Application of Polydopamine Functionalized Zinc Oxide for Glucose Biosensor Design
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Synthesis of ZnO Nanorods and the Deposition of Polydopamine
2.3. Photoelectrochemical Detection of Glucose
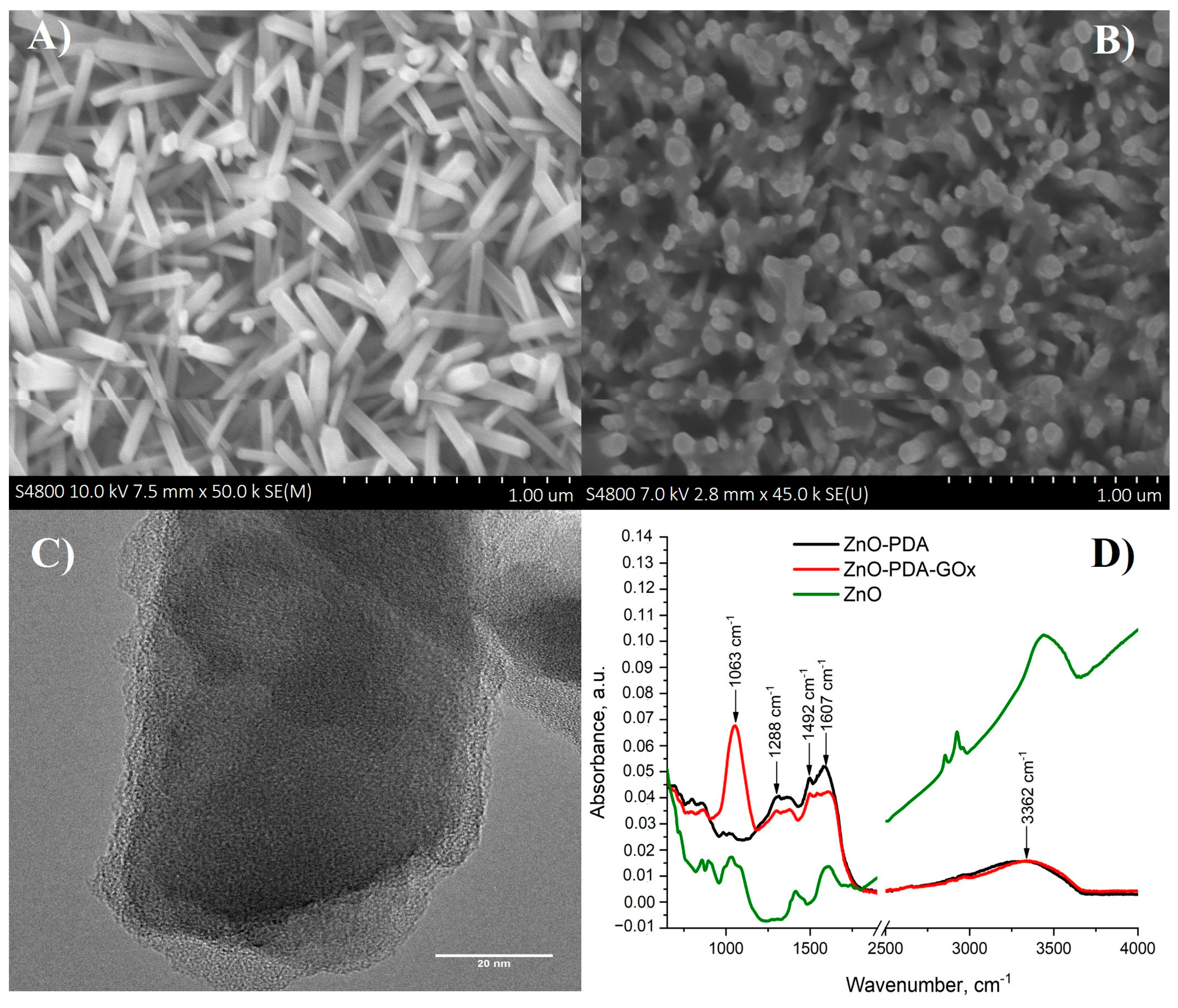
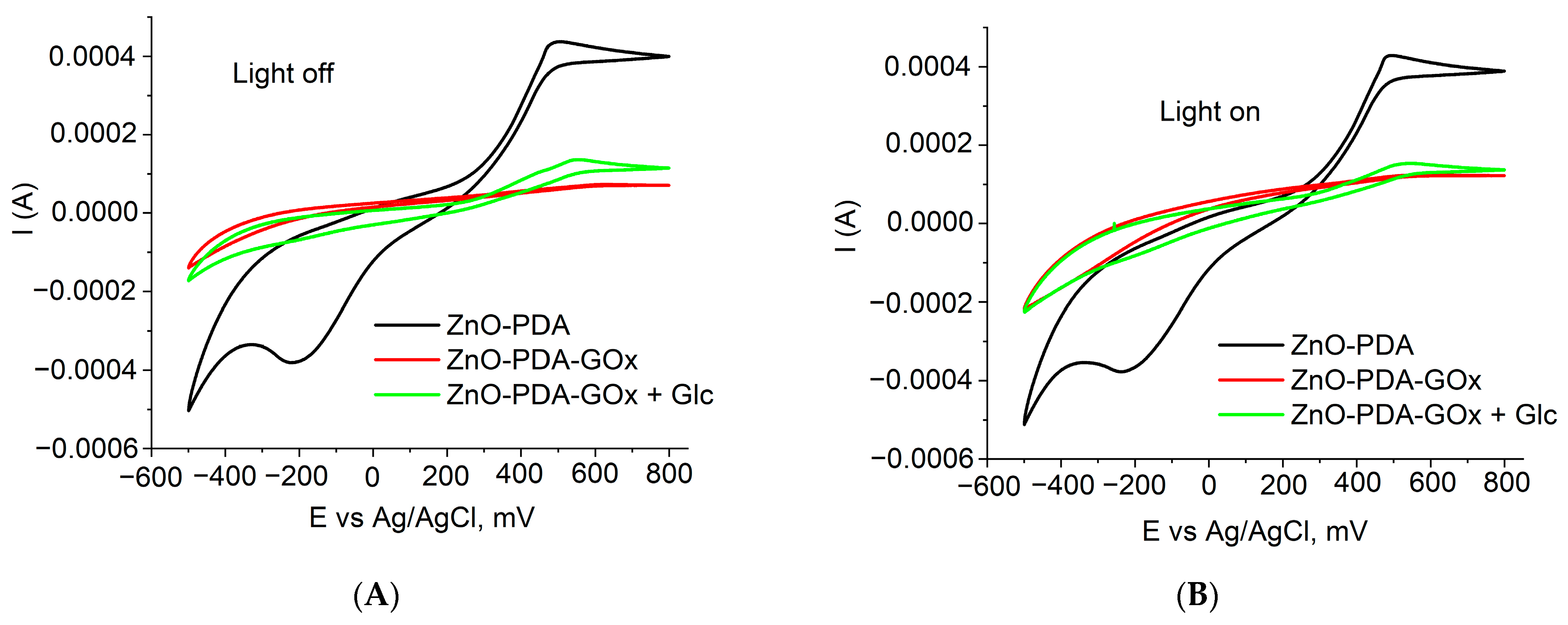
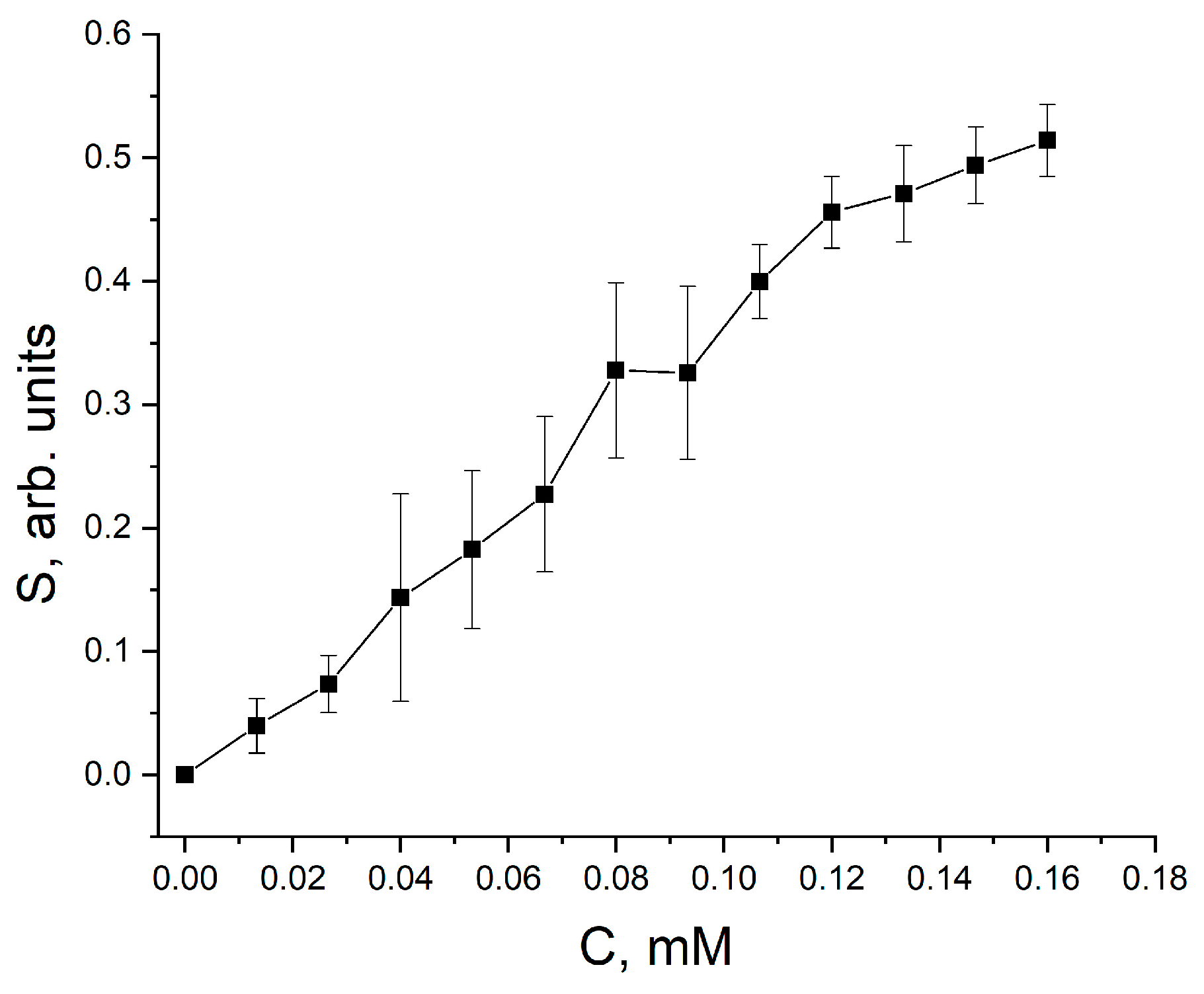
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Tang, R.; Chen, C. Photoelectrochemical Detection of Chromium (VI) Using Layered MoS2 Modified BiOI. J. Chem. Sci. 2020, 132, 1–9. [Google Scholar] [CrossRef]
- Luo, J.; Liang, D.; Zhao, D.; Yang, M. Photoelectrochemical Detection of Circulating Tumor Cells Based on Aptamer Conjugated Cu2O as Signal Probe. Biosens. Bioelectron. 2020, 151, 111976. [Google Scholar] [CrossRef] [PubMed]
- del Barrio, M.; Luna-López, G.; Pita, M. Enhancement of Biosensors by Implementing Photoelectrochemical Processes. Sensors 2020, 20, 3281. [Google Scholar] [CrossRef]
- He, X.; Ying, Y.; Zhao, X.; Deng, W.; Tan, Y.; Xie, Q. Cobalt-Doped Tungsten Trioxide Nanorods Decorated with Au Nanoparticles for Ultrasensitive Photoelectrochemical Detection of Aflatoxin B1 Based on Aptamer Structure Switch. Sens. Actuators B Chem. 2021, 332, 129528. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Feng, L.; Chen, X.; Jiang, J.; Zhang, S.; Zhang, C.; Zhang, A.; Chen, G.; Wang, H. Synergetic Ag2S and ZnS Quantum Dots as the Sensitizer and Recognition Probe: A Visible Light-Driven Photoelectrochemical Sensor for the “Signal-on” Analysis of Mercury (II). J. Hazard. Mater. 2020, 387, 121715. [Google Scholar] [CrossRef]
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison between Electrochemical and Photoelectrochemical Detection of Dopamine Based on Titania-Ceria-Graphene Quantum Dots Nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical Enzymatic Biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A Photoelectrochemical Glucose Sensor Based on Gold Nanoparticles as a Mimic Enzyme of Glucose Oxidase. RSC Adv. 2019, 9, 15307–15313. [Google Scholar] [CrossRef] [Green Version]
- German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The Use of Different Glucose Oxidases for the Development of an Amperometric Reagentless Glucose Biosensor Based on Gold Nanoparticles Covered by Polypyrrole. Electrochim. Acta 2015, 169, 326–333. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Amperometric Glucose Biosensor Based on Electrochemically Deposited Gold Nanoparticles Covered by Polypyrrole. Electroanalysis 2017, 29, 1267–1277. [Google Scholar] [CrossRef]
- Vashist, S.K. Non-Invasive Glucose Monitoring Technology in Diabetes Management: A Review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Park, K.H.; Lee, Y.R. An Ultrasensitive Photoelectrochemical Biosensor for Glucose Based on Bio-Derived Nitrogen-Doped Carbon Sheets Wrapped Titanium Dioxide Nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Hao, W.; Wu, Q.; Peng, J.; Tu, J.; Cao, Y. 3D Hollow-out TiO2 Nanowire Cluster/GOx as an Ultrasensitive Photoelectrochemical Glucose Biosensor. J. Mater. Chem. B 2020, 8, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Çakıroğlu, B.; Özacar, M. A Photoelectrochemical Biosensor Fabricated Using Hierarchically Structured Gold Nanoparticle and MoS 2 on Tannic Acid Templated Mesoporous TiO2. Electroanalysis 2020, 32, 166–177. [Google Scholar] [CrossRef]
- Çakıroğlu, B.; Demirci, Y.C.; Gökgöz, E.; Özacar, M. A Photoelectrochemical Glucose and Lactose Biosensor Consisting of Gold Nanoparticles, MnO2 and g-C3N4 Decorated TiO2. Sens. Actuators B Chem. 2019, 282, 282–289. [Google Scholar] [CrossRef]
- He, L.; Yang, Z.; Gong, C.; Liu, H.; Zhong, F.; Hu, F.; Zhang, Y.; Wang, G.; Zhang, B. The Dual-Function of Photoelectrochemical Glucose Oxidation for Sensor Application and Solar-to-Electricity Production. J. Electroanal. Chem. 2021, 882, 114912. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Zhang, K.; Cao, Y.; Tu, J.; Xiao, D.; Wu, Q. Glucose Photoelectrochemical Enzyme Sensor Based on Competitive Reaction of Ascorbic Acid. Biosens. Bioelectron. 2020, 166, 112466. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yang, M.; Cheng, Y.; Liu, J.; Xiao, B.; Chen, S.; Huang, J.; Xie, Q.; Wu, G.; Wu, H. Dielectric Properties and Thermal Conductivity of Epoxy Composites Using Quantum-Sized Silver Decorated Core/Shell Structured Alumina/Polydopamine. Compos. Part A Appl. Sci. Manuf. 2019, 118, 302–311. [Google Scholar] [CrossRef]
- Wang, R.; Feng, D.; Chen, T.; Chen, S.; Liu, Y. Mussel-Inspired Polydopamine Treated Si/C Electrode as High-Performance Anode for Lithium-Ion Batteries. J. Alloys Compd. 2020, 825, 154081. [Google Scholar] [CrossRef]
- Gao, J.; Li, H.; Li, M.; Wang, G.; Long, Y.; Li, P.; Li, C.; Yang, B. Polydopamine/Graphene/MnO2 Composite-Based Electrochemical Sensor for in Situ Determination of Free Tryptophan in Plants. Anal. Chim. Acta 2021, 1145, 103–113. [Google Scholar] [CrossRef]
- Arroquia, A.; Acosta, I.; Armada, M.P.G. Self-Assembled Gold Decorated Polydopamine Nanospheres as Electrochemical Sensor for Simultaneous Determination of Ascorbic Acid, Dopamine, Uric Acid and Tryptophan. Mater. Sci. Eng. C 2020, 109, 110602. [Google Scholar] [CrossRef] [PubMed]
- Sukeri, A.; Arjunan, A.; Bertotti, M. New Strategy to Fabricate a Polydopamine Functionalized Self-Supported Nanoporous Gold Film Electrode for Electrochemical Sensing Applications. Electrochem. Commun. 2020, 110, 106622. [Google Scholar] [CrossRef]
- Fedorenko, V.; Viter, R.; Mrówczyński, R.; Damberga, D.; Coy, E.; Iatsunskyi, I. Synthesis and Photoluminescence Properties of Hybrid 1D Core-Shell Structured Nanocomposites Based on ZnO/Polydopamine. RSC Adv. 2020, 10, 29751–29758. [Google Scholar] [CrossRef]
- Damberga, D.; Fedorenko, V.; Grundšteins, K.; Altundal, Ş.; Šutka, A.; Ramanavičius, A.; Coy, E.; Mrówczyński, R.; Iatsunskyi, I.; Viter, R. Influence of PDA Coating on the Structural, Optical and Surface Properties of ZnO Nanostructures. Nanomaterials 2020, 10, 2438. [Google Scholar] [CrossRef] [PubMed]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2020, 221, 1900232. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile Synthesis of Novel Size-Controlled Antibacterial Hybrid Spheres Using Silver Nanoparticles Loaded with Poly-Dopamine Spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, Morphological and Biological Analysis of Zinc Oxide Nanoparticles (ZnO NPs) Using: Papaver Somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhang, Q.; Gong, C.; Liu, H.; Hu, F.; Zhong, F.; Wang, G.; Su, H.; Wen, S.; Xiang, S.; et al. The Dual-Function of Hematite-Based Photoelectrochemical Sensor for Solar-to-Electricity Conversion and Self-Powered Glucose Detection. Sens. Actuators B Chem. 2020, 310, 127842. [Google Scholar] [CrossRef]
- Yang, W.; Xu, W.; Wang, Y.; Chen, D.; Wang, X.; Cao, Y.; Wu, Q.; Tu, J.; Zhen, C. Photoelectrochemical Glucose Biosensor Based on the Heterogeneous Facets of Nanocrystalline TiO2/Au/Glucose Oxidase Films. ACS Appl. Nano Mater. 2020, 3, 2723–2732. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Wang, X.; Huang, L.; Zhang, Y.; Yao, S. A CeO2@MnO2 Core–Shell Hollow Heterojunction as Glucose Oxidase-like Photoenzyme for Photoelectrochemical Sensing of Glucose. Sens. Actuators B Chem. 2020, 304, 127389. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorenko, V.; Damberga, D.; Grundsteins, K.; Ramanavicius, A.; Ramanavicius, S.; Coy, E.; Iatsunskyi, I.; Viter, R. Application of Polydopamine Functionalized Zinc Oxide for Glucose Biosensor Design. Polymers 2021, 13, 2918. https://doi.org/10.3390/polym13172918
Fedorenko V, Damberga D, Grundsteins K, Ramanavicius A, Ramanavicius S, Coy E, Iatsunskyi I, Viter R. Application of Polydopamine Functionalized Zinc Oxide for Glucose Biosensor Design. Polymers. 2021; 13(17):2918. https://doi.org/10.3390/polym13172918
Chicago/Turabian StyleFedorenko, Viktoriia, Daina Damberga, Karlis Grundsteins, Arunas Ramanavicius, Simonas Ramanavicius, Emerson Coy, Igor Iatsunskyi, and Roman Viter. 2021. "Application of Polydopamine Functionalized Zinc Oxide for Glucose Biosensor Design" Polymers 13, no. 17: 2918. https://doi.org/10.3390/polym13172918
APA StyleFedorenko, V., Damberga, D., Grundsteins, K., Ramanavicius, A., Ramanavicius, S., Coy, E., Iatsunskyi, I., & Viter, R. (2021). Application of Polydopamine Functionalized Zinc Oxide for Glucose Biosensor Design. Polymers, 13(17), 2918. https://doi.org/10.3390/polym13172918









