A Dyciandiamine-Based Methacrylate-Epoxy Dual-Cure Blend-System for Stereolithography
Abstract
:1. Introduction
2. Materials and Methods
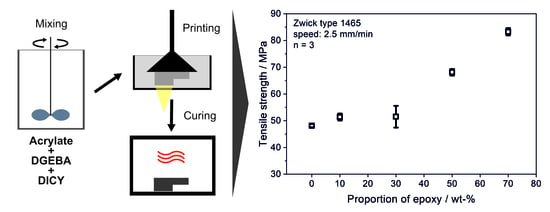
2.1. Material
2.2. Printing Process and Thermal Post-Curing
2.3. Methods
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Tensile Testing
2.3.4. Dynamic Mechanical Testing (DMA)
2.3.5. Microscopy
3. Results and Discussion
3.1. DSC
3.2. Tensile Testing
3.3. Microscopy
3.4. Temperature-Dependent Stability
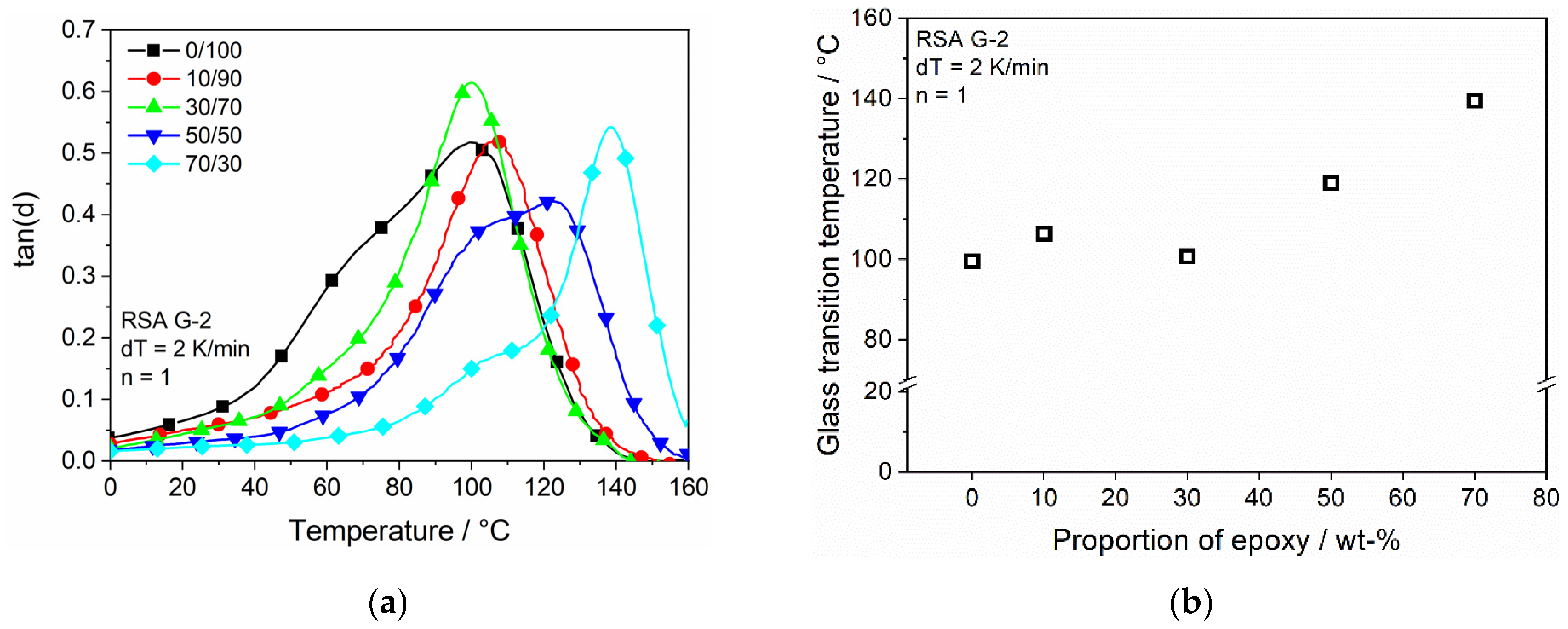
3.4.1. Dynamical Mechanical Testing
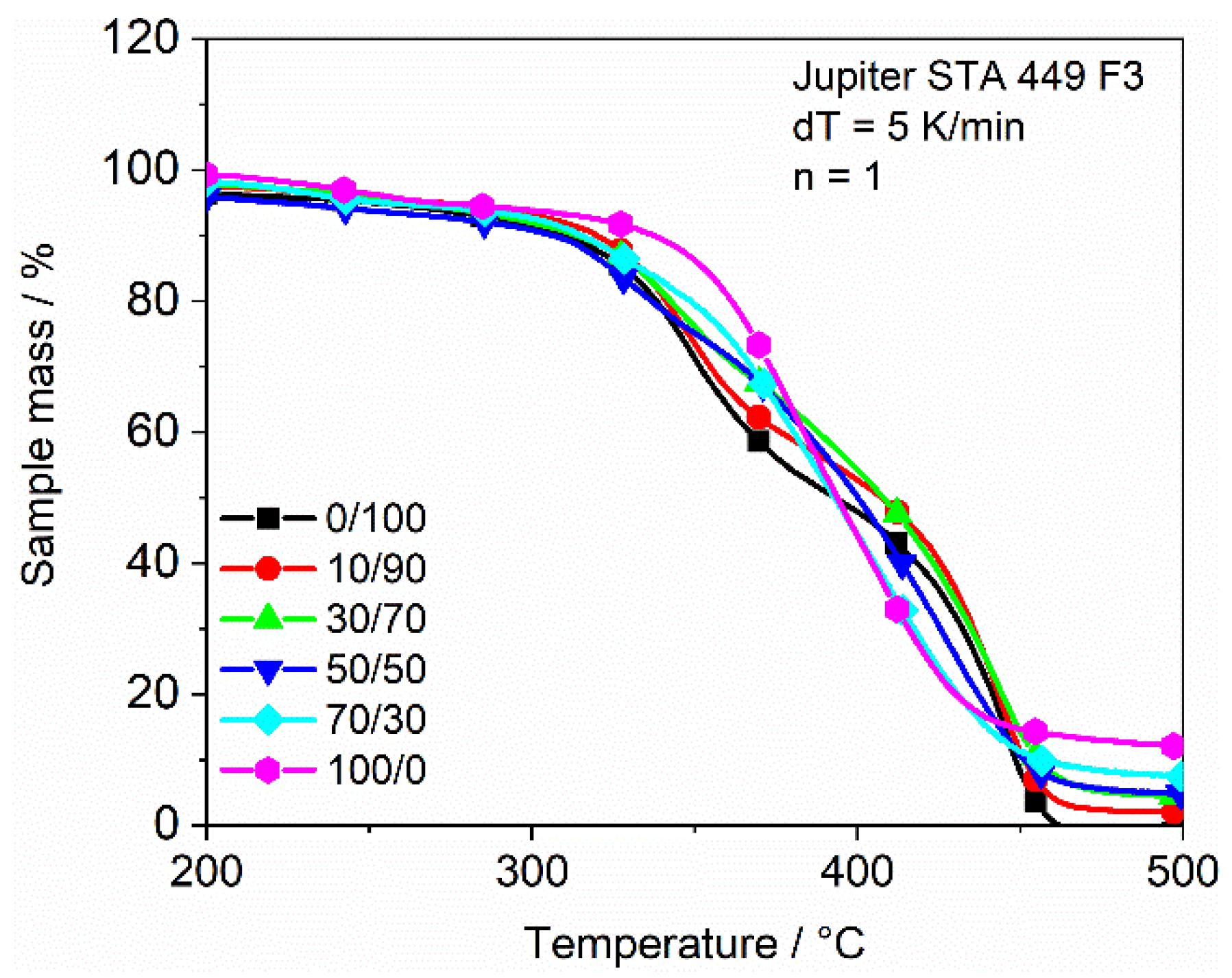
3.4.2. Thermographic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hull, C. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330A, 11 March 1986. [Google Scholar]
- Gebhardt, A.; Kessler, J.; Thurn, L. 3D Printing, 2nd ed.; Carl Hanser Verlag: Munich, Germany, 2019. [Google Scholar]
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Dorfinger, P.; Stampfl, J.; Liska, R. Toughening of photopolymers for stereolithography (SL). Mater. Sci. Forum 2015, 825, 53–59. [Google Scholar] [CrossRef]
- Bentivoglio Ruiz, C.S.; Machado, L.D.B.; Volponi, J.E.; Segura Pino, E. Oxygen inhibition and coating thickness effects on uv radiation curing of weatherfast clearcoats studied by Photo-DSC. J. Therm. Anal. Calorim. 2004, 75, 507–512. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Patrocinio, D.; Laza, J.M.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Evaluation of postcuring process on the thermal and mechanical properties of the Clear02™ resin used in stereolithography. Polym. Test. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Ravve, A. Light-Associated Reactions of Synthetic Polymers, 1st ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Edeleva, M.; Marien, Y.W.; Van Steenberge, P.H.M.; D’Hooge, D.R. Jacket temperature regulation allowing well-defined non-adiabatic lab-scale solution free radical polymerization of acrylates. React. Chem. Eng. 2021, 6, 1053–1069. [Google Scholar] [CrossRef]
- White, S.R.; Kim, Y.K. Staged curing of composite materials. Compos. Part A Appl. Sci. Manuf. 1996, 27, 219–227. [Google Scholar] [CrossRef]
- Waenawae, W.; Pumkrachang, S.; bin Zainudin, S.; Vongsetskul, T.; Osotchan, T. Curing mechanism study for dual cure of epoxy adhesive by differential scanning calorimetry. Mater. Sci. Forum 2016, 864, 3–7. [Google Scholar] [CrossRef]
- Flosbach, C.; Fugier, R. Epoxy functional acrylic polymers for high performance coating applications. In Epoxy Polymers; Pascault, J.P., Williams, R.J.J., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2010; pp. 39–54. [Google Scholar]
- Konuray, O.; Fernandez-Francos, X.; Ramis, X.; Serra, A. State of the art in dual-curing acrylate systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-H.; Lee, S.-W.; Park, J.-W.; Kim, H.-J. Preparation and characterization of dual curable adhesives containing epoxy and acrylate functionalities. React. Funct. Polym. 2013, 73, 641–646. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Li, L.; Yan, B.; Bao, J.; Zhang, A. Acrylate-based photosensitive resin for stereolithographic three-dimensional printing. J. Appl. Polym. Sci. 2019, 136, 47487. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Yu, R.; Zhang, Y.; Yang, X.; Zhao, X.; Wang, L.; Huang, W. Silicone–epoxy-based hybrid photopolymers for 3D printing. Macromol. Chem. Phys. 2018, 219, 5101–5111. [Google Scholar] [CrossRef]
- Campbell, J.A.; Inglis, H.; Ng WeiLong, E.; McKinley, C.; Lewis, D.A. Morphology control in a dual-cure system for potential applications in additive manufacturing. Polymers 2019, 11, 420. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Li, Y.; Zheng, J.; Lu, M.; Wu, K. Modified epoxy acrylate resin for photocurable temporary protective coatings. Prog. Org. Coat. 2015, 89, 17–25. [Google Scholar] [CrossRef]
- Velankar, S.; Pazos, J.; Cooper, S.L. High-performance UV-curable urethane acrylates via deblocking chemistry. J. Appl. Polym. Sci. 1996, 62, 1361–1376. [Google Scholar] [CrossRef]
- Rolland, J.; Chen, K.; Poelma, J.; Goodrich, J.; Pinschmidt, R.; DeSimone, J.; Robertson, L. Polyurethane Resins Having Multiple Mechanisms of Hardening for Use in Producing Three-Dimensional Objects. U.S. Patent WO2015/200,201Al, 30 December 2015. [Google Scholar]
- Obst, P.; Riedelbauch, J.; Oehlmann, P.; Rietzel, D.; Launhardt, M.; Schmölzer, S.; Osswald, T.A.; Witt, G. Investigation of the influence of exposure time on the dual-curing reaction of RPU 70 during the DLS process and the resulting mechanical part properties. Addit. Manuf. 2020, 32, 101002. [Google Scholar] [CrossRef]
- Bachmann, J.; Gleis, E.; Fruhmann, G.; Riedelbauch, J.; Schmölzer, S.; Hinrichsen, O. Investigation of the temperature influence on the dual curing urethane-methacrylate resin Rigid Polyurethane 70 (RPU 70) in digital light synthesis (DLS). Addit. Manuf. 2021, 37, 101677. [Google Scholar] [CrossRef]
- Chen, K.; Kuang, X.; Li, V.; Kang, G.; Qi, H.J. Fabrication of tough epoxy with shape memory effects by UV-assisted direct-ink write printing. Soft Matter 2018, 14, 1879–1886. [Google Scholar] [CrossRef]
- Griffini, G.; Invernizzi, M.; Levi, M.; Natale, G.; Postiglione, G.; Turri, S. 3D-printable CFR polymer composites with dual-cure sequential IPNs. Polymers 2016, 91, 174–179. [Google Scholar] [CrossRef]
- Redmann, A.; Osswald, T.A. A model for modulus development of dual-cure resin systems. Polym. Eng. Sci. 2020, 61, 830–835. [Google Scholar] [CrossRef]
- Redmann, A.; Oehlmann, P.; Scheffler, T.; Kagermeier, L.; Osswald, T.A. Thermal curing kinetics optimization of epoxy resin in Digital Light Synthesis. Addit. Manuf. 2020, 32, 101018. [Google Scholar] [CrossRef]
- Kuang, X.; Zhao, Z.; Chen, K.; Fang, D.; Kang, G.; Qi, H.J. High-speed 3D printing of high-performance thermosetting polymers via two-stage curing. Macromol. Rapid Commun. 2018, 39, e1700809. [Google Scholar] [CrossRef] [PubMed]
- Lantean, S.; Roppolo, I.; Sangermano, M.; Pirri, C.; Chiappone, A. Development of new hybrid acrylic/epoxy DLP-3D printable materials. Inventions 2018, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Dornbusch, M.; Christ, U.; Rasing, R. Epoxy Resins; Vincentz Network: Hannover, Germany, 2016. [Google Scholar]
- Guo, Q. Structure, Properties, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 21. [Google Scholar]
- Wu, F.; Zhou, X.; Yu, X. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M. Mechanism and Kinetics of the Dicyandiamide Cure of Epoxy Resins; University of Massachusetts: Boston, MA, USA, 1988. [Google Scholar]
- Braun, H.; Yagci, Y.; Nuyken, O. Copolymerization of butyl vinyl ether and methyl methacrylate by combination of radical and radical promoted cationic mechanisms. Eur. Polym. J. 2002, 38, 151–156. [Google Scholar] [CrossRef]
- Bae, C.-J.; Ramachandran, A.; Chung, K.; Park, S. Ceramic stereolithography: Additive manufacturing for 3D complex ceramic structures. J. Korean Ceram. Soc. 2017, 54, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Nesvadba, P. Radical Polymerization in Industry. In Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons, Ltd.: New York, NY, USA, 2012. [Google Scholar]
- Ramos, J.A.; Pagani, N.; Riccardi, C.C.; Borrajo, J.; Goyanes, S.N.; Mondragon, I. Cure kinetics and shrinkage model for epoxy-amine systems. Polymers 2005, 46, 3323–3328. [Google Scholar] [CrossRef]
- d’Almeida, J.R.M.; de Menezes, G.W.; Monteiro, S.N. Ageing of the DGEBA/TETA epoxy system with off-stoichiometric compositions. Mater. Res. 2003, 6, 415–420. [Google Scholar] [CrossRef]

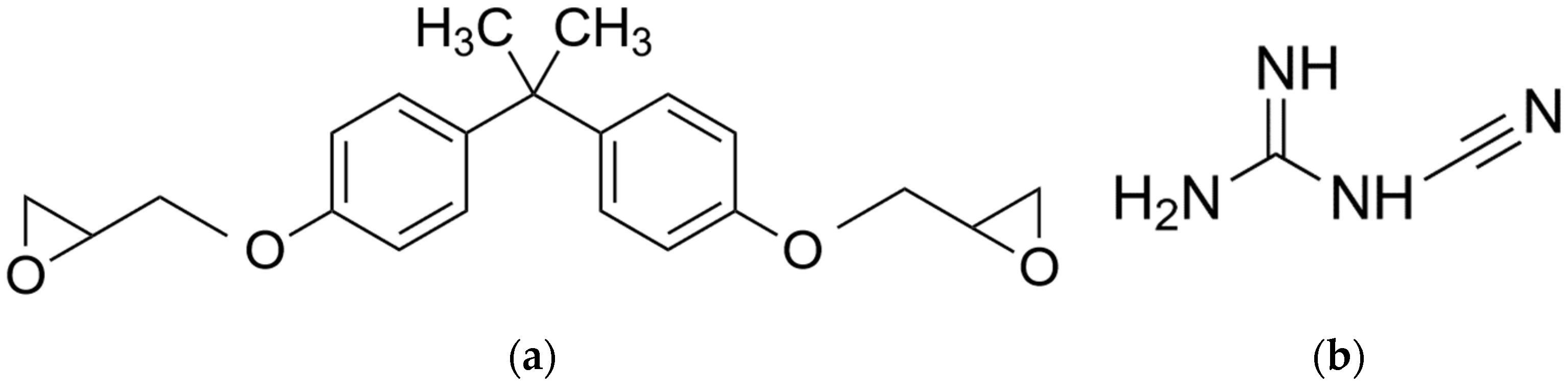

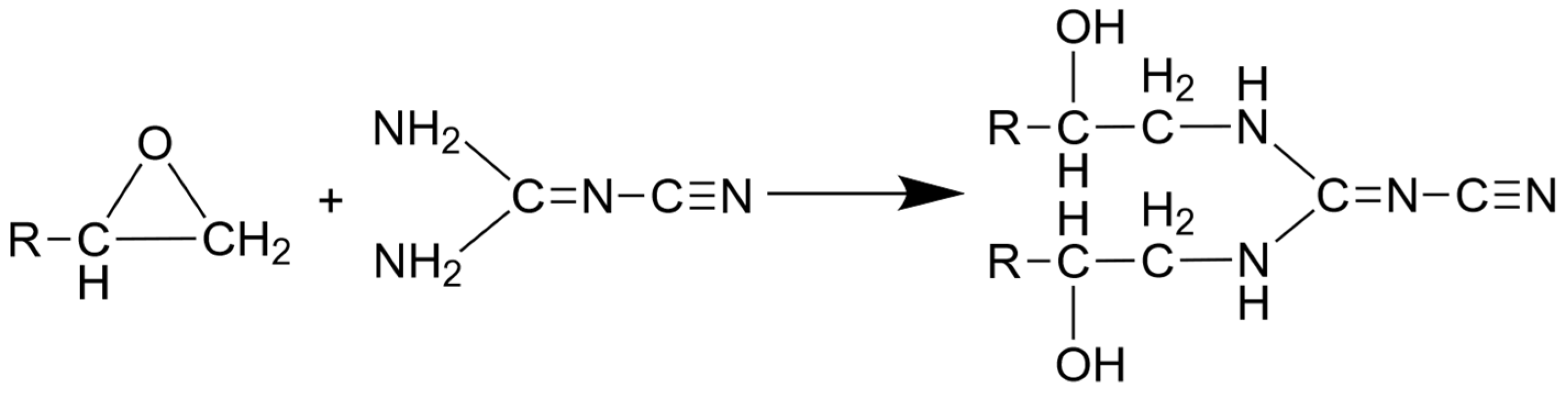
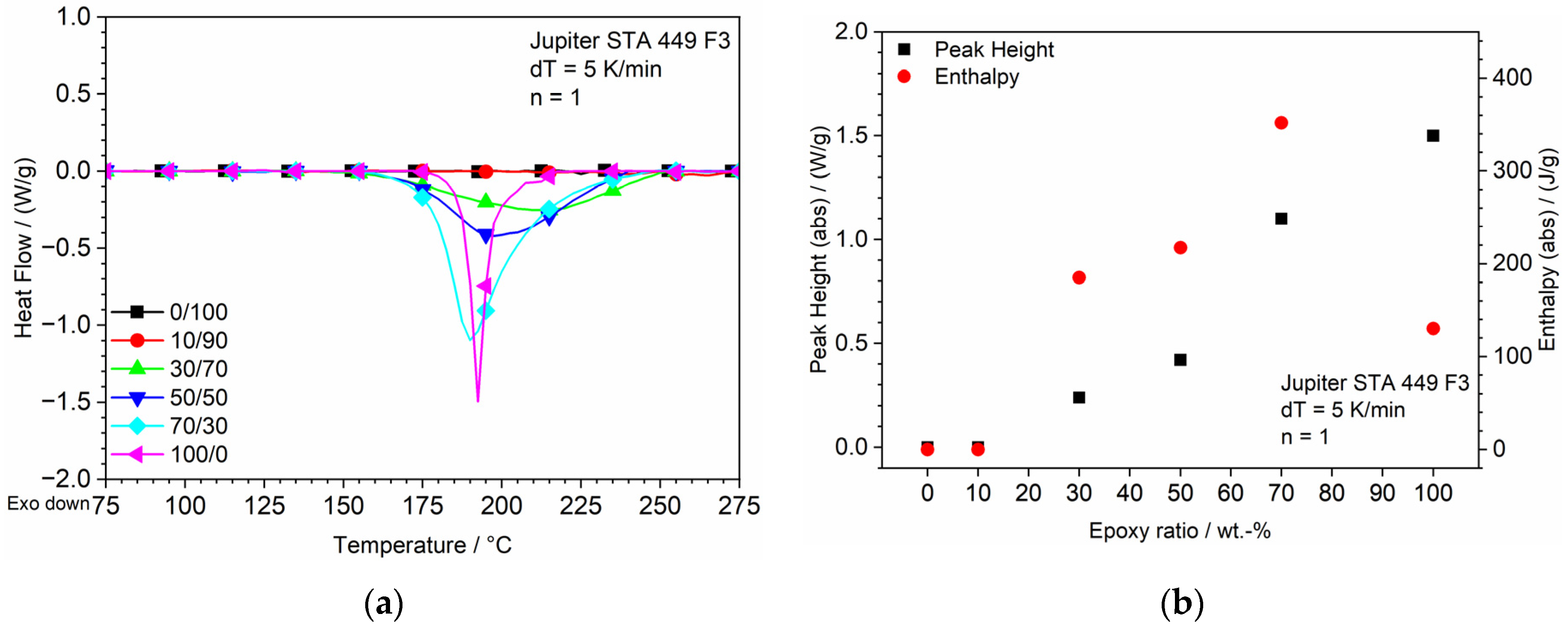



| Sample No. | Sample Label | DGEBA/DICY (Epoxy) (wt.-%) | Clear (Methacrylate) (wt.-%) |
|---|---|---|---|
| 1 | 0/100 | 0 | 100 |
| 2 | 10/90 | 10 | 90 |
| 3 | 30/70 | 30 | 70 |
| 4 | 50/50 | 50 | 50 |
| 5 | 70/30 | 70 | 30 |
| 6 * | 100/0 | 100 | 0 |
| Properties | Amount of Epoxy (wt-%) | ||||
|---|---|---|---|---|---|
| 0 | 10 | 30 | 50 | 70 | |
| Elastic modulus, ED (MPa) | 2050 ± 226 | 2770 ± 34 | 3020 ± 20 | 3217 ± 36 | 2947 ± 86 |
| Tensile strength, σmax (MPa) | 48.1 ± 1.3 | 51.4 ± 1.3 | 51.5 ± 4.0 | 68.1 ± 1.2 | 83.3 ± 1.4 |
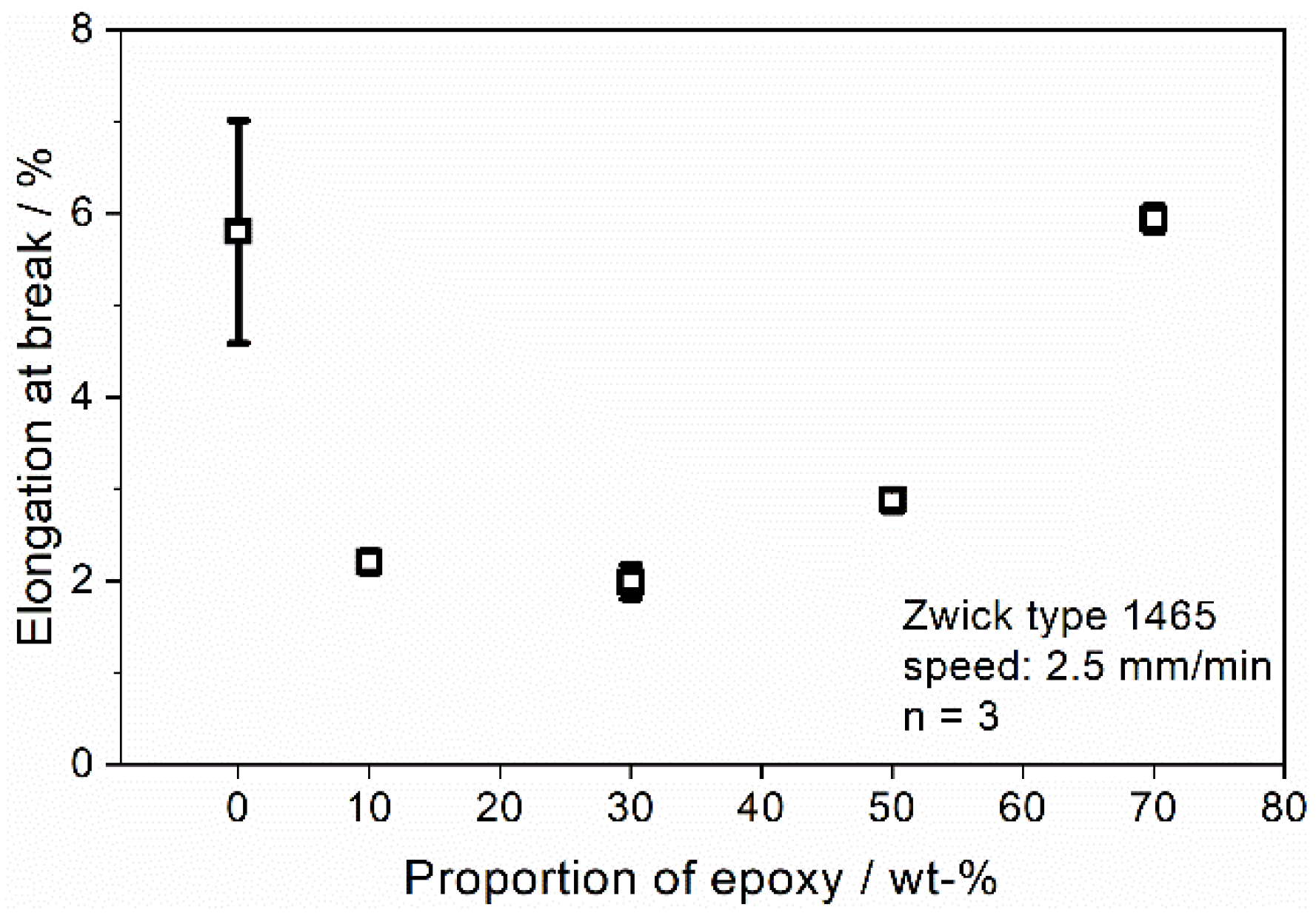
| Elongation at break, εr (%) | 5.8 ± 1.2 | 2.2 ± 0.1 | 2.0 ± 0.2 | 2.9 ± 0.1 | 5.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeis, M.; Drummer, D. A Dyciandiamine-Based Methacrylate-Epoxy Dual-Cure Blend-System for Stereolithography. Polymers 2021, 13, 3139. https://doi.org/10.3390/polym13183139
Romeis M, Drummer D. A Dyciandiamine-Based Methacrylate-Epoxy Dual-Cure Blend-System for Stereolithography. Polymers. 2021; 13(18):3139. https://doi.org/10.3390/polym13183139
Chicago/Turabian StyleRomeis, Manuel, and Dietmar Drummer. 2021. "A Dyciandiamine-Based Methacrylate-Epoxy Dual-Cure Blend-System for Stereolithography" Polymers 13, no. 18: 3139. https://doi.org/10.3390/polym13183139
APA StyleRomeis, M., & Drummer, D. (2021). A Dyciandiamine-Based Methacrylate-Epoxy Dual-Cure Blend-System for Stereolithography. Polymers, 13(18), 3139. https://doi.org/10.3390/polym13183139