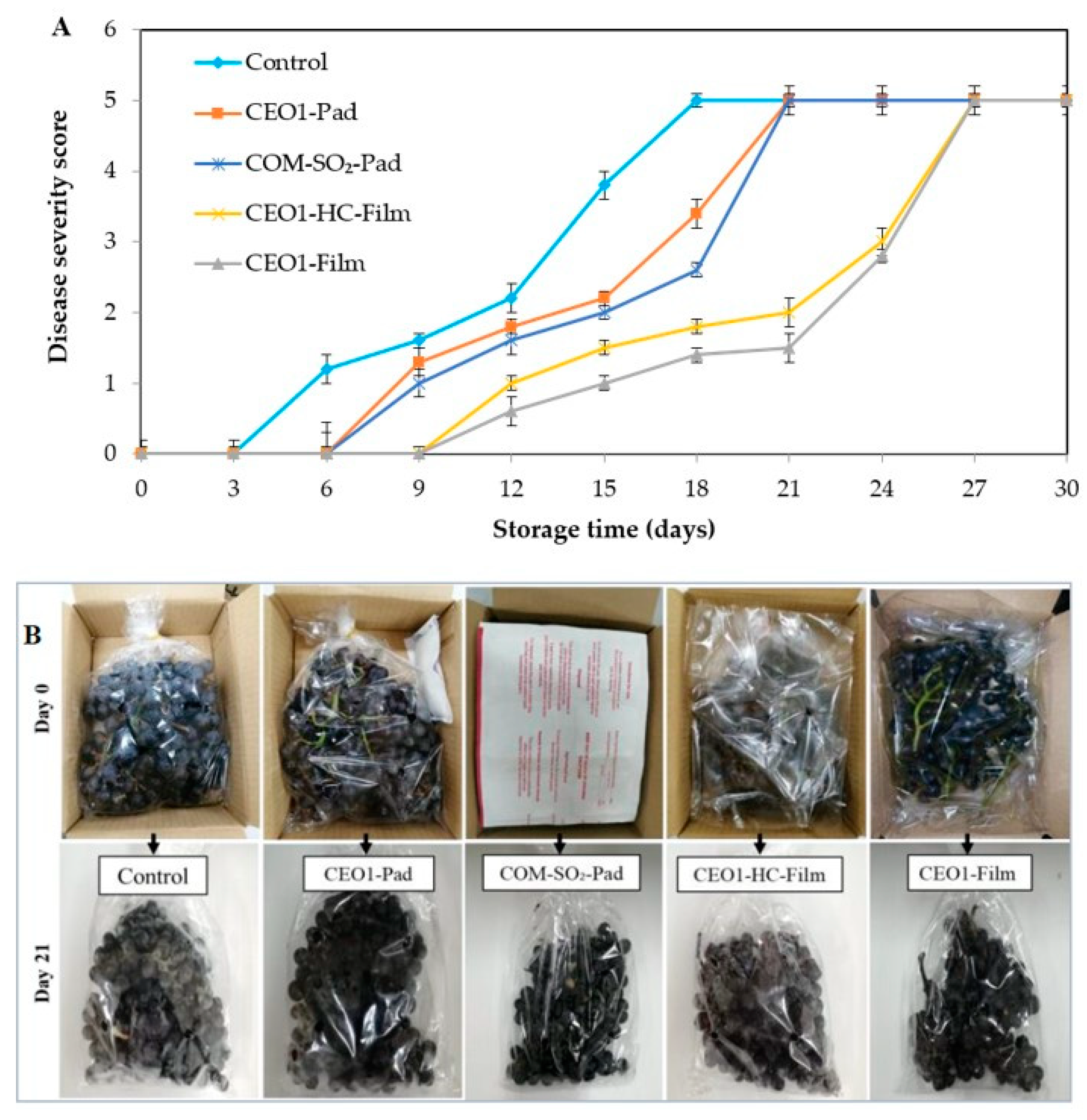
3.3.1. Disease Severity
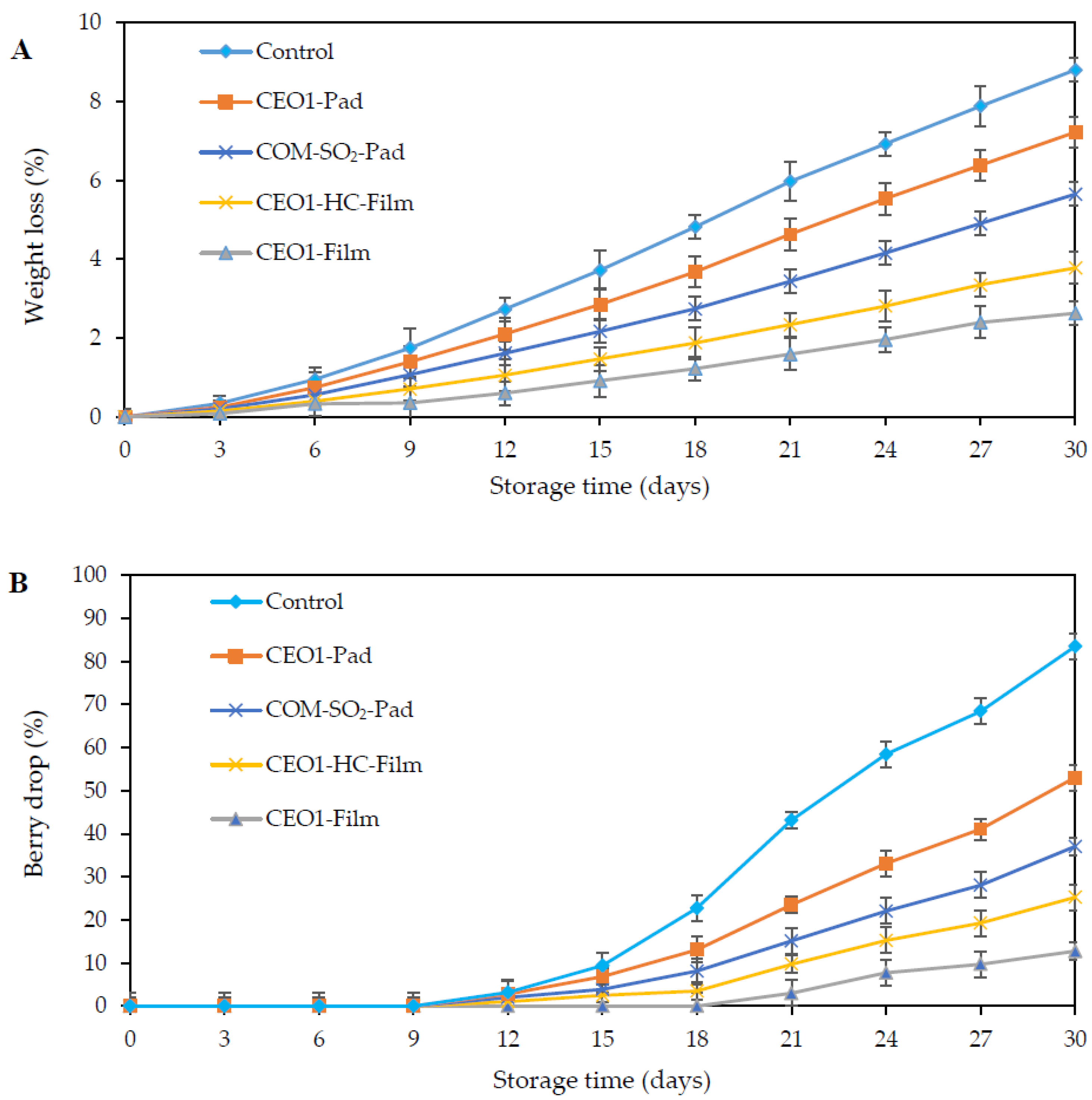
3.3.2. Weight Loss
3.3.3. Berry Drop
3.3.4. Sensory Acceptance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayawardena, R.S.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef]
- Guerra, I.C.D.; de Oliveira, P.D.L.; Santos, M.M.F.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. Technol. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Vertuccio, L.; Vittoria, V.; Pace, B.; Cefola, M.; Quintieri, L.; Bernardo, P.; Clarizia, G. Active packaging for table grapes: Evaluation of antimicrobial performances of packaging for shelf life of the grapes under thermal stress. Food Packag. Shelf Life 2020, 25, 100545. [Google Scholar] [CrossRef]
- Xu, W.-T.; Huang, K.-L.; Guo, F.; Qu, W.; Yang, J.-J.; Liang, Z.-H.; Luo, Y.-B. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 2007, 46, 86–94. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; Chiarotti, F.; Koyama, R.; Hussain, I.; de Souza, R.T. Control of Botrytis mold of the new seedless grape ‘BRS Vitoria’ during cold storage. Sci. Hortic. 2015, 193, 316–321. [Google Scholar] [CrossRef]
- Ahmed, S.; Roberto, S.R.; Domingues, A.R.; Shahab, M.; Junior, O.J.C.; Sumida, C.H.; De Souza, R.T. Effects of Different Sulfur Dioxide Pads on Botrytis Mold in ‘Italia’ Table Grapes under Cold Storage. Horticulturae 2018, 4, 29. [Google Scholar] [CrossRef]
- Sortino, G.; Allegra, A.; Gianguzzi, G.; Passafiume, R.; Gallotta, A.; Gullo, G.J.C.e.t. Postharvest application of sulphur dioxide fumigation to improve quality and storage ability of “red globe” grape cultivar during long cold storage. Chem. Eng. Trans. 2017, 58, 403–408. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.d.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Abifarin, T.O.; Otunola, G.A.; Afolayan, A.J. Chemical Composition of Essential Oils Obtained from Heteromorpha arborescens (Spreng.) Cham. and Schltdl Leaves Using Two Extraction Methods. Sci. World J. 2020, 2020, 9232810. [Google Scholar] [CrossRef] [PubMed]
- Echegoyen, Y.; Nerín, C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef]
- Anderson, E.W.; Fornell, C.; Rust, R.T. Customer Satisfaction, Productivity, and Profitability: Differences Between Goods and Services. Maket. Sci. 1997, 16, 129–145. [Google Scholar] [CrossRef]
- Kaur, K. Chemical Composition, Antioxidant and Antifungal Potential of Clove (Syzygium aromaticum) Essential Oil, its Major Compound and its Derivatives. J. Essent. Oil-Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; FernÁNdez-LÓPez, J.; PÉRez-ÁLvarez, J.A. Antifungal activities of thyme, clove and oregano essential oils. J. Food Safety 2007, 27, 91–101. [Google Scholar] [CrossRef]
- Gayoso, C.W.; Lima, E.O.; Oliveira, V.T.; Pereira, F.O.; Souza, E.L.; Lima, I.O.; Navarro, D.F. Sensitivity of fungi isolated from onychomycosis to Eugenia cariophyllata essential oil and eugenol. Fitoterapia 2005, 76, 247–249. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczyńska, A. Effect of clove and thyme essential oils on candida biofilm formation and the oil distribution in yeast cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, X.; Sun, K. Rice straw cellulose nanofibrils reinforced poly(vinyl alcohol) composite films. Carbohydr. Polym. 2018, 197, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Li, J. Antibacterial activity of polyvinyl alcohol (PVA)/ε-polylysine packaging films and the effect on longan fruit. Food Sci. Technol. 2020, 40, 838–843. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Peng, Z.Y.; Wang, J.H.; Li, W.; Ding, Q.M.; Lin, Q.L.; Liu, D.M.; Wang, S.S.; Shi, Y.; et al. Nano-silver-containing polyvinyl alcohol composite film for grape fresh-keeping. Mater. Express 2019, 9, 985–992. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W.J.T.C. Fabrication and Testing of PVA/Chitosan Bilayer Films for Strawberry Packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- Putri, K.N.A.; Siswanta, D.; Suherman, S. Synthesis of edta-crosslinked chitosancarboxymethyl cellulose film as cu (ii) adsorbent. In Proceedings of the ASEAN/Asian Academic Society International Conference Proceeding Series, Songkhla, Thailand, 11–14 November 2019; pp. 75–85. [Google Scholar]
- Putri, K.N.A.; Keereerak, A.; Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 2020, 156, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Putri, K.N.A.; Chinpa, W. Physical properties and crystal violet adsorption of biosorbent prepared from lemon grass fiber coated with polylactic acid/cellulose acetate blend. In Proceedings of the The Pure and Applied Chemistry International Conference 2020 (PACCON2020); IMPACT Forum: Nonthaburi, Thailand, 2020; pp. 31–36. [Google Scholar]
- Mateo, E.M.; Gómez, J.V.; Domínguez, I.; Gimeno-Adelantado, J.V.; Mateo-Castro, R.; Gavara, R.; Jiménez, M. Impact of bioactive packaging systems based on EVOH films and essential oils in the control of aflatoxigenic fungi and aflatoxin production in maize. Int. J. Food Microbiol. 2017, 254, 36–46. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Bari, M.R.; Hamishehkar, H.; Kafil, H.S.; Lim, L.-T. Essential oils-loaded electrospun chitosan-poly(vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. LWT. 2021, 144, 111217. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Deng, J.; Tang, J.; Jiang, P.; Ma, L.; Zeng, X.J.J.o.C.; Nanoscience, T. Research on properties of polyvinyl alcohol antifungal film based on clove essential oil nano-microcapsule. J. Comput. Theor. Nanosci. 2017, 14, 2981–2985. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal Activity of Plant Essential Oils Against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 86–92. [Google Scholar] [CrossRef]
- Jakowienko, P.; WÓJcik-StopczyŃSka, B.; Jadczak, D. Antifungal activity of essential oils from two varieties of sweet basil (Ocimum basilicum L.). Veg. Crop. Res. Bull. 2011, 74, 97–106. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.H.; Salem, M.F.; Mazrou, K.E.; El-Tras, W.F. Control of citrus molds using bioactive coatings incorporated with fungal chitosan/plant extracts composite. J. Sci. Food Agric. 2016, 96, 1306–1312. [Google Scholar] [CrossRef]
- Fan, X. Involvement of Volatile Sulfur Compounds in Ionizing Radiation-induced Off-odor of Fresh Orange Juice. J. Food Sci. 2004, 69, C593–C598. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S.; O’Brien, N.M. Effect of Pretreatments and Defatting of Seabass Skins on Properties and Fishy Odor of Gelatin. J. Food Biochem. 2016, 40, 741–753. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; Caba, K.d.l.; Benjakul, S.; Prodpran, T. Fish gelatin films laminated with emulsified gelatin film or poly(lactic) acid film: Properties and their use as bags for storage of fried salmon skin. Food Hydrocoll. 2021, 111, 106199. [Google Scholar] [CrossRef]
- El-Garhy, H.A.S.; Abdel-Rahman, F.A.; S Shams, A.; Osman, G.H.; Moustafa, M.M.A. Comparative analyses of four chemicals used to control black mold disease in tomato and its effects on defense signaling pathways, productivity and quality traits. Plants 2020, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.d.S.; Silva, S.D.M.; Dantas, A.L.; Silva, A.F.d.; Rodrigues, A.A.M.; Silva, G.C.D.; Nascimento, L.C.; Mendonça, R.M.N.J.S.-c.A. Quality of IsabeŒ grape treated pre-harvest with CaCl2 and citrus biomass-based elicitor. Semin. Ciências Agrárias (Londrina) 2017, 38, 2945–2956. [Google Scholar] [CrossRef][Green Version]
- Amaral, G.V.; Silva, E.K.; Costa, A.L.R.; Alvarenga, V.O.; Cavalcanti, R.N.; Esmerino, E.A.; Guimarães, J.T.; Freitas, M.Q.; Sant’Ana, A.S.; Cunha, R.L.; et al. Whey-grape juice drink processed by supercritical carbon dioxide technology: Physical properties and sensory acceptance. LWT-Food Sci. Technol. 2018, 92, 80–86. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Zhou, P.; Benjakul, S. Combined effects of pulsed electric field, Chamuang leaf extract and cold plasma on quality and shelf-life of Litopenaeus vannamei. Food Biosci. 2021, 41, 100975. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 2018, 7, e00613. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Latorre, B.A.; Naranjo, P. Hairline, a postharvest cracking disorder in table grapes induced by sulfur dioxide. Postharvest Biol. Technol. 2008, 47, 90–97. [Google Scholar] [CrossRef]
- Blanckenberg, A.; Opara, U.L.; Fawole, O.A. Postharvest losses in quantity and quality of table grape (cv. Crimson seedless) along the supply chain and associated economic, environmental and resource impacts. Sustainability 2021, 13, 4450. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against Botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.; Yang, M.; Shi, C.; Zheng, C. Studies of postharvest berry abscission of ‘Kyoho’ table grapes during cold storage and high oxygen atmospheres. Postharvest Biol. Technol. 2007, 43, 95–101. [Google Scholar] [CrossRef]
- Harindra Champa, W.A.; Gill, M.I.S.; Mahajan, B.V.C.; Bedi, S. Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless under low temperature storage. LWT-Food Sci. Technol. 2015, 60, 412–419. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
| EOs | Radial Growth (mm) | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5% | 1% | 2% | 5% | 0.5% | 1% | 2% | 5% | |
| Control | 7.7 ± 0.4 a | 8.1 ± 0.6 a | 8.9 ± 0.6 a | 9.1 ± 0.3 a | - | - | - | - |
| Lemongrass | 6.7 ± 0.7 b | 6.1 ± 0.9 b | 5.5 ± 0.8 c | 5.4 ± 0.7 c | 0.4 ± 0.02 b | 0.8 ± 0.04 b | 1.3 ± 0.07 b | 1.4 ± 0.2 b |
| Spearmint | 7.8 ± 0.2 a | 6.2 ± 0.4 b | 6.3 ± 0.8 bc | 6.2 ± 0.6 b | 0.5 ± 0.01 b | 0.8 ± 0.03 b | 0.8 ± 0.02 c | 0.9 ± 0.04 c |
| Ginger | 6.9 ± 0.8 ab | 5.6 ± 0.9 bc | 6.1 ± 0.8 bc | 5.7 ± 0.9 c | 0.5 ± 0.03 b | 0.6 ± 0.03 b | 0.7 ± 0.09 c | 0.8 ± 0.02 c |
| Bergamot | 6.3 ± 0.9 b | 6.9 ± 0.6 b | 5.7 ± 0.7 c | 5.4 ± 0.8 c | 0.5 ± 0.01 b | 0.7 ± 0.02 b | 0.7 ± 0.02 c | 0.9 ± 0.05 c |
| Lemon | 7.5 ± 0.7 a | 7.7 ± 0.5 a | 7.5 ± 0.5 b | 6.9 ± 0.7 b | 0.4 ± 0.02 b | 0.6 ± 0.01 b | 0.6 ± 0.01 c | 0.6 ± 0.01 c |
| Peppermint | 7.6 ± 0.4 a | 7.7 ± 0.9 a | 7.4 ± 0.8 b | 6.7 ± 0.7 b | 0.3 ± 0.01 b | 0.7 ± 0.02 b | 0.8 ± 0.01 c | 0.8 ± 0.02 c |
| Thyme | 6.0 ± 0.8 b | 5.6 ± 0.9 bc | 4.1 ± 0.3 d | 3.1 ± 0.8 d | 0.4 ± 0.04 b | 0.7 ± 0.04 b | 1.4 ± 0.06 b | 1.6 ± 0.03 b |
| Cinnamon | 6.7 ± 0.3 b | 5.3 ± 0.8 bc | 4.1 ± 0.6 d | 3.3 ± 0.6 d | 0.5 ± 0.03 b | 0.8 ± 0.05 b | 1.1 ± 0.01 b | 1.5 ± 0.08 b |
| Clove | 5.2 ± 0.8 c | 4.7 ± 0.7 c | 2.5 ± 0.4 e | 1.3 ± 0.3 e | 0.9 ± 0.05 ab | 1.5 ± 0.04 a | 1.9 ± 0.05 a | 2.9 ± 0.09 a |
| EOs | 0.5% | 1% | 2% | 5% | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| Lemongrass | 25 b | 13 b | 25 c | 13 c | 13 c | 6 c | 6 d | 3 d |
| Spearmint | 100 a | 50 a | 50 b | 25 b | 25 b | 13 b | 25 b | 13 b |
| Ginger | 100 a | 50 a | 100 a | 50 a | 50 a | 25 a | 50 a | 25 a |
| Bergamot | 100 a | 50 a | 100 a | 50 a | 50 a | 25 a | 25 b | 13 b |
| Lemon | 100 a | 50 a | 100 a | 50 a | 50 a | 25 a | 50 a | 25 a |
| Peppermint | 100 a | 50 a | 50 b | 25 b | 13 c | 6 c | 13 c | 6 c |
| Thyme | 25 b | 13 b | 25 c | 13 c | 6 d | 3 d | 6 d | 3 d |
| Cinnamon | 100 a | 50 a | 25 c | 13 c | 6 d | 3 d | 6 d | 3 d |
| Clove | 25 b | 13 b | 13 d | 6 d | 6 d | 3 d | 3 e | 2 e |
| Samples | Storage Time (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 21 | |||||||
| Appearance | Firmness | Odor | Overall | Appearance | Firmness | Odor | Overall | |
| Control | 8.5 ± 0.2 aA | 8.6 ± 0.3 aA | 8.5 ± 0.2 aA | 8.5 ± 0.3 aA | 3.5 ± 0.1 aB | 3.8 ± 0.3 aB | 3.2 ± 0.2 aB | 3.5 ± 0.1 aB |
| CEO1-Pad | 8.6 ± 0.1 aA | 8.7 ± 0.1 aA | 8.6 ± 0.3 aA | 8.6 ± 0.2 aA | 4.4 ± 0.3 bB | 4.3 ± 0.2 bB | 4.1 ± 0.4 bB | 5.3 ± 0.3 bB |
| COM-SO2-Pad | 8.5 ± 0.2 aA | 8.6 ± 0.2 aA | 8.5 ± 0.3 aA | 8.5 ± 0.2 aA | 5.5 ± 0.2 cB | 5.7 ± 0.1 cB | 5.6 ± 0.3 cB | 5.6 ± 0.2 cB |
| CEO1-HC-Film | 8.6 ± 0.1 aA | 8.7 ± 0.3 aA | 8.7 ± 0.2 aA | 8.6 ± 0.1 aA | 6.3 ± 0.1 dB | 6.4 ± 0.2 dB | 6.1 ± 0.3 dB | 6.3 ± 0.2 dB |
| CEO1-Film | 8.8 ± 0.3 aA | 8.8 ± 0.2 aA | 8.8 ± 0.1 aA | 8.8 ± 0.3 aA | 7.2 ± 0.2 eB | 7.3 ± 0.1 eB | 7.2 ± 0.2 eB | 7.2 ± 0.1 eB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luesuwan, S.; Naradisorn, M.; Shiekh, K.A.; Rachtanapun, P.; Tongdeesoontorn, W. Effect of Active Packaging Material Fortified with Clove Essential Oil on Fungal Growth and Post-Harvest Quality Changes in Table Grape during Cold Storage. Polymers 2021, 13, 3445. https://doi.org/10.3390/polym13193445
Luesuwan S, Naradisorn M, Shiekh KA, Rachtanapun P, Tongdeesoontorn W. Effect of Active Packaging Material Fortified with Clove Essential Oil on Fungal Growth and Post-Harvest Quality Changes in Table Grape during Cold Storage. Polymers. 2021; 13(19):3445. https://doi.org/10.3390/polym13193445
Chicago/Turabian StyleLuesuwan, Siriporn, Matchima Naradisorn, Khursheed Ahmad Shiekh, Pornchai Rachtanapun, and Wirongrong Tongdeesoontorn. 2021. "Effect of Active Packaging Material Fortified with Clove Essential Oil on Fungal Growth and Post-Harvest Quality Changes in Table Grape during Cold Storage" Polymers 13, no. 19: 3445. https://doi.org/10.3390/polym13193445
APA StyleLuesuwan, S., Naradisorn, M., Shiekh, K. A., Rachtanapun, P., & Tongdeesoontorn, W. (2021). Effect of Active Packaging Material Fortified with Clove Essential Oil on Fungal Growth and Post-Harvest Quality Changes in Table Grape during Cold Storage. Polymers, 13(19), 3445. https://doi.org/10.3390/polym13193445









