Scaled Synthesis of Polyamine-Modified Cellulose Nanocrystals from Bulk Cotton and Their Use for Capturing Volatile Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Protocol for Synthesis of CNC from Bulk Cotton
2.2.1. One-Gram Scale
2.2.2. One-Hundred-Gram Scale
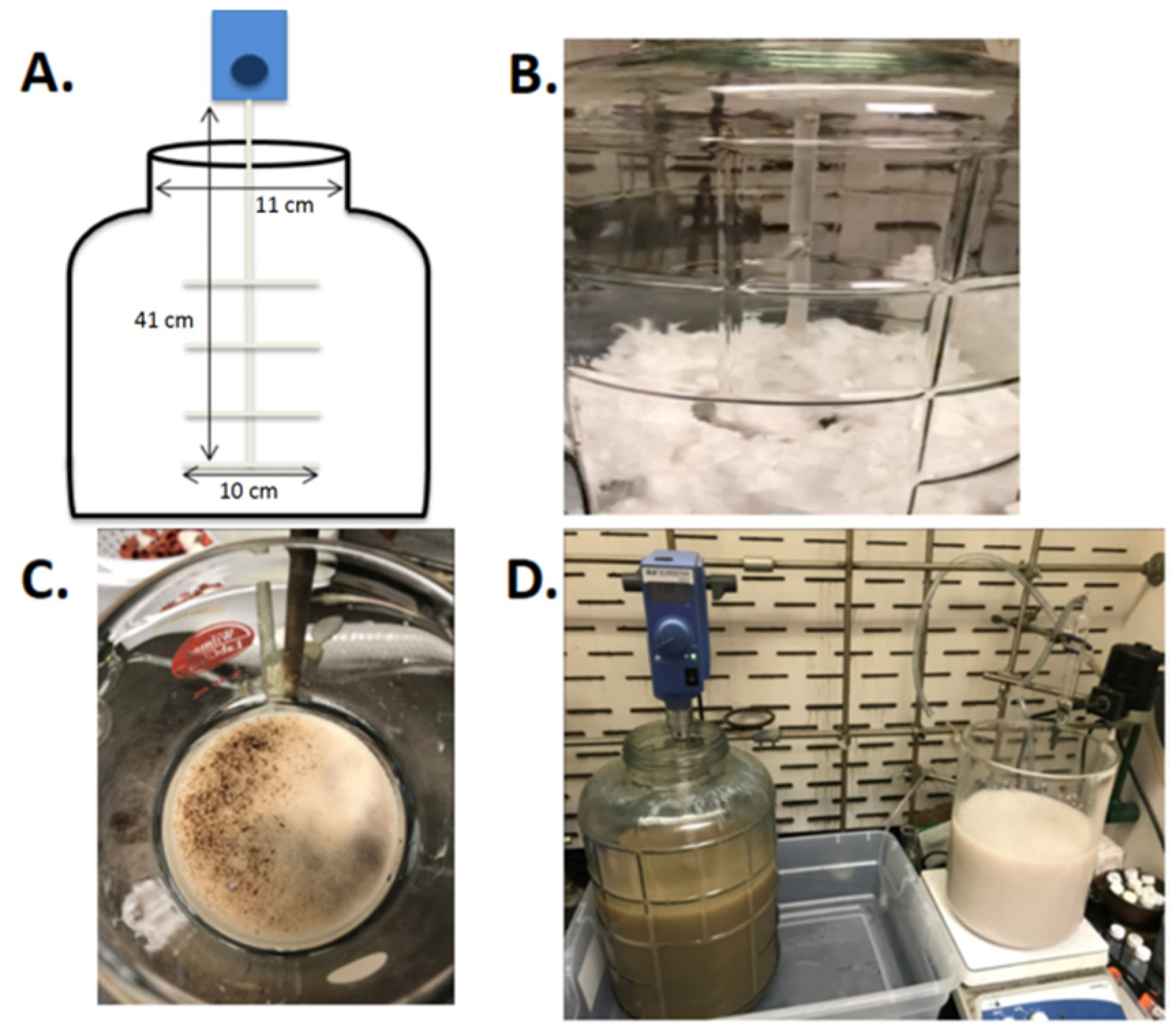
2.2.3. Five-Hundred-Gram Scale
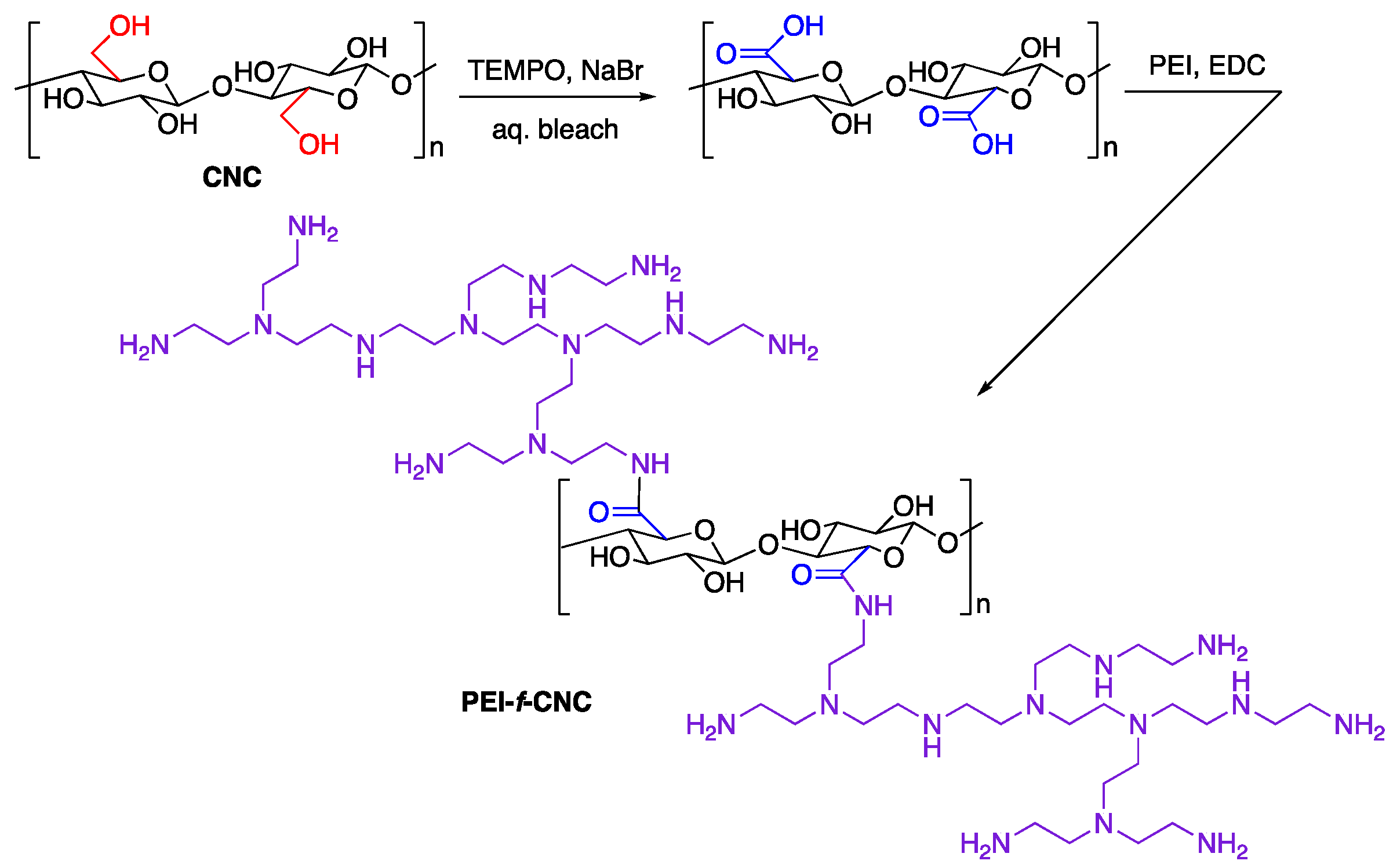
2.3. TEMPO-Mediated Oxidation of CNCs (100 g Scale)
2.4. PEI-Functionalization (100 g Scale)
2.5. Characterization of PEI-f-CNC Materials
2.5.1. Dynamic Light Scattering (DLS) Measurements
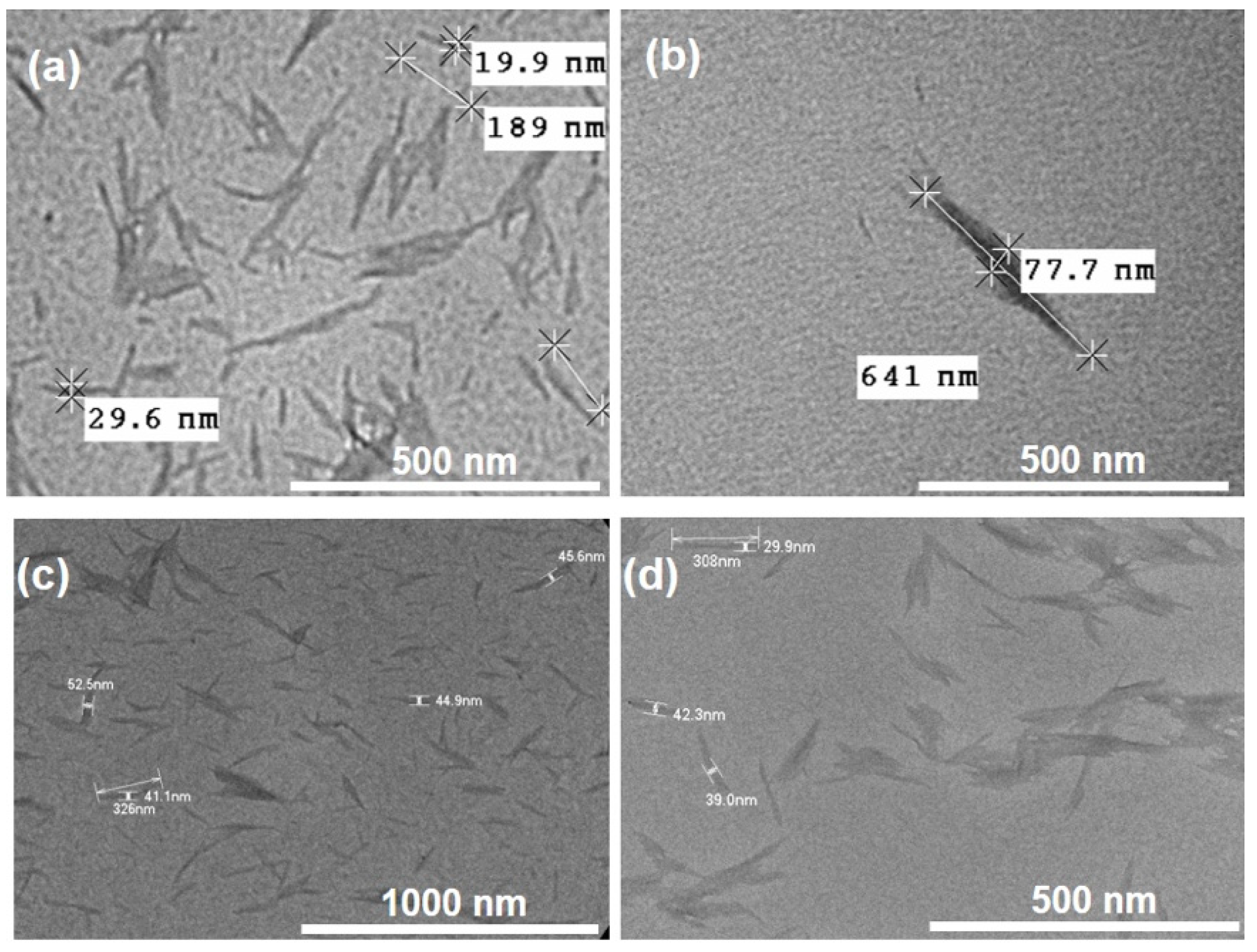
2.5.2. Transmission Electron Microscopy (TEM)
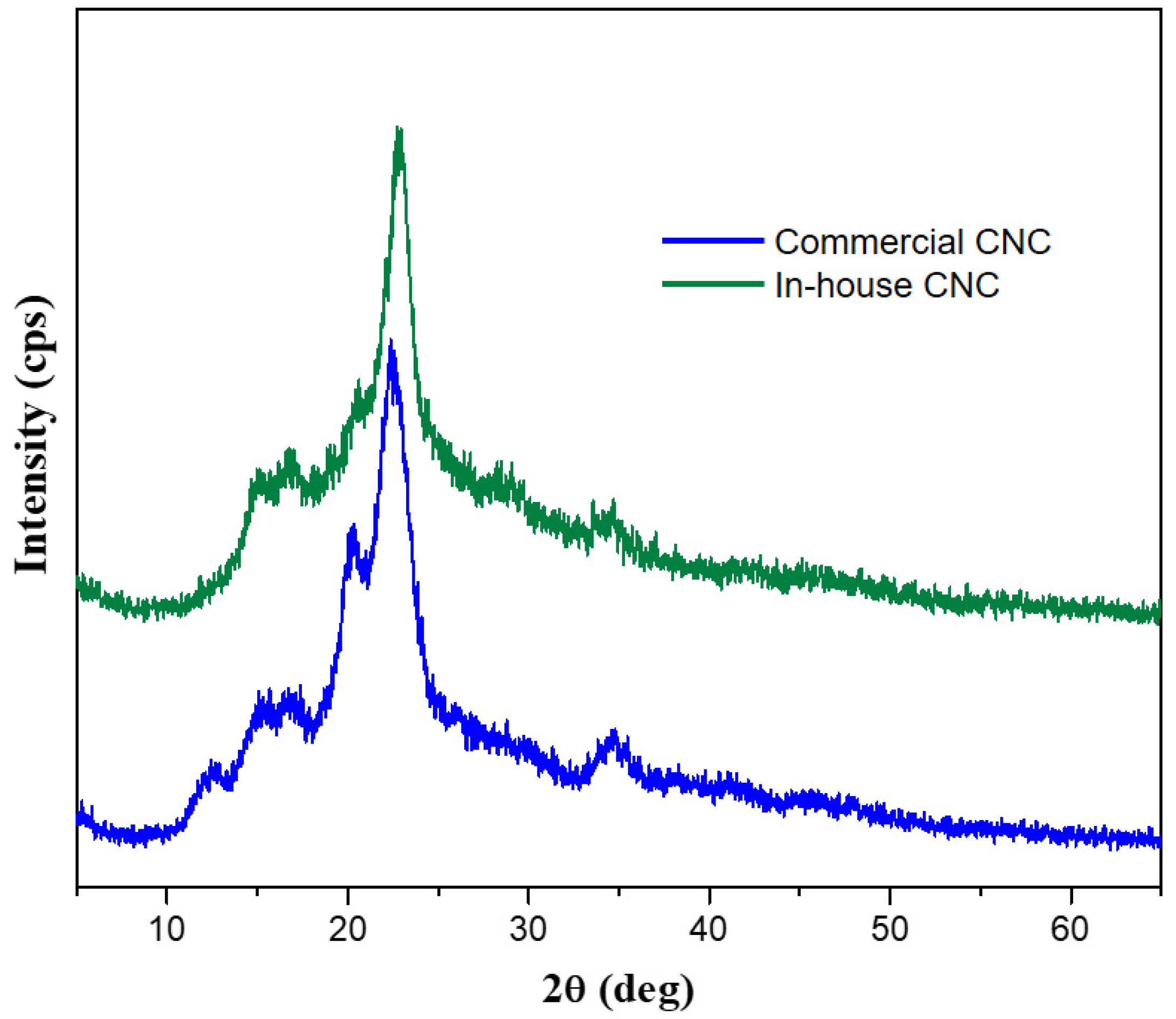
2.5.3. Powder X-ray Diffraction of Commercial and in-House CNC
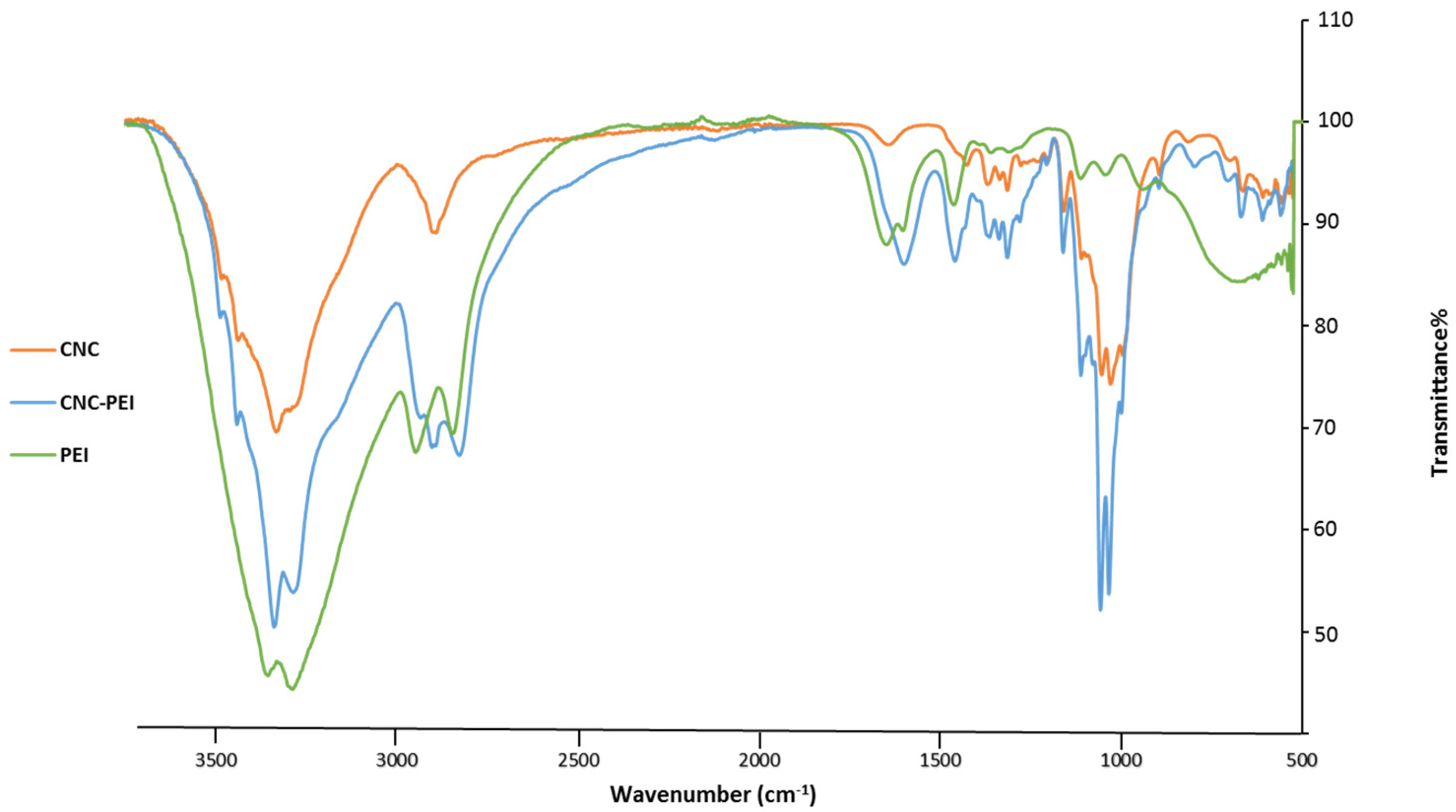
2.5.4. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5.5. Thermo Gravimetric Analysis (TGA)
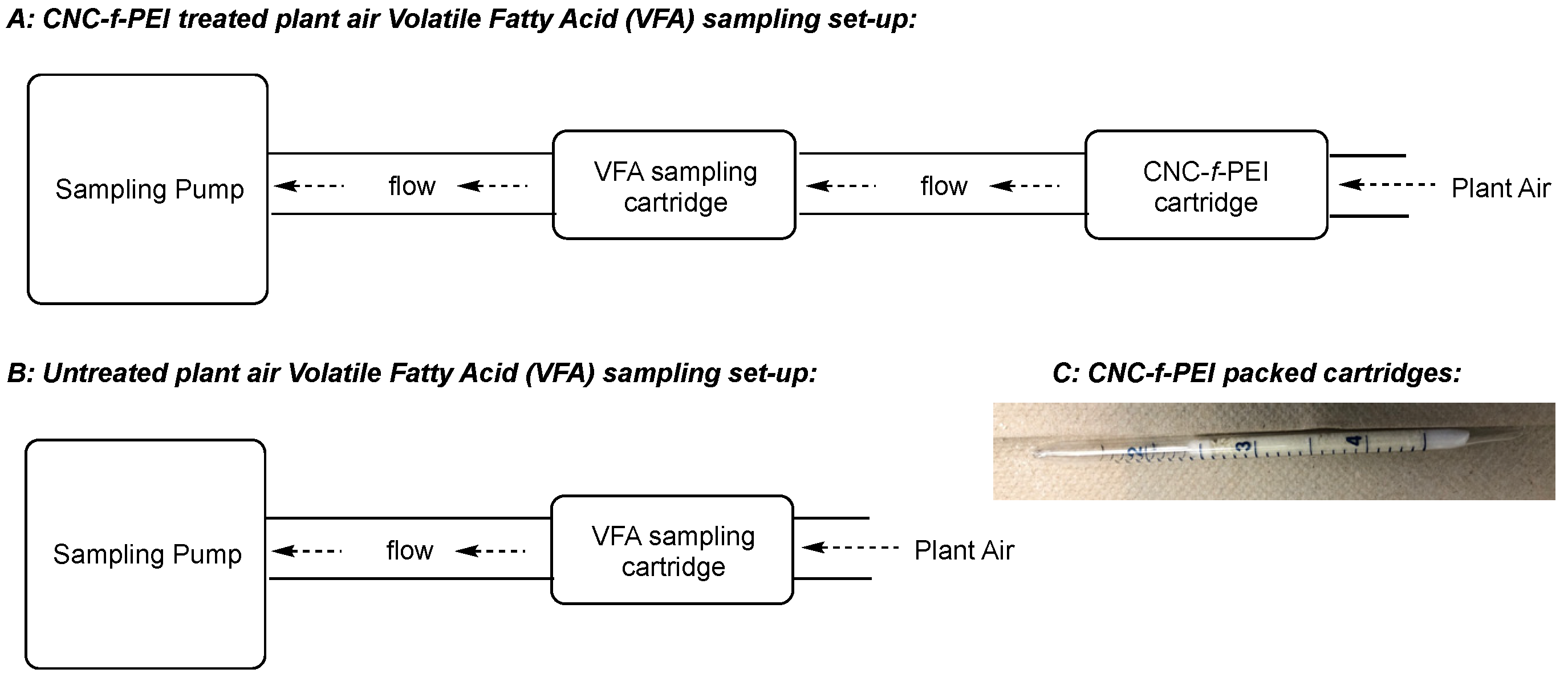
2.6. CNC-f-PEI Cartridge Preparation
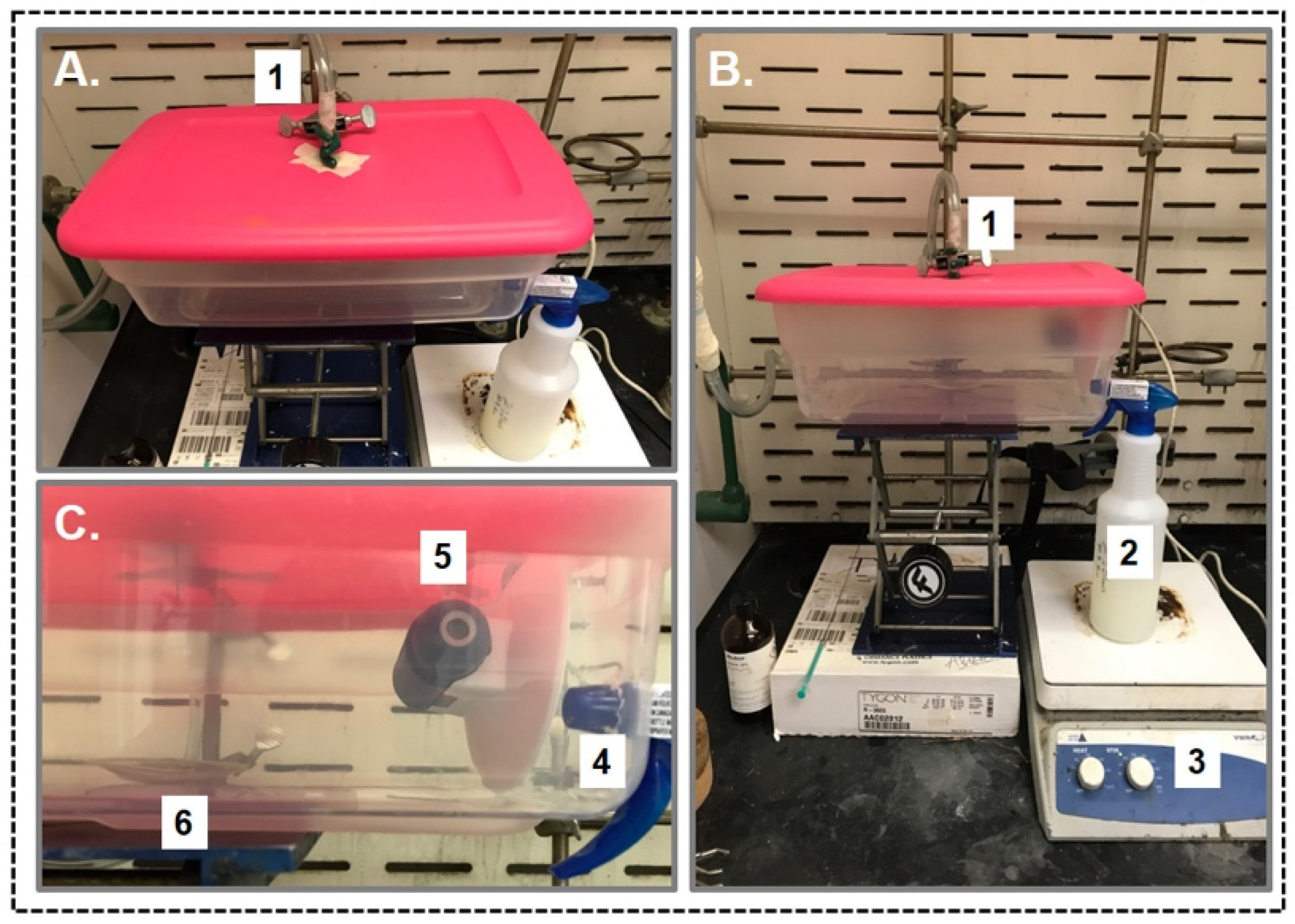
2.7. In-Plant Sampling Experiment:
2.8. General Procedure for the Spray Experiment of with Aqueous Suspension of PEI-CNC Materials
3. Results and Discussion
3.1. Scaled Synthesis of CNC from Bulk Cotton
3.2. Scaled Synthesis of PEI-f-CNC from In-House Prepared CNC
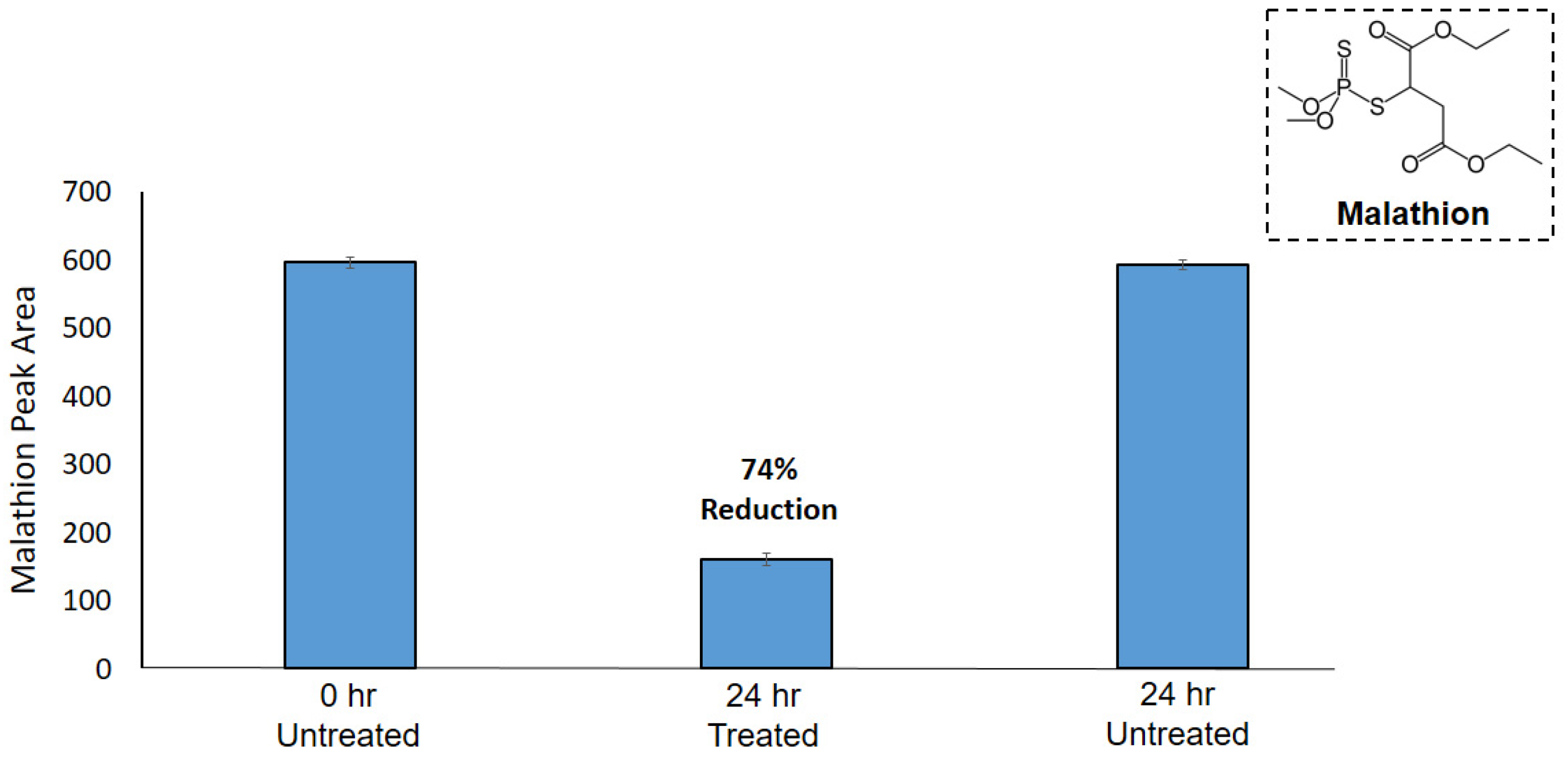
3.3. Application of PEI-f-CNC Packed Bed Filter Cartridges for VOC Remediation
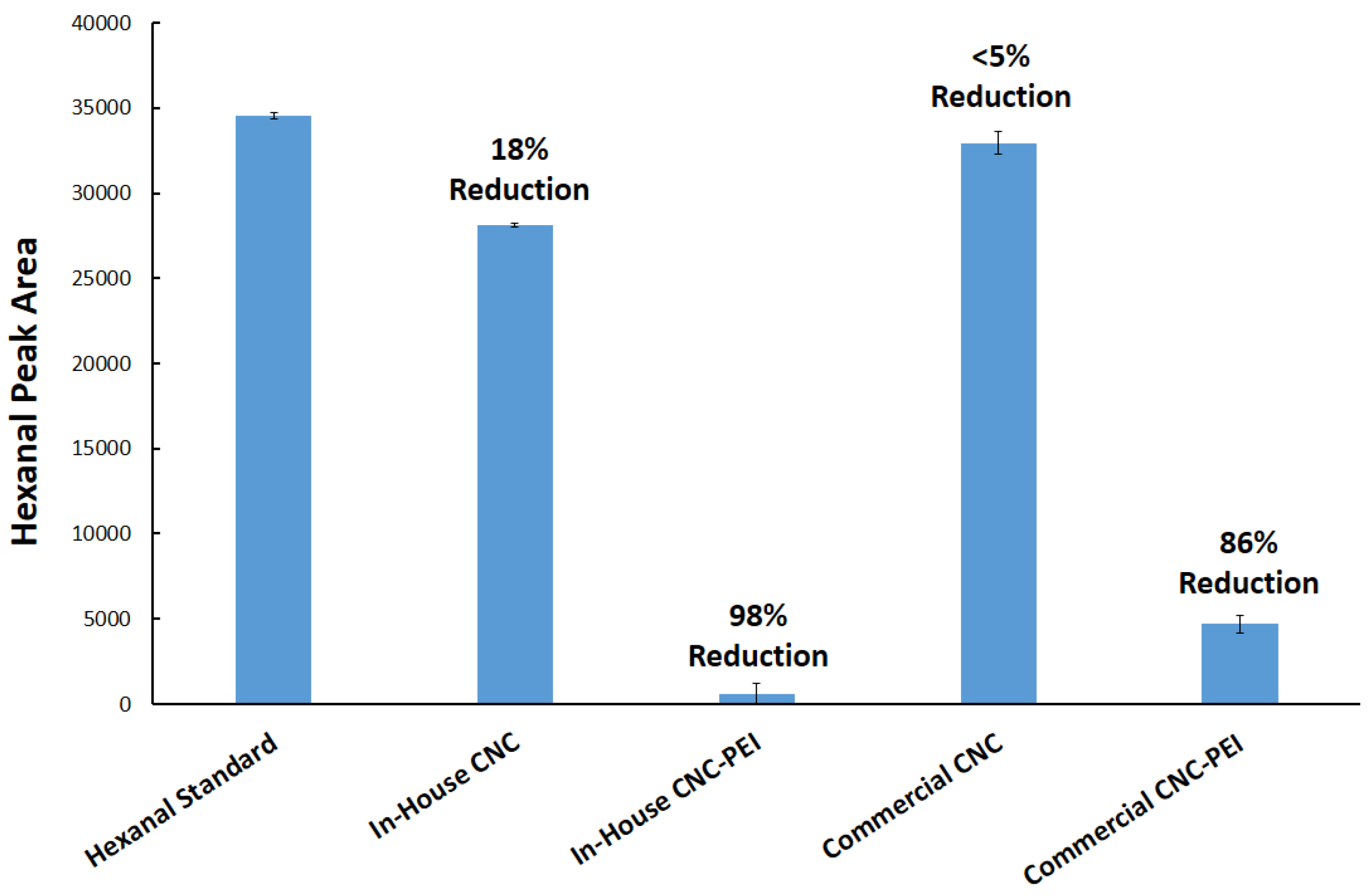
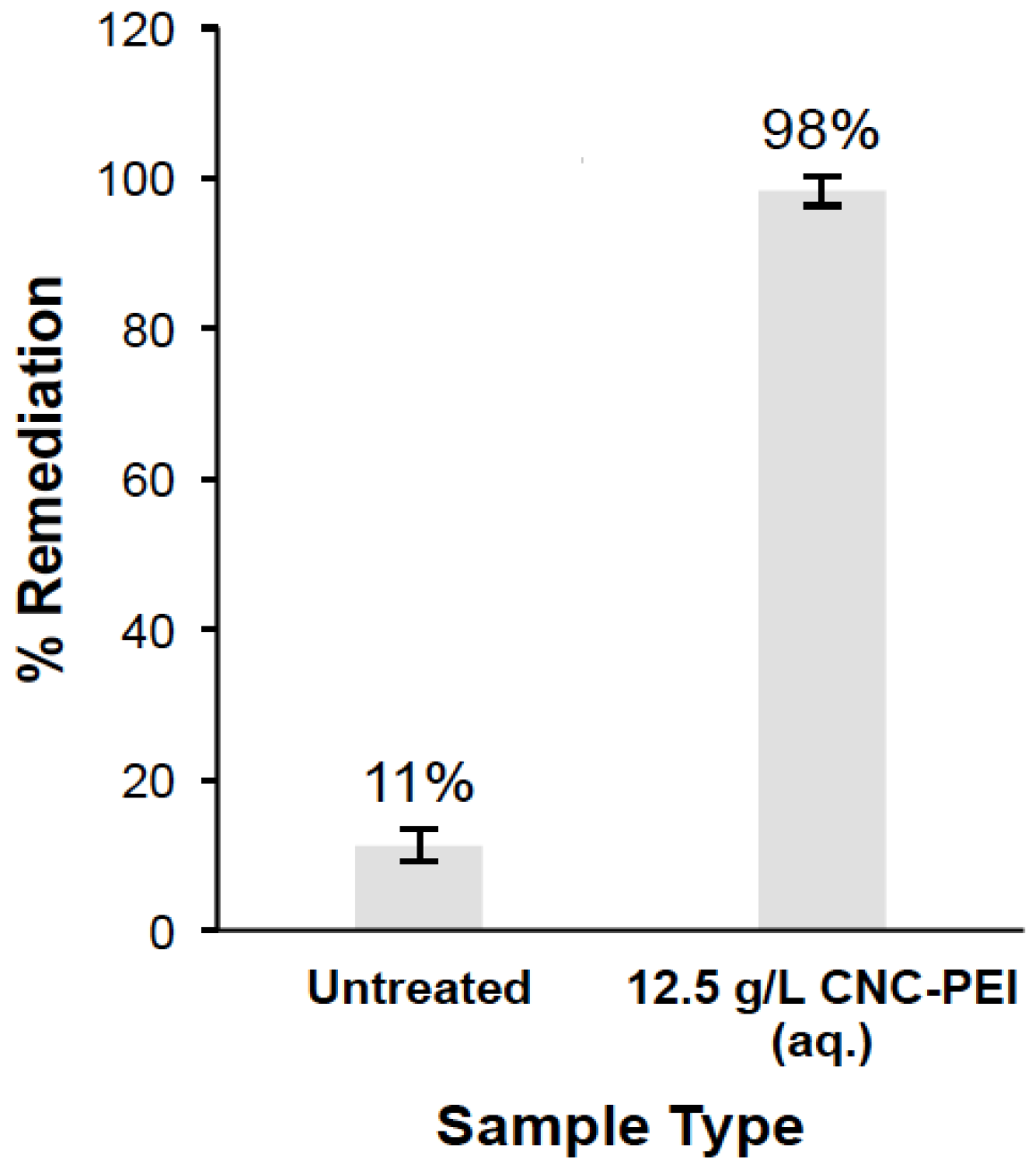
3.4. Application of PEI-f-CNC in A Spray-Based Delivery Method for VOC Remediation
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nanocomposites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergstrom, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Jin, L.Q.; Li, W.G.; Xu, Q.H.; Sun, Q.C. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Jin, L.Q.; Sun, Q.C.; Xu, Q.H.; Xu, Y.J. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef]
- Pei, A.H.; Butchosa, N.; Berglund, L.A.; Zhou, Q. Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 2013, 9, 2047–2055. [Google Scholar] [CrossRef]
- Zhu, W.J.; Liu, L.; Liao, Q.; Chen, X.; Qian, Z.Q.; Shen, J.Y.; Liang, J.L.; Yao, J.M. Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose 2016, 23, 3785–3797. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Li, J.; Zuo, K.M.; Wu, W.B.; Xu, Z.Y.; Yi, Y.G.; Jing, Y.; Dai, H.Q.; Fang, G.G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef]
- Tang, C.X.; Brodie, P.; Li, Y.Z.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Xi, C.Y.; Wang, R.K.; Rao, P.H.; Zhang, W.Q.; Yan, L.L.; Li, G.H.; Chai, F.; Cai, Y.Y.; Luo, T.T.; Zhou, X.Y. The fabrication and arsenic removal performance of cellulose nanocrystal-containing absorbents based on the "bridge joint" effect of iron ions. Carbohydr. Polym. 2020, 237, 116129. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. ChemPlusChem 2015, 80, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Wayland, H.A.; Boury, S.N.; Chhetri, B.P.; Brandt, A.; Proskurnin, M.A.; Filichkina, V.A.; Zharov, V.P.; Biris, A.S.; Ghosh, A. Advanced Cellulosic Materials for Treatment and Detection of Industrial Contaminants in Wastewater. ChemistrySelect 2016, 1, 4472–4488. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Clemons, C.; Reiner, R.; Sabo, R.; Agarwal, U.P.; Bissessur, R.; Sojoudiasli, H.; Carreau, P.J.; Acharya, B. Towards the scalable isolation of cellulose nanocrystals from tunicates. Sci. Rep. 2020, 10, 19090. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.L.; Guerra, F.D.; Dhulekar, J.; Alexis, F.; Whitehead, D.C. Target-Specific Capture of Environmentally Relevant Gaseous Aldehydes and Carboxylic Acids with Functional Nanoparticles. Chem. Eur. J. 2015, 21, 14834–14842. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Campbell, M.L.; Whitehead, D.C.; Alexis, F. Tunable Properties of Functional Nanoparticles for Efficient Capture of VOCs. ChemistrySelect 2017, 2, 9889–9894. [Google Scholar] [CrossRef]
- Ateia, M.; Attia, M.F.; Maroli, A.; Tharayil, N.; Alexis, F.; Whitehead, D.C.; Karanfil, T. Rapid Removal of Poly- and Perfluorinated Alkyl Substances by Poly(ethylenimine)-Functionalized Cellulose Microcrystals at Environmentally Relevant Conditions. Environ. Sci. Technol. Lett. 2018, 5, 764–769. [Google Scholar] [CrossRef]
- Guerra, F.D.; Campbell, M.L.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Capture of Aldehyde VOCs Using a Series of Amine-Functionalized Cellulose Nanocrystals. ChemistrySelect 2018, 3, 5495–5501. [Google Scholar] [CrossRef]
- Swasy, M.I.; Campbell, M.L.; Brummel, B.R.; Guerra, F.D.; Attia, M.F.; Smith, G.D.; Alexis, F.; Whitehead, D.C. Poly(amine) modified kaolinite clay for VOC capture. Chemosphere 2018, 213, 19–24. [Google Scholar] [CrossRef]
- Attia, M.F.; Swasy, M.I.; Alexis, F.; Whitehead, D.C. Controllable Design of Naked and Poly(Amine)-Capped Porous and Nonporous Microparticles of Sustainable Polymers That Exhibit Dual Modalities for Volatile Organic Compound Adsorption. ACS Appl. Polym. Mater. 2019, 1, 3459–3469. [Google Scholar] [CrossRef]
- Attia, M.F.; Swasy, M.I.; Ateia, M.; Alexis, F.; Whitehead, D.C. Periodic mesoporous organosilica nanomaterials for rapid capture of VOCs. Chem. Commun. 2020, 56, 607–610. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, F.; Swasy, M.I.; Attia, M.F.; Ateia, M.; Encalada, D.; Vizuete, K.; Galeas, S.; Guerrero, V.H.; Debut, A.; et al. Cellulose particles capture aldehyde VOC pollutants. RSC Adv. 2020, 10, 7967–7975. [Google Scholar] [CrossRef]
- Swasy, M.I.; Brummel, B.R.; Narangoda, C.; Attia, M.F.; Hawk, J.M.; Alexis, F.; Whitehead, D.C. Degradation of pesticides using amine-functionalized cellulose nanocrystals. RSC Adv. 2020, 10, 44312–44322. [Google Scholar] [CrossRef]
- Meeker, D.L. Essential Rendering: All About the Animal By-Products Industry; Meeker, D.L., Ed.; Kirby Lithographic Company, Inc.: Arlington, VA, USA, 2006. [Google Scholar]
- Guerra, F.D.; Smith, G.D.; Alexis, F.; Whitehead, D.C. A Survey of VOC Emissions from Rendering Plants. Aerosol Air Qual. Res. 2017, 17, 209–217. [Google Scholar] [CrossRef]
- Vanlangenhove, H.R.; Vanacker, M.; Schamp, N.M. Quantitative determination of carbonyl compounds in rendering emissions by reversed-phase high-performance liquid chromatography of 2,4-dinitrophenylhydrazones. Analyst 1983, 108, 329–334. [Google Scholar] [CrossRef]
- Vanlangenhove, H.R.; Vanwassenhove, F.A.; Coppin, J.K.; Vanacker, M.R.; Schamp, N.M. Gas-Chromatography Mass-Spectrometry Identification of Organic Volatiles Contributing to Rendering Odors. Environ. Sci. Technol. 1982, 16, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal structure and hydrogen bonding system in cellulose 1(alpha), from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, Z.A.; Maqbool, F.; Langenhove, H.V. Rendering plant emissions of volatile organic compounds during sterilization and cooking processes. Environ. Technol. 2014, 35, 1321–1327. [Google Scholar] [CrossRef] [PubMed]



| Entry | Cellulose Source | Acid | Temperature (°C) | Time (h) | Diameter (nm) 4 |
|---|---|---|---|---|---|
| 1 | CMC 1 (20 um) | 2.5 M HCl | 100 | 0.5 | 93 |
| 2 | Cotton-A 2 | 2.5 M HCl | 100 | 0.5 | 560 |
| 3 | Cotton-B 3 | 2.5 M HCl | 100 | 4 | 1800 5 |
| 4 | Cotton-B | 50% H2SO4 | 20 | 4 | 175 |
| 5 | Cotton-B | 50% H2SO4 | 45 | 4 | 52 (375) 6 |
| 6 | Cotton-B | 25% H2SO4 | 45 | 4 | 9970 |
| 7 | Cotton-B | 50% H2SO4 | 60 | 4 | 77 |
| 8 | CMC (20 µm) | 64% H2SO4 | 45 | 1 | 432 |
| 9 | Cotton-A | 64% H2SO4 | 45 | 1 | 91 |
| 10 | Cotton-B | 64% H2SO4 | 45 | 1 | 416 |
| Entry | Scale | Hydrodynamic Size (nm) 1 | Yield (%) |
|---|---|---|---|
| 1 | 1 g | 52 | 20 |
| 2 | 100 g | 60 | 50 |
| 3 | 500 g | 177 | 65 |
| VFA | Untreated (ppb) | Treated (ppb) | % Reduction |
|---|---|---|---|
| acetic acid | 290 | ND 1 | >97% |
| propanoic acid | 190 | 2.03 ± 0.55 | 99% |
| isobutyric acid | 31 | ND | >98% |
| butyric acid | 250 | 2.53 ± 0.11 | 99% |
| 2-methylbutanoic acid | 20 | ND | >97% |
| isovaleric acid | 15 | ND | >96% |
| valeric acid | 18 | ND | >97% |
| isocaproic acid | 19 | ND | >97% |
| caproic acid | 7.2 | ND | >93% |
| enanthoic acid | 0.51 | ND | >8% |
| caprylic acid | 0.55 | ND | >24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummel, B.R.; Narangoda, C.J.; Attia, M.F.; Swasy, M.I.; Smith, Jr., G.D.; Alexis, F.; Whitehead, D.C. Scaled Synthesis of Polyamine-Modified Cellulose Nanocrystals from Bulk Cotton and Their Use for Capturing Volatile Organic Compounds. Polymers 2021, 13, 3060. https://doi.org/10.3390/polym13183060
Brummel BR, Narangoda CJ, Attia MF, Swasy MI, Smith, Jr. GD, Alexis F, Whitehead DC. Scaled Synthesis of Polyamine-Modified Cellulose Nanocrystals from Bulk Cotton and Their Use for Capturing Volatile Organic Compounds. Polymers. 2021; 13(18):3060. https://doi.org/10.3390/polym13183060
Chicago/Turabian StyleBrummel, Beau R., Chandima J. Narangoda, Mohamed F. Attia, Maria I. Swasy, Gary D. Smith, Jr., Frank Alexis, and Daniel C. Whitehead. 2021. "Scaled Synthesis of Polyamine-Modified Cellulose Nanocrystals from Bulk Cotton and Their Use for Capturing Volatile Organic Compounds" Polymers 13, no. 18: 3060. https://doi.org/10.3390/polym13183060
APA StyleBrummel, B. R., Narangoda, C. J., Attia, M. F., Swasy, M. I., Smith, Jr., G. D., Alexis, F., & Whitehead, D. C. (2021). Scaled Synthesis of Polyamine-Modified Cellulose Nanocrystals from Bulk Cotton and Their Use for Capturing Volatile Organic Compounds. Polymers, 13(18), 3060. https://doi.org/10.3390/polym13183060








