Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HIXs
2.3. Thermogravimetric Analysis
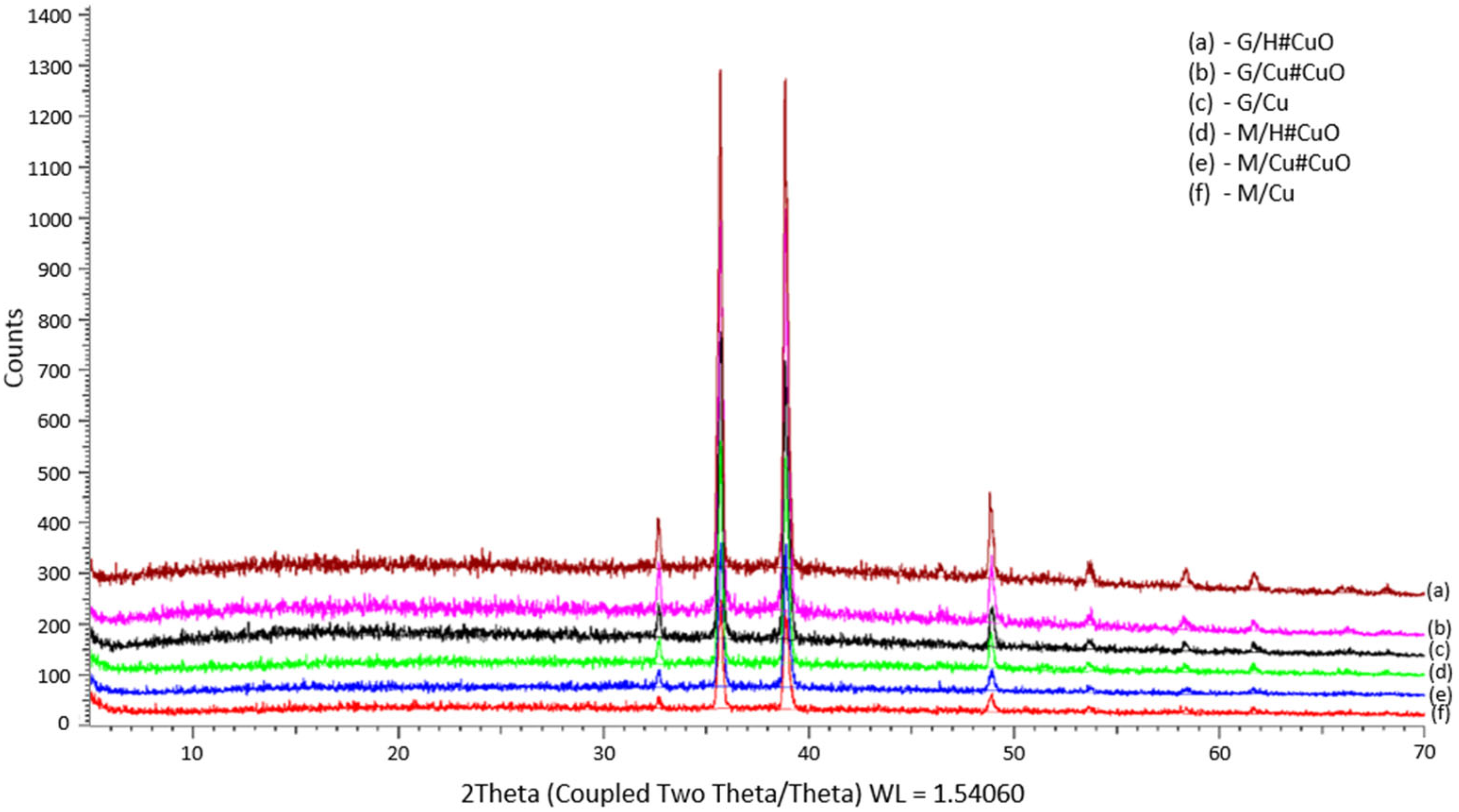
2.4. X-ray Powder Diffraction Analysis
3. Results and Discussion
3.1. Hybrid Polymer Formation
3.2. Thermal Analysis in Air
3.3. Thermal Analysis in Nitrogen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.; Meng, X.; Fang, S.; Cao, H.; Jv, W.; Zheng, X.; Liu, C.; Chen, M.; Sun, Z. MnO2 functionalized amorphous carbon sorbents from spent lithium-ion batteries for highly efficient removal of cadmium from aqueous solutions. Ind. Eng. Chem. Res. 2020, 59, 10210–10220. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Hui, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Ghosh, B.K.; Hazra, S.; Naik, B.; Nath Ghosh, N.N. Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 2015, 269, 371–378. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Ociński, D. Optimization of hybrid polymer preparation by ex situ embedding of waste Fe/Mn oxides into chitosan matrix as an effective As (III) and As(V) sorbent. Environ. Sci. Pollut. Res. 2019, 26, 26026–26038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, D.; Venkatanarasimhan, S. Copper (II) oxide nanoparticles coated cellulose sponge—An effective heterogeneous catalyst for the reduction of toxic organic dyes. Environ. Sci. Pollut. Res. 2019, 26, 22958–22970. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Hu, Z.; Wang, Z.; Yang, X.; Xie, X.; Zhao, J. Effective removal of carbamazepine and diclofenac by CuO/Cu2O/Cu-biochar composite with different adsorption mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 45435–45446. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Environmentally benign production of cupric oxide nanoparticles and various utilization of their polymeric hybrids in different technologies. Coordin. Chem. Rev. 2020, 419, 213378. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coordin. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- SenGupta, A.K. Ion Exchange in Environmental Processes: Fundamentals, Applications and Sustainable Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 345–390. [Google Scholar]
- Hua, M.; Yang, B.; Shan, C.; Zhang, W.; He, S.; Lv, L.; Pan, B. Simultaneous removal of As(V) and Cr(VI) from water by macroporous anion exchanger supported nanoscale hydrous ferric oxide composite. Chemosphere 2017, 171, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Shuang, C.; Wang, Y.; Wang, J.; Su, Y.; Li, A. Effect of pore structure on the removal of clofibric acid my magnetic anion exchange resin. Chemosphere 2018, 191, 817–824. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Pan, S.; Zhang, X.; Zhang, W.; Pan, B. Utilization of gel-type polystyrene host for immobilization of nano-sized hydrate zirconium oxides: A new strategy for enhanced phosphate removal. Chemosphere 2021, 263, 127938. [Google Scholar] [CrossRef]
- Bui, T.H.; Hong, S.P.; Kim, C.; Yoon, J. Performance analysis of hydrated Zr (IV) oxide nanoparticle-impregnated anion exchange resin for selective phosphate removal. J. Colloid Interf. Sci. 2021, 586, 741–747. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Wilk, Ł.J.; Drabent, K.; Kociołek-Balawejder, E. Synthesis and characterization of hybrid materials containing iron oxide for removal of sulfides from water. J. Colloid. Interf. Sci. 2015, 460, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Wilk, Ł.J.; Ciechanowska, A.; Kociołek-Balawejder, E. Adsorptive-oxidative removal of sulfides from water by MnO2-loaded carboxylic cation exchangers. Materials 2020, 13, 5124. [Google Scholar] [CrossRef] [PubMed]
- Jacukowicz-Sobala, I.; Ociński, D.; Mazur, P.; Stanisławska, E.; Kociołek-Balawejder, E. Cu(II)-Fe(III) oxide doped anion exchangers—Multifunctional composites for arsenite removal from water via As(III) adsorption and oxidation. J. Hazard. Mater. 2020, 394, 122527. [Google Scholar] [CrossRef] [PubMed]
- Jacukowicz-Sobala, I.; Kociołek-Balawejder, E.; Stanisławska, E.; Dworniczek, E.; Seniuk, A. Antimicrobial activity of anion exchangers containing cupric compounds against Enterococcus faecalis. Colloid Surf. A 2019, 576, 103–109. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Zhang, Y.; Cai, J.; Zhang, W.; Pan, B. Arsenate adsorption by hydrous oxide nanoparticles embedded in cross-linked anion exchanger; effect of the host pore structure. ACS Appl. Mater. Interfaces 2016, 8, 3012–3020. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lee, T.-S. Oxidative pyrolysis of organic ion exchange resins in the presence of metal oxide catalysts. J. Hazard. Mater. 2002, 92, 301–314. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Gao, X. The co-combustion and pollutant emission characteristics of the three kinds of waste ion exchange resins and coal. Int. J. Chem. React. Eng. 2020, 18, 20200053. [Google Scholar] [CrossRef]
- Kinoshita, K.; Hirata, M.; Yahata, T. Treatment of ion-exchange resins by fluidized bed incinerator equipped with copper oxide catalyst. J. Nucl. Sci. Technol. 1991, 28, 228–238. [Google Scholar] [CrossRef]
- Chun, U.-K.; Choi, K.; Yang, K.-H.; Park, J.-K.; Song, M.-J. Waste minimization pretreatment via pyrolysis and oxidative pyrolysis of organic ion exchange resin. Waste Manage. 1998, 18, 183–196. [Google Scholar] [CrossRef]
- Wojtaszek, M.; Wasielewski, R. The use of spent ion exchange resins as components of the coal charge for the production of metallurgical coke. Fuel 2021, 286, 119249. [Google Scholar] [CrossRef]
- Luca, V.; Bianchi, H.L.; Allevatto, F.; Vaccaro, J.O.; Alvarado, A. Low temperature pyrolysis of simulated spent anion exchange resins. J. Environ. Chem. Eng. 2017, 5, 4165–4172. [Google Scholar] [CrossRef]
- Scheithauer, D.; Heschel, W.; Meyer, B.; Krzack, S. Pyrolysis of undoped and multi-element doped ion exchange resins with regard to storage properties. J. Anal. Appl. Pyrol. 2017, 124, 276–284. [Google Scholar] [CrossRef]
- You, Y.-W.; Moon, E.-H.; Heo, I.; Park, H.; Hong, J.-S.; Suh, J.-K. Preparation and characterization of porous carbons from ion-exchange resins with different degree of cross-linking for hydrogen storage. J. Ind. Eng. Chem. 2017, 45, 164–170. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Q.; Duan, W.; Zuo, Z.; Huo, L.; Dai, J. CO2 adsorption and desorption performance of waste ion-exchange resin-based activated carbon. Environ. Prog. Sust. Ener. 2018, 37, 703–711. [Google Scholar] [CrossRef]
- Matsumura, T.; Takagi, H.; Tanaike, O.; Sakane, H.; Miyajima, N. Iodine-assisted control of the pore and morphology in the porous carbons prepared by the carbonization of ion-exchange resins. Micropor. Mesopor. Mater. 2019, 282, 237–242. [Google Scholar] [CrossRef]
- He, P.; Haw, K.-G.; Yan, S.; Tang, L.; Fang, Q.; Qiu, S.; Valtchev, V. Carbon beads with a well-defined pore structure derived from ion-exchange resin beads. J. Mater. Chem. A 2019, 32, 18285–18294. [Google Scholar] [CrossRef]
- Oh, J.-Y.; You, Y.-W.; Park, J.; Hong, J.-S.; Heo, I.; Lee, C.-H.; Suh, J.-K. Adsorption characteristic of benzene on resin-based activated carbon under humid conditions. J. Ind. Eng. Chem. 2019, 71, 242–249. [Google Scholar] [CrossRef]
- Eun, H.C.; Yang, H.C.; Cho, Y.Z.; Lee, H.S. Study of stable destruction method of radioactive waste ion exchanges. J. Radioanal. Nucl. Chem. 2009, 281, 585–590. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog. Nucl. Ener. 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef]
- Choi, W.N.; Lee, U.; Kim, H.R. Radiological assessment on spent resin treatment facility and transportation for radioactive waste disposal. Prog. Nucl. Ener. 2020, 118, 103125. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Baszczuk, A.; Jasiorski, M. Deposition of spherical and bracelet-like Cu2O nanoparticles within the matrix of anion exchanges via reduction of tetrachlorocuprate anions. J. Environ. Chem. Eng. 2020, 8, 103722. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Stanisławska, E.; Baszczuk, A.; Jasiorski, M.; Kociołek-Balawejder, E. Size-controlled transformation of Cu2O into zero valent copper within the matrix of anion exchangers via green chemical reduction. Polymers 2020, 12, 2629. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, E.; Jasiorski, M. Anomalous effect of Cu2O and CuO deposit on the porosity of a macromolecular anion exchanger. J. Nanopart. Res. 2021, 23, 126. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Effect of the kind of cupric compounds deposit on thermal decomposition of anion exchangers. Thermochimica Acta 2021, 695, 178812. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Weakly hydrated anion exchangers doped with Cu2O and Cu0 particles- thermogravimetric studies. Materials 2021, 14, 925. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Pelpin, F.X.; Aselfa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Shi, R.; Quan, J.; Liu, J.; Wang, J.; Pei, Y.; Wang, X.; Li, Z.; Ren, J. Highly efficient synthesis of dimethyl carbonate over copper catalysts supported on resin-derived carbon microspheres. Chem. Eng. Sci. 2019, 207, 1060–1071. [Google Scholar] [CrossRef]
- Deka, P.; Borah, J.B.; Saikia, H.; Bharali, P. Cu-based nanoparticles as emerging environmental catalysis. Chem. Rec. 2019, 19, 462–473. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I. Synthesis and characterization of CuO-loaded macroreticular anion exchange hybrid polymer. React. Funct. Polym. 2016, 100, 107–115. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Ociński, D. CuO-loaded macroreticular anion exchange hybrid polymers obtained via tetrachlorocuprate(II) ionic form. Int. J. Polym. Sci. 2017, 2017, 4574397. [Google Scholar] [CrossRef] [Green Version]
- Kociołek-Balawejder, E.; Stanisławska, E.; Ociński, D.; Winiarska, K. CuO and Cu2(OH)3Cl loaded gel-type anion exchange hybrid polymers obtained via tetrachlorocuprate ionic form. J. Environ. Chem. Eng. 2017, 5, 5668–5676. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, T. A novel selective hybrid cation exchanger for low-concentration ammonia nitrogen removal from natural water and secondary wasterwater. Chem. Eng. J. 2015, 281, 295–302. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Chayka, M.Y.; Konev, D.V.; Polyanskiy, L.N.; Krysanov, V.A. The influence of the ion-exchange groups nature and the degree of chemical activation by silver on the process of copper electrodeposition into the ion exchanger. Electrochimica Acta 2007, 53, 330–336. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Sakardina, E.A.; Kalinichev, A.I.; Zolotukhina, E.V. Stabilization of copper nanoparticles with volume- and surface-distribution inside ion-exchange matrices. Russ. J. Phys. Chem. 2015, 89, 1648–1654. [Google Scholar] [CrossRef]
- Cavaco, S.A.; Fernandes, S.; Quina, M.M.; Ferreira, L.M. Removal of chromium from electroplating industry effluents by ion exchange resins. J. Hazard. Mater. 2007, 144, 634–638. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ogden, M.D.; Moon, E.M.; Soldenhoff, K.L. REE behavior and sorption on weak acid resins from buffered media. J. Ind. Eng. Chem. 2018, 59, 440–455. [Google Scholar] [CrossRef]
- Choi, J.-W.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Sequential recovery of gold and copper from bioleached wastewater using ion exchange resins. Environ. Pollut. 2020, 266, 115167. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; He, H.; Zhang, J.; Mao, Z. Effect of ion form of the ion-exchange resin on ϵ-poly-l-lysine purification from microbial fermentation broth. RSC Adv. 2019, 9, 12174–12188. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, H.; Xia, Z.; Zhao, X.; Wu, Y.; An, M. Purification and structural analysis of the effective anti-TMV compound ε-poly-L-lysine produced by Streptomyces ahygroscopicus. Molecules 2019, 24, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chugunov, A.S.; Nechaev, A.F. Potential use of carboxyl ion exchangers for operational optimization of special water purification systems in NPP with VVER. Atom. Energy 2015, 118, 224–228. [Google Scholar] [CrossRef]
- Vinnitskii, V.A.; Chugunov, A.S.; Nechaev, A.F. Prospects for using weakly dissociated ion exchange resins in special water treatment systems at VVER-based nuclear power plants for reducing the volume of radioactive waste generated. Therm. Eng. 2018, 65, 212–216. [Google Scholar] [CrossRef]
- Dorfner, K. Synthetic ion exchange resins. In Ion Exchangers; Dorfner, K., Ed.; Walter de Gruyter: Berlin, Germany, 1991; p. 222. [Google Scholar]
- Dubois, M.A.; Dozol, J.F.; Nicotra, C.; Sereze, J.; Massiani, C. Pyrolysis and incineration of cationic and anionic ion-exchange resins—Identification of volatile degradation compounds. J. Anal. Appl. Pyrol. 1995, 31, 129–140. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Freeze dried and thermally dried anion exchanger doped with iron(III) (hydr)oxide—Thermogravimetric studies. Thermochimica Acta 2019, 680, 178359. [Google Scholar] [CrossRef]
- Idiţoiu, C.; Segal, E.; Chambree, D. Kinetics of non-isothermal behavior of synthetic cationites with low acidity. J. Therm. Anal. Calorim. 1999, 56, 407–417. [Google Scholar] [CrossRef]
- Chambree, D.; Idiţoiu, C.; Segal, E.; Cesario, A. The study of non-isothermal degradation of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2005, 82, 803–811. [Google Scholar] [CrossRef]
- Chambree, D.; Idiţoiu, C.; Segal, E. Non-isothermal dehydration kinetics of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2007, 88, 673–679. [Google Scholar] [CrossRef]
- Bogoczek, R.; Pińkowska, H. Covalent reactions on carboxylic cation exchangers poly(acrylic acid–dvb/esters). React. Funct. Polym. 2003, 54, 117–130. [Google Scholar] [CrossRef]
- Bogoczek, R.; Pińkowska, H. Chemical modification of acrylic acid and divinylbenzene copolymers. In Synthesis of polymeric acrylic anhydrides. Part I; Lviv Polytechnic National University: Lviv, Ukraine, 2000; pp. 42–57. [Google Scholar]
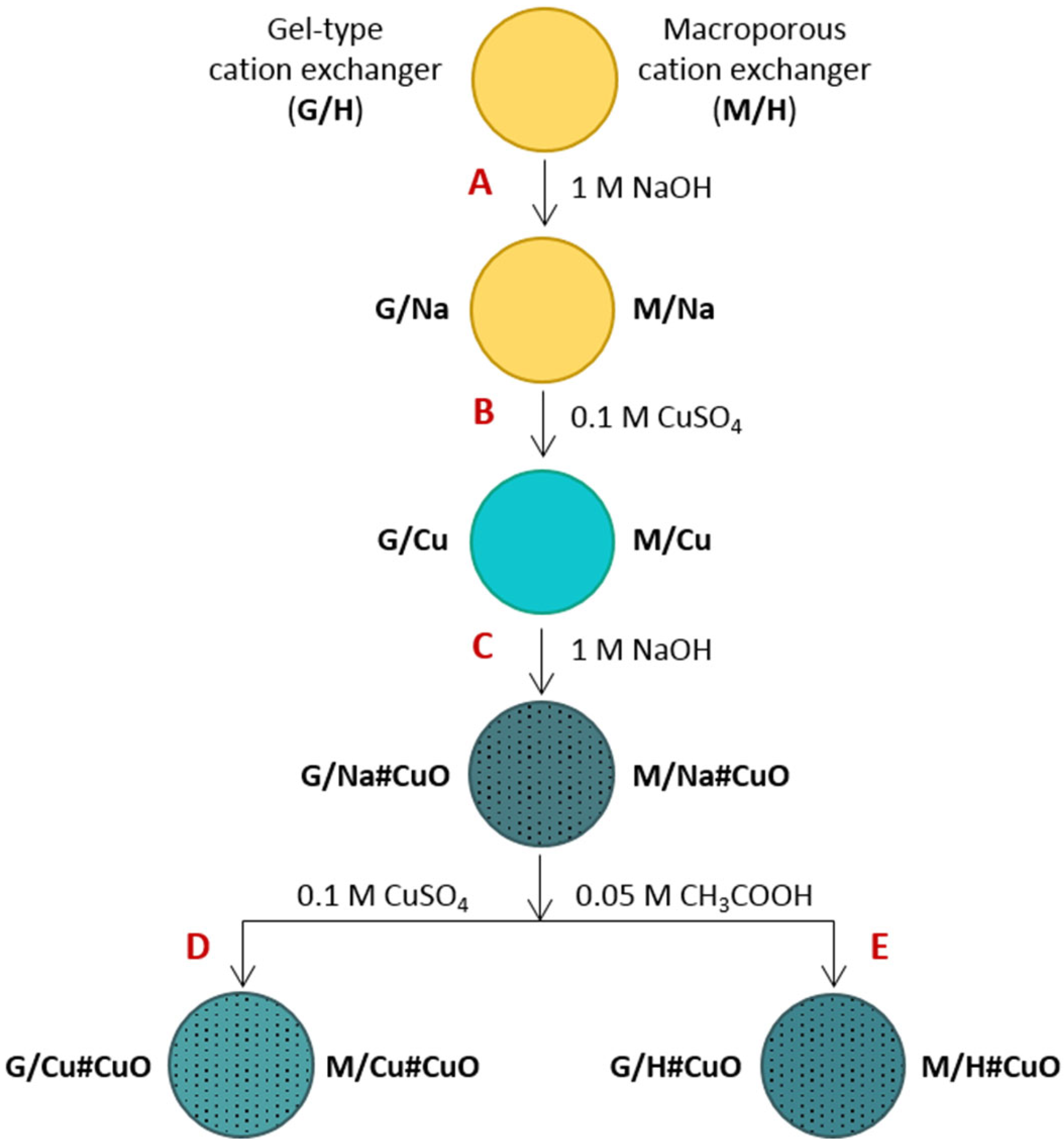






| Properties | Amberlite IRC 50 | Amberlite IRC 86 |
|---|---|---|
| Appearance | Beige opaque spherical beads | Clear amber translucent spherical beads |
| Particle size, mesh | 16–50 | 20–50 |
| Matrix type | Methacrylic acid–DVB | Acrylic acid–DVB |
| Matrix structure | Macroporous (M) | Gel-like (G) |
| Functional groups | Carboxylic in H+ form (M/H, G/H) | |
| Total exchange capacity by dry weight, meq/g | 10.5 | 10.7 |
| Total reversible swelling,% | H+ → Na+, 100 | H+ → Na+, 80 |
| Stage | Substrate | Product | Mass Growth, % | Cu Content in Sample | Colour of the Product | |||
|---|---|---|---|---|---|---|---|---|
| Code | Mass, g | Code | Mass, g | meq/g | mg/g | |||
| Macroreticular cation exchanger as polymeric support | ||||||||
| A, B | M/H | 9.36 | M/Cu | 13.54 | +44.5 | 7.10 | 225.6 | Light blue |
| C | M/Cu | 10.01 | M/Na#CuO | 11.85 | +18.2 | 5.07 | 161.0 | Dark grey |
| D | M/Na#CuO | 4.08 | M/Cu#CuO | 4.26 | +4.4 | 10.30 | 327.3 | Grey blue |
| E | M/Na#CuO | 4.03 | M/H#CuO | 3.10 | -23.1 | 6.15 | 195.4 | Grey |
| Gel-like cation exchanger as polymeric support | ||||||||
| A, B | G/H | 9.42 | G/Cu | 13.71 | +45.5 | 7.21 | 228.9 | Dark green |
| C | G/Cu | 10.35 | G/Na#CuO | 12.52 | +20.1 | 5.76 | 182.9 | Black |
| D | G/Na#CuO | 4.31 | G/Cu#CuO | 4.49 | +4.17 | 11.16 | 354.4 | Black |
| E | G/Na#CuO | 4.17 | G/H#CuO | 3.22 | −22.8 | 7.35 | 233.3 | Black |
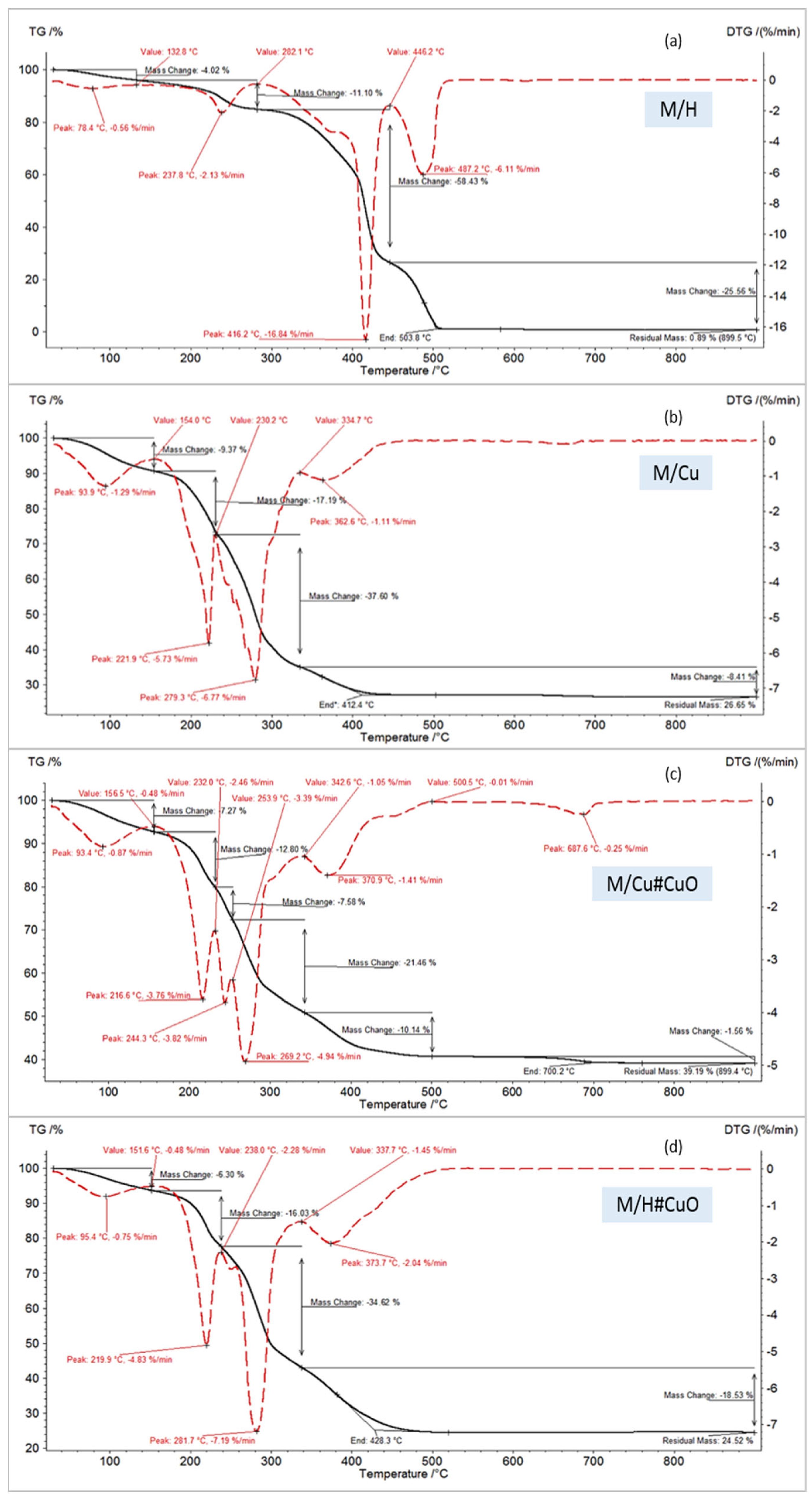
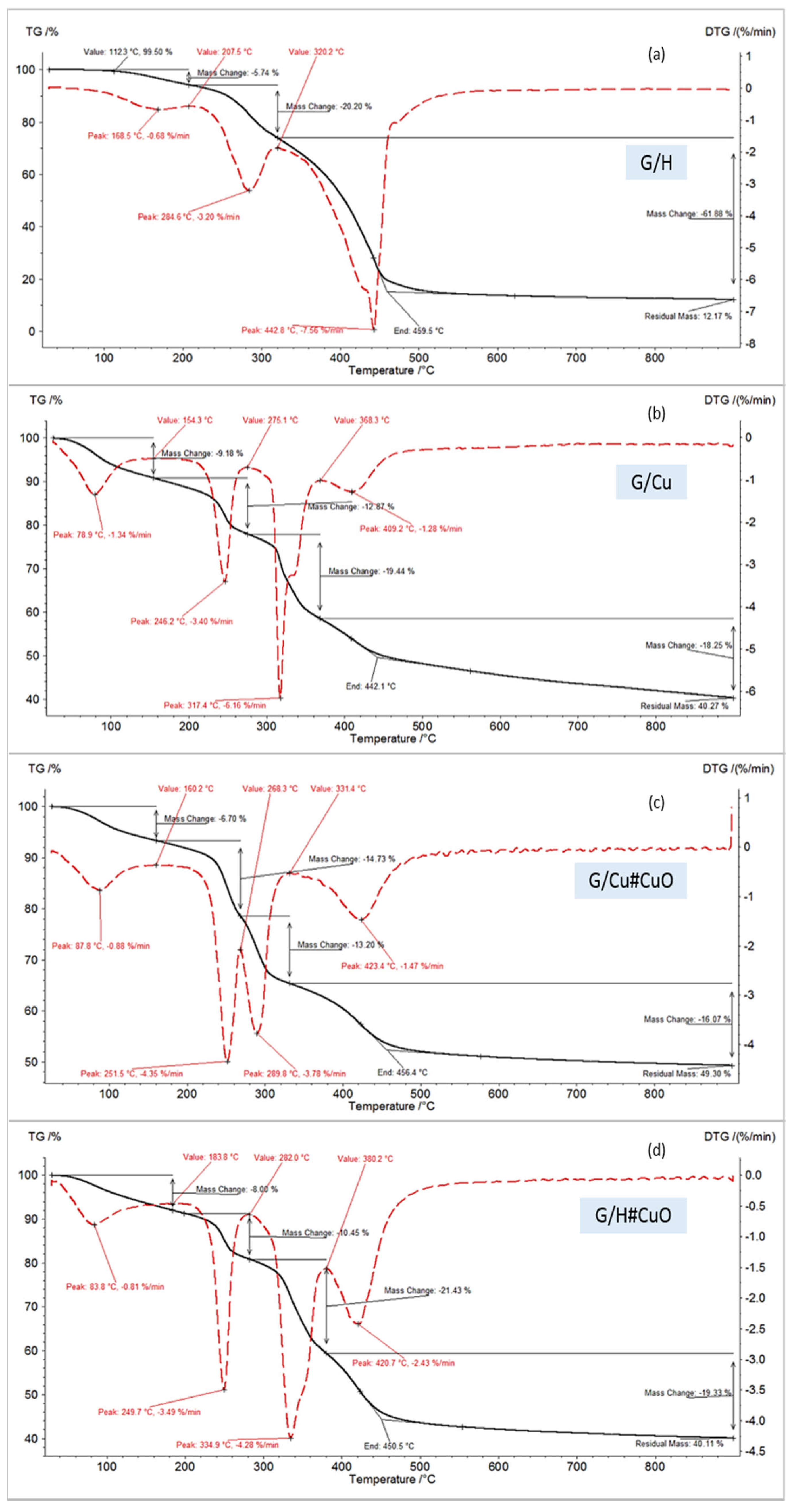
| Code | Water Mass Loss, % | Peak Temperature of Polymer Degradation, °C | End Temperature, °C | Residual Mass, % |
|---|---|---|---|---|
| decomposition in air | ||||
| M/H | 4.02 | 237.8, 416.2, 487.2 | 503.8 | 0.89 |
| M/Cu | 9.37 | 221.9, 279.3, 362.6 | 412.4 | 26.65 |
| M/Cu#CuO | 7.27 | 216.6, 269.2 370.9 | 420.0 | 39.19 |
| M/H#CuO | 6.30 | 219.9, 281.7, 373.7 | 428.3 | 24.52 |
| G/H | 6.04 | 285.0, 427.1, 527.6 | 551.7 | 0 |
| G/Cu | 10.22 | 246.4, 313.9, 381.7 | 464.5 | 29.86 |
| G/Cu#CuO | 7.55 | 251.7, 276.4, 380.6 | 427.7 | 46.31 |
| G/H#CuO | 9.26 | 250.3, 318.5, 391.6 | 440.1 | 30.13 |
| decomposition in N2 | ||||
| M/H | 4.73 | 238.8, 429.5 | 440.4 | 6.10 |
| M/Cu | 9.28 | 203.5, 302.2, 369.3 | 446.2 | 27.43 |
| M/Cu#CuO | 7.14 | 213.4, 251.3, 290.7, 392.2 | 448.7 | 40.98 |
| M/H#CuO | 6.27 | 220.7. 296.2, 403.5 | 434.0 | 27.67 |
| G/H | 5.74 | 284.6, 442.8 | 459.5 | 12.17 |
| G/Cu | 9.18 | 246.2, 317.4, 409.2 | 442.1 | 40.27 |
| G/Cu#CuO | 6.70 | 251.5, 289.8, 423.4 | 456.4 | 49.30 |
| G/H#CuO | 8.08 | 249.7, 334.9, 420.7 | 450.5 | 40.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Mucha, I. Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation. Polymers 2021, 13, 3199. https://doi.org/10.3390/polym13183199
Kociołek-Balawejder E, Stanisławska E, Jacukowicz-Sobala I, Mucha I. Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation. Polymers. 2021; 13(18):3199. https://doi.org/10.3390/polym13183199
Chicago/Turabian StyleKociołek-Balawejder, Elżbieta, Ewa Stanisławska, Irena Jacukowicz-Sobala, and Igor Mucha. 2021. "Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation" Polymers 13, no. 18: 3199. https://doi.org/10.3390/polym13183199
APA StyleKociołek-Balawejder, E., Stanisławska, E., Jacukowicz-Sobala, I., & Mucha, I. (2021). Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation. Polymers, 13(18), 3199. https://doi.org/10.3390/polym13183199







