Effect of Powder on Tribological and Electrochemical Properties of Nylon 66 and Ultra-High Molecular Weight Polyethylene in Water and Seawater Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Friction and Wear Tests
2.2. Electrochemical Experimental Parameters
3. Results
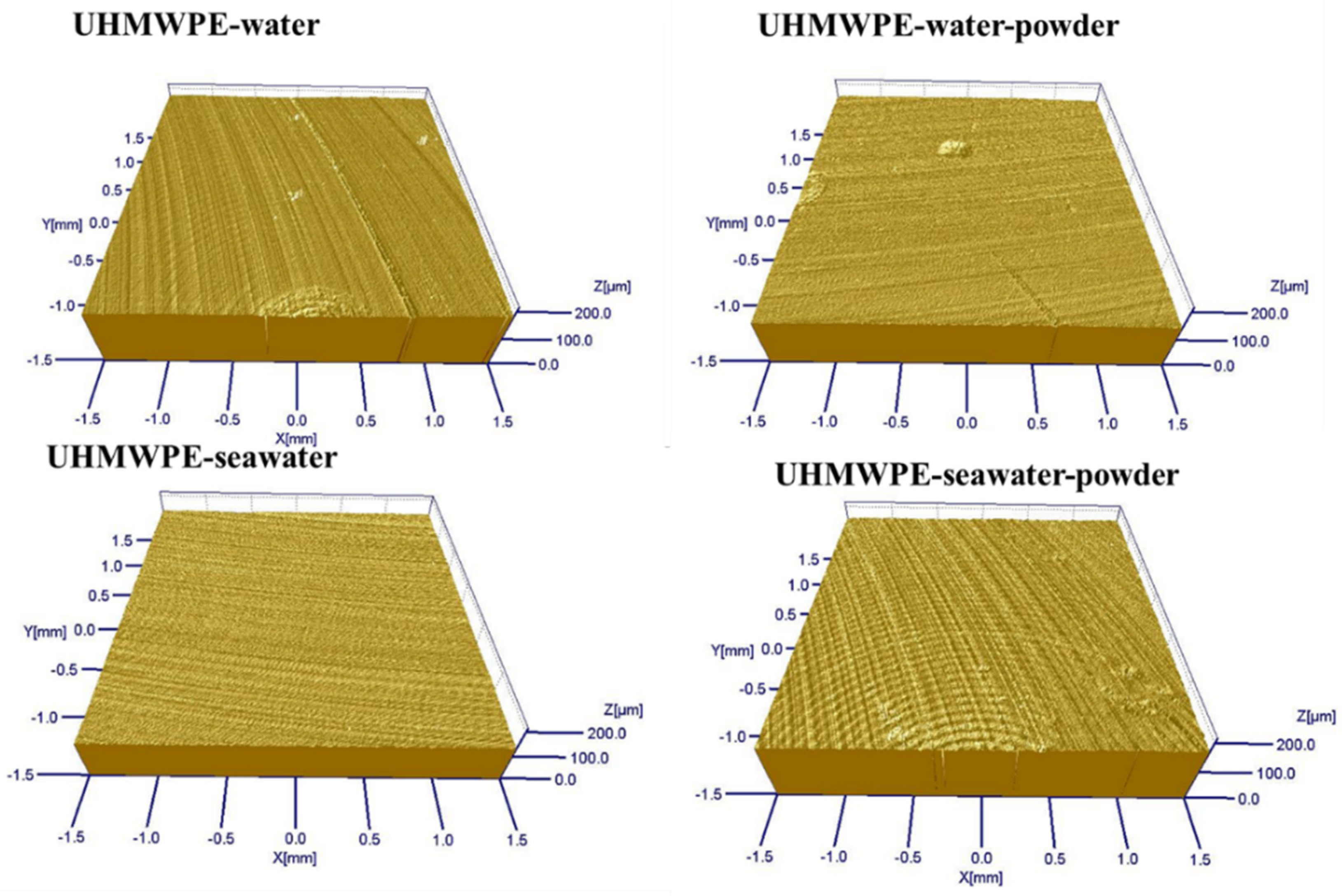
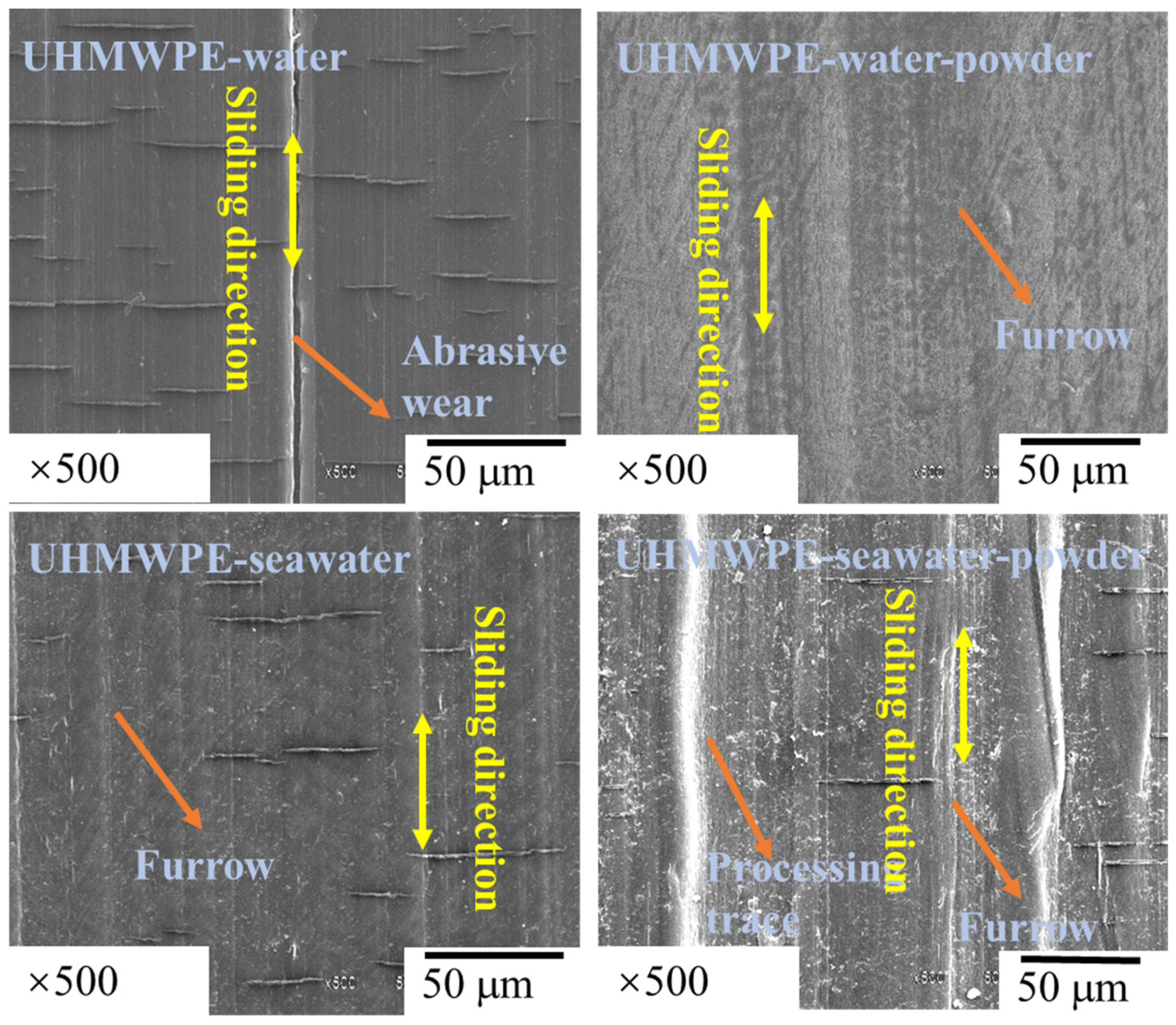
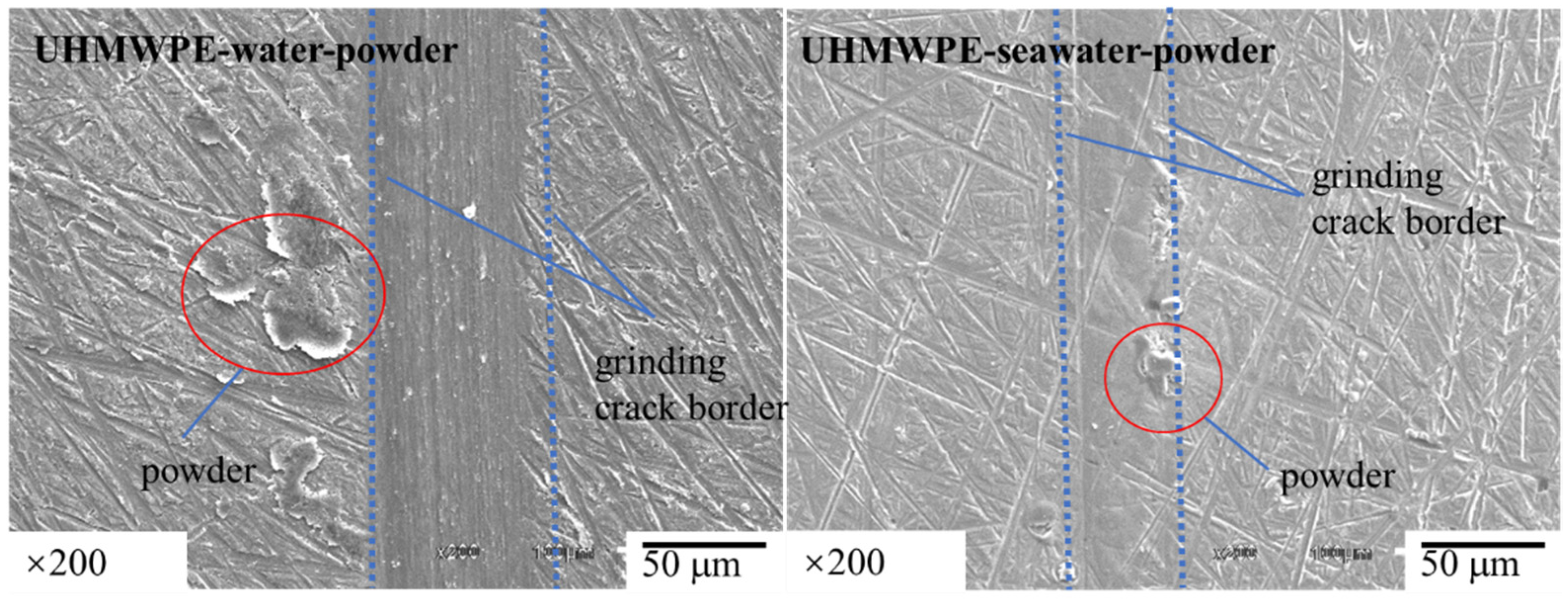
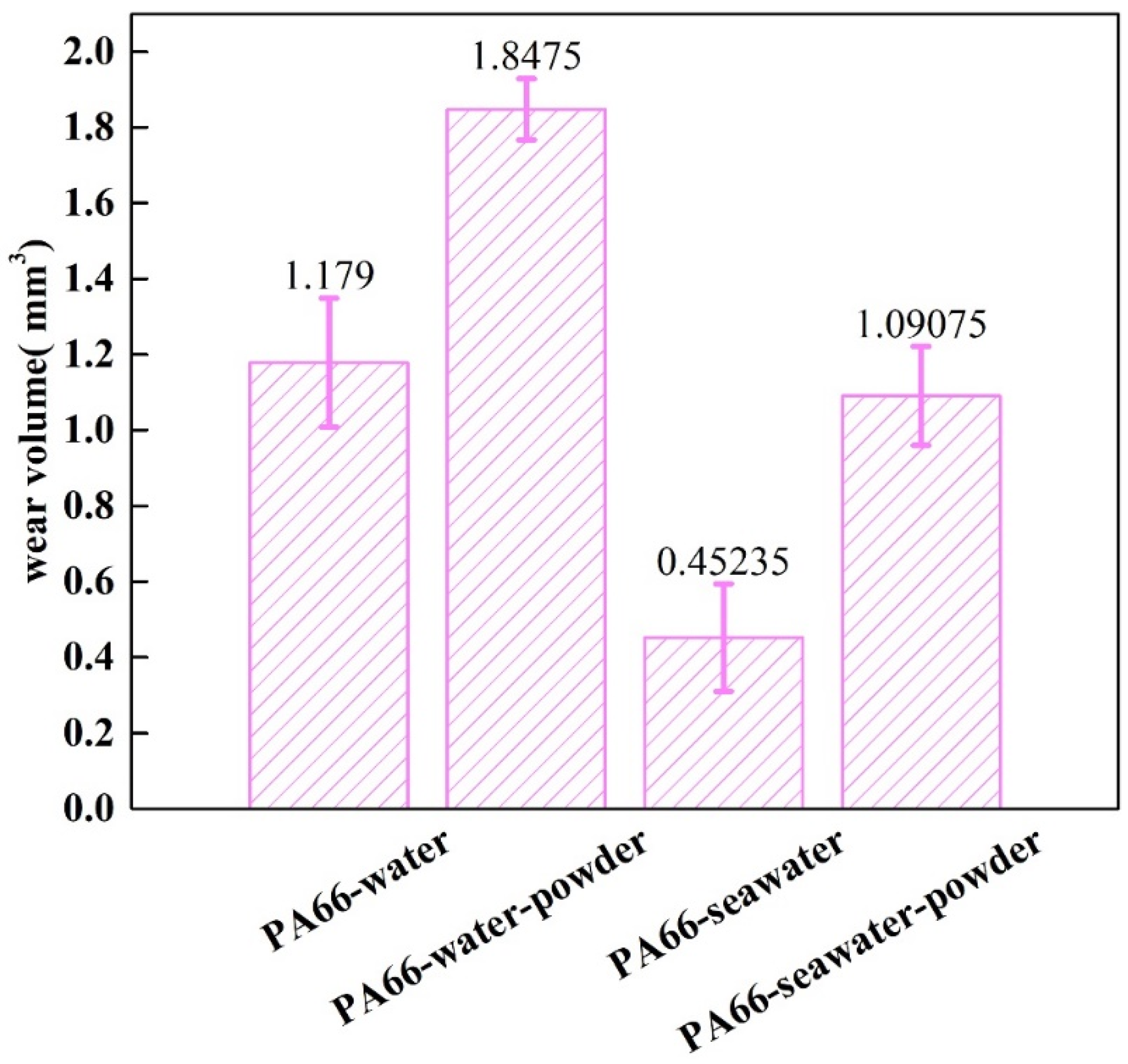
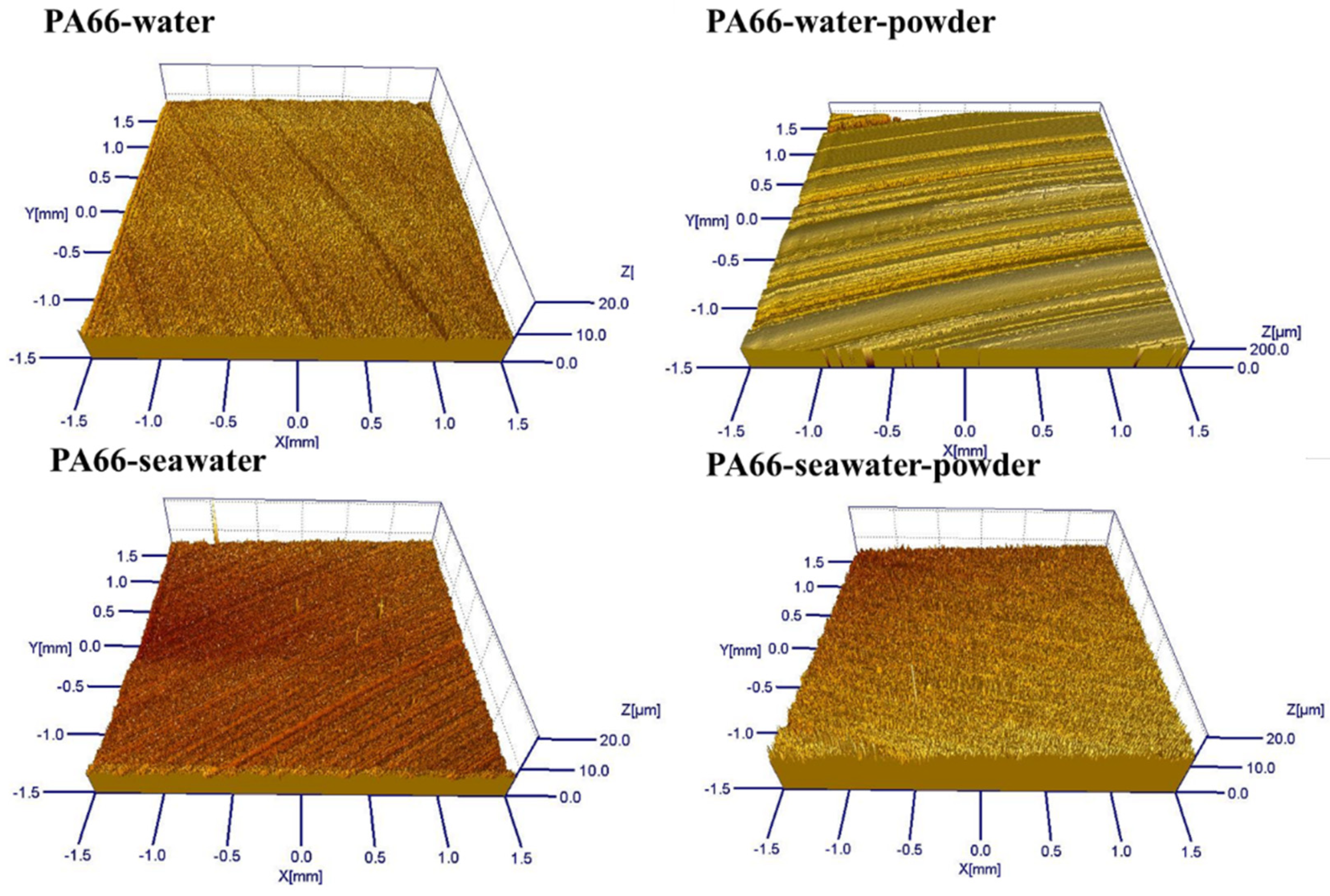
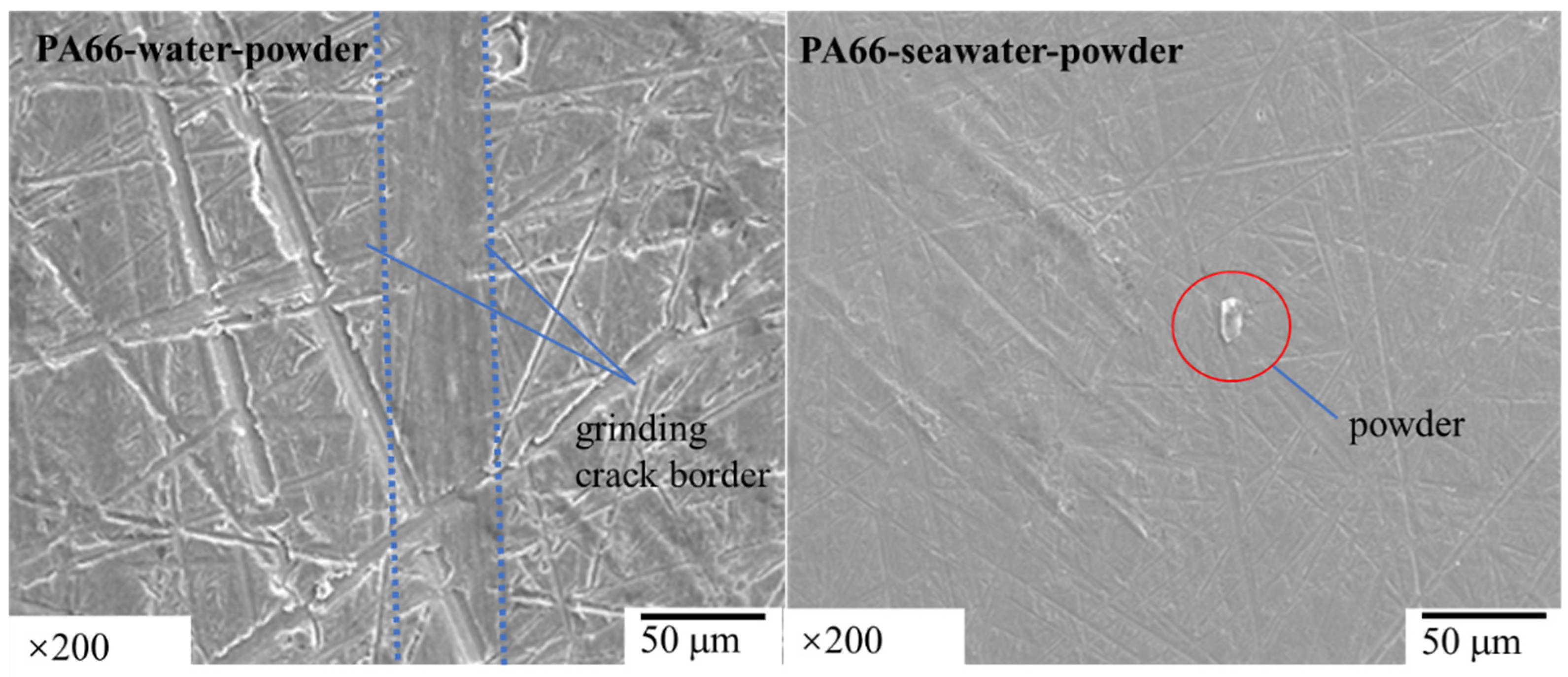
3.1. Effect of Powder on Friction and Wear
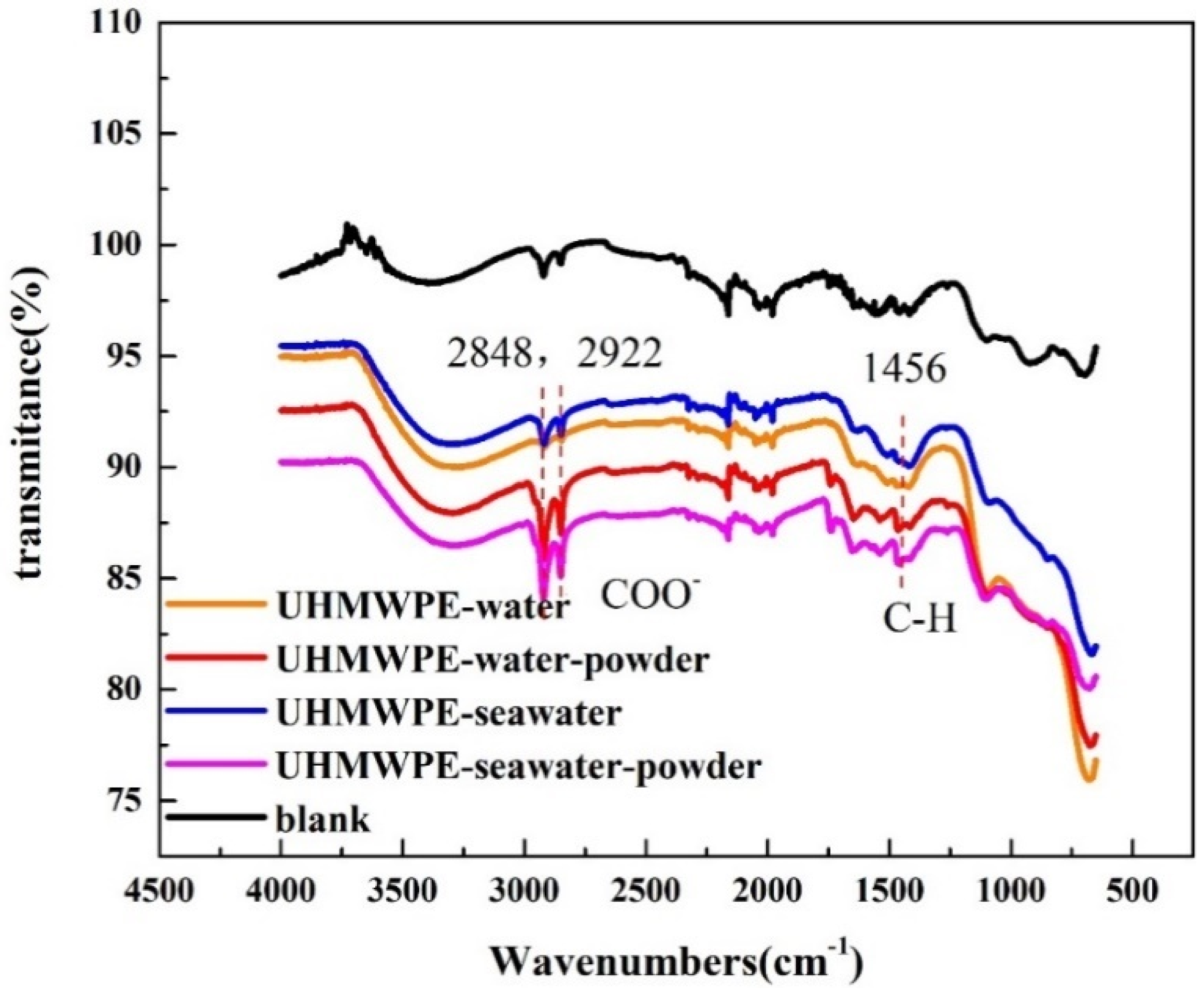
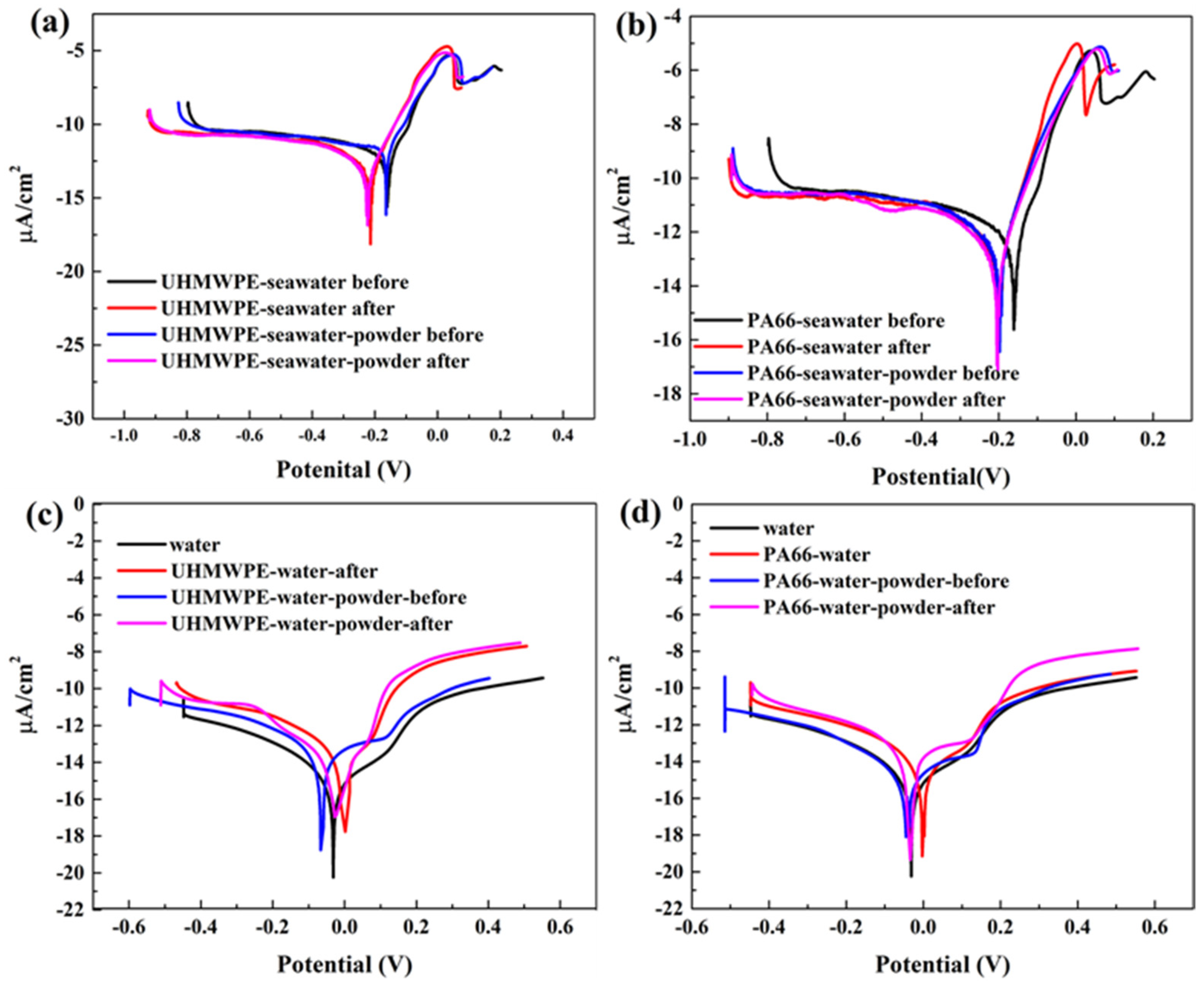
3.2. Effect of Powder on Solution Corrosivity
4. Conclusions
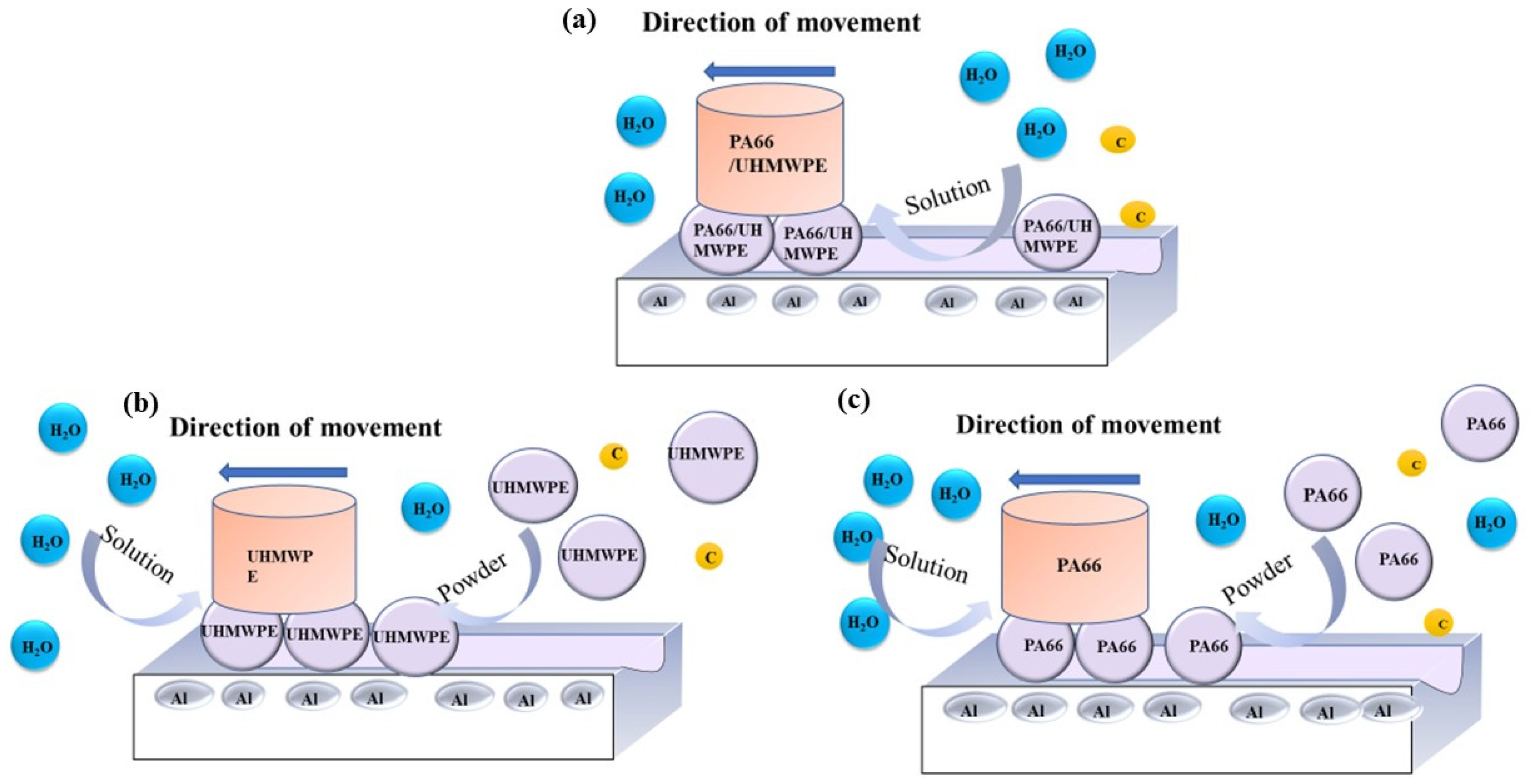
- (a)
- Adding powder decreased the wear volume and friction coefficient of the UHMWPE specimen. After adding powder into pure water, the average wear amount decreased from 0.57045 to 0.3269 mm3. After adding powder into seawater, the average wear amount decreased from 0.27435 to 0.1963 mm3. For the PA66 specimens, the addition of powder increased the wear amounts of all samples, but adding powder increased the coefficient of the pin in the water environment and decreased that in the seawater environment. After adding powder into pure water, the average wear amount increased from 1.179 to 1.8475 mm3. After adding powder into seawater, the average wear amount increased from 0.45235 to 1.09075 mm3.
- (b)
- The results of tribological experiments indicated that wear debris can improve the tribological properties of UHMWPE in water and seawater environments by shielding the friction surface as well as reduce wear. However, the wear debris shielding the surface of the PA66 pin could not reduce wear in the water environment, the powder in the water stimulated abrasive wear, and the friction reduction and wear resistance of PA66 in the water environment decreased. This is due to the high hardness of PA66 powder, resulting in abrasive wear.
- (c)
- The results of electrochemical experiments demonstrated that powder can form a physical barrier on the surface and reduce the corrosion current to protect the material. In UHMWPE tests, the corrosion current density of seawater decreased from 6.342 to 2.199 μA/cm2 before and after friction test, and from 11.530 to 2.083 μA/cm2 in seawater with powder, reflecting the synergistic effect of seawater and powder. In PA66 tests, the corrosion current density of PA66 decreased from 6.342 to 3.286 μA/cm2 before and after tribological test in seawater, and from 10.420 to 3.229 μA/cm2 in seawater with powder. Moreover, in the salt-containing seawater solution, the powder is easier to adhere to the surface, and the law of friction reduction and corrosion resistance of the powder is similar, which indicates that the interaction of electrochemistry, friction, and wear occur. It also shows the synergistic effect of powder and solution environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.J.; Wani, M.F.; Gupta, R. Friction and wear characterization of graphite/Polytetrafluoroethylene composites against stainless steel: A comparative investigation under different environments. J. Phys. Conf. Ser. 2019, 1240, 012107. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Yin, Z.W.; Li, H.L.; Gao, G.Y.; Zhang, X.L. Friction and wear characteristics of ultrahigh molecular weight polyethylene (UHMWPE) composites containing glass fibers and carbon fibers under dry and water-lubricated conditions. Wear 2017, 380–381, 42–51. [Google Scholar] [CrossRef]
- Wang, J.Z.; Yan, F.Y.; Xue, Q.J. Tribological behavior of PTFE sliding against steel in sea water. Wear 2009, 267, 1634–1641. [Google Scholar] [CrossRef]
- Chen, B.B.; Wang, J.Z.; Yan, F.Y. Microstructure of PTFE-Based Polymer Blends and Their Tribological Behaviors Under Aqueous Environment. Tribol. Lett. 2012, 45, 387–395. [Google Scholar] [CrossRef]
- Du, Q.; Hu, Y.; Cui, W. Safety assessment of the acrylic conical frustum viewport structure for a deep-sea manned submersible. Ships Offshore Struct. 2017, 12, 221–229. [Google Scholar] [CrossRef]
- Wang, F. Theory and practice of maritime power. J. Eng. Sci. 2016, 18, 55–60. (In Chinese) [Google Scholar] [CrossRef]
- Baets, P.D. Comparison of the wear behaviour of six bearing materials for a heavily loaded sliding system in seawater. Wear 1995, 180, 61–72. [Google Scholar] [CrossRef]
- Chen, B.B.; Wang, J.Y.; Yan, F.Y. Synergism of carbon fiber and polyimide in polytetrafluoroethylene-based composites: Friction and wear behavior under sea water lubrication. Mater. Des. 2012, 36, 366–371. [Google Scholar] [CrossRef]
- Khan, M.J.; Wani, M.F.; Gupta, R. Tribological properties of bronze filled PTFE under dry sliding conditions and aqueous environments (distilled water and sea water). Int. J. Surf. Sci. Eng. 2018, 12, 348–364. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, D. Comparative investigation on the tribological behavior of reinforced plastic composite under natural seawater lubrication. Mater. Des. 2013, 51, 983–988. [Google Scholar] [CrossRef]
- Chen, B.B.; Wang, J.Y.; Yan, F.Y. Comparative investigation on the tribological behaviors of CF/PEEK composites under sea water lubrication. Tribol. Int. 2012, 52, 170–177. [Google Scholar] [CrossRef]
- Qi, H.M.; Hu, C.; Zhang, G. Comparative study of tribological properties of carbon fibers and aramid particles reinforced polyimide composites under dry and sea water lubricated conditions. Wear 2019, 436–437, 203001. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.Z.; Jiang, P.F.; Yan, F.Y. Hydrostatic pressure–dependent wear behavior of thermoplastic polymers in deep sea. Polyms Adv. Technol. 2018, 29, 2410–2415. [Google Scholar] [CrossRef]
- Litwin, W. Influence of Surface Roughness Topography on Properties of Water-Lubricated Polymer Bearings: Experimental Research. Tribol. Trans. 2011, 54, 351–361. [Google Scholar] [CrossRef]
- Guo, Z.W.; Xie, X.; Yuan, C.Q.; Bai, X. Study on influence of micro convex textures on tribological performances of UHMWPE material under the water-lubricated conditions. Wear 2019, 426–427, 1327–1335. [Google Scholar] [CrossRef]
- Kazemi, N.S.; Hamed, Y. Effect of sea water on water absorption and flexural properties of wood-polypropylene composites. Eur. J. Wood Wood Prod. 2011, 69, 553–556. [Google Scholar] [CrossRef]
- Velkavrh, I.; Klien, S.; Voyer, J.; Ausserer, F.; Diem, A. Influence of Water Absorption on Static Friction of Pure and Friction-Modified PA6 Polymers. Key Eng. Mater. 2019, 4873, 59–64. [Google Scholar] [CrossRef]
- Chen, S.; Xu, H.P.; Duan, H.T.; Hua, M.; Wei, L.; Shang, H.F.; Li, J. Influence of hydrostatic pressure on water absorption of polyoxymethylene: Experiment and molecular dynamics simulation. Polym. Adv. Technol. 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.Y.; Chen, B.B.; Yan, F.Y. Tribocorrosion Behaviors of Inconel 625 Alloy Sliding against 316 Steel in Seawater. Tribol. Trans. 2011, 54, 514–522. [Google Scholar] [CrossRef]
- Kim, J.; Jang, H.; Kim, J. Friction and wear of monolithic and glass-fiber reinforced PA66 in humid conditions. Wear 2014, 309, 82–88. [Google Scholar] [CrossRef]
- Ye, Y.W.; Zhang, D.W.; Li, J.Y.; Liu, T.; Pu, J.B. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Jia, Y. Study on the Extraction and Isolation of Artificial Joint Wear Debris under Ultrasonic Irradiation. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2015. [Google Scholar]
- Wu, Z.J. Research and Prediction of Tribological Properties of Ptfe Matrix Composites in Fresh Water Environment; Yanshan University: Qinhuangdao, China, 2020. [Google Scholar]
- Liu, H.T.; Ge, S.R. Study on the relationship between wear debris thickness and positive pressure of UHMWPE. Sci. Bull. 2008, 53, 605–613. [Google Scholar] [CrossRef][Green Version]
- Qi, H.; Zhang, L.; Zhang, G.; Wang, T.M.; Wang, Q.H. Comparative study of tribochemistry of ultrahigh molecular weight polyethylene, polyphenylene sulfide and polyetherimide in tribo-composites. J. Colloid Interface Sci 2018, 514, 615–624. [Google Scholar] [CrossRef]
- Przedlacki, M.; Kajdas, C. Tribochemistry of Fluorinated Fluids Hydroxyl Groups on Steel and Aluminum Surfaces. Tribol. Trans. 2007, 49, 202–214. [Google Scholar] [CrossRef]
- Zhang, L.G.; Qi, H.M.; Li, G.T.; Zhang, G.; Wang, T.M.; Wang, Q.H. Impact of reinforcing fillers’ properties on transfer film structure and tribological performance of POM-based materials. Tribol. Int. 2016, 109, 58–68. [Google Scholar] [CrossRef]
- Qi, H.M.; Zhang, G.; Chang, L.; Zhao, F.Y.; Wang, T.M.; Wang, Q.H. Ultralow Friction and Wear of Polymer Composites under Extreme Unlubricated Sliding Conditions. Adv. Mater. Interfaces 2017, 4, 16011717. [Google Scholar] [CrossRef]
- Kajdas, C.K. Importance of the triboemission process for tribochemical reaction. Tribol. Int. 2008, 38, 337–353. [Google Scholar] [CrossRef]
- Kathryn, L.H.; Pitenis, A.A.; Sawyer, W.G.; Krick, B.A.; Blackman, G.S.; Kasprzak, D.J.; Junk, C.P. PTFE Tribology and the Role of Mechanochemistry in the Development of Protective Surface Films. Macromolecules 2015, 48, 3739–3745. [Google Scholar] [CrossRef]
- Lauer, J.L.; Bunting, B.G.; Jones, W.R., Jr. Investigation of Frictional Transfer Films of PTFE by Infrared Emission Spectroscopy and Phase-Locked Ellipsometry. Tribol. Trans. 2008, 31, 282–288. [Google Scholar] [CrossRef]
- Li, H. Identification of nylon 6 and nylon 66 by Fourier Transform Infrared Spectroscopy. Aging Appl. Synth. Mater. 2020, 49, 59–60. [Google Scholar]
- Guo, L.H.; Li, G.T.; Guo, Y.X.; Zhao, F.Y.; Zhang, L.G.; Wang, C.; Zhang, G. Extraordinarily low friction and wear of epoxy-metal sliding pairs lubricated with ultra-low sulfur diesel. ACS Sustain. Chem. Eng. 2018, 6, 15781–15790. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liu, G. Grain size effect on the electrochemical corrosion behavior of surface nanocrystallized low-carbon steel. Corrosion 2004, 60, 891–896. [Google Scholar] [CrossRef]
- Li, L. Corrosion Monitoring of Reinforced Concrete Structure Based on Electrochemical Theory. J. Nanoelectron. Optoelectron. 2018, 13, 572–577. [Google Scholar] [CrossRef]
- Lu, C.G.; Wei, Z.Q.; Qiao, H.X.; Li, K. Reliability life analysis of reinforced concrete in a salt corrosion environment based on a three-parameter Weibull distribution. J. Eng. Sci. 2021, 43, 512–520. [Google Scholar] [CrossRef]
- Tao, G.Y. Feasibility of Ultrasonic Rolling Treatment for Surface Protection of Pipeline Steel. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2020. [Google Scholar]
- Cui, G.; Bi, Q.; Zhu, S.; Fu, L.; Yang, J.; Qiao, Z.; Liu, W. Synergistic effect of alumina and graphite on bronze matrix composites: Tribological behaviors in sea water. Wear 2013, 4, 216–224. [Google Scholar] [CrossRef]
- Wang, L.; Jun, C.; Qiao, Z.; Yang, J.; Liu, W. Tribological behaviors of in situ TiB2 ceramic reinforced TiAl-based composites under sea water environment. Ceram. Int. 2017, 43, 4314–4323. [Google Scholar] [CrossRef]
- Wu, D.F.; Liu, Y.S.; Zhao, D.L.; Yong, X.F. Tribo-corrosion properties of WC-10Co-4Cr coating in natural silt-laden waters when sliding against Si3N4. Int. J. Refract. Met. Hard Mater. 2016, 58, 143–151. [Google Scholar] [CrossRef]







| Density(g/cm3) | Tensile Strength (MPa) | Elongation at Exercise (%) | |
|---|---|---|---|
| PA66 | 1.26 | 37 | 3.8 |
| UHMWPE | 0.93 | 22 | >50 |
| Element | Si | Fe | Cu | Mg | Zn | Mn | Ti | v |
|---|---|---|---|---|---|---|---|---|
| Mass fraction | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.005 | 0.005 |
| Element | Ca2+ | Mg2+ | I3- | K+ |
|---|---|---|---|---|
| Concentration(mg/L) | 410 | 1200 | 0.01 | 360 |
| Pin-Solution Environment | UHMWPE -Water | UHMWPE -Water-Powder | UHMWPE -Seawater | UHMWPE -Seawater-Powder |
|---|---|---|---|---|
| Ra(nm) | 280 | 234 | 350 | 302 |
| Pin Solution Environment | Empty | UHMWPE-Water | UHMWPE- Water-Powder | UHMWPE- Seawater | UHMWPE -Seawater-Powder |
|---|---|---|---|---|---|
| C | 0.22 | 1.72 | 2.14 | 1.61 | 3.16 |
| O | 1.11 | 5.36 | 4.85 | 52.96 | 26.76 |
| Pin-Solution Environment | PA66 -Water | PA66 -Water-Powder | PA66 -Seawater | PA66 -Seawater-Powder |
|---|---|---|---|---|
| Ra(nm) | 146 | 4311 | 181 | 275 |
| Pin-Solution Environment | Empty | PA66 -Water | PA66 -Water-Powder | PA66 -Seawater | PA66 -Seawater-Powder |
|---|---|---|---|---|---|
| C | 0.22 | 1.73 | 1.12 | 1.78 | 2.75 |
| O | 1.11 | 5.1 | 19.45 | 13.22 | 7.91 |
| Solution Environment | Corrosion Current Density(μA/cm2) |
|---|---|
| seawater | 6.342 |
| seawater after friction test | 2.199 |
| seawater with powder before friction test | 11.530 |
| seawater with powder after friction test | 2.083 |
| water | 0.704 |
| water after friction test | 1.214 |
| water with powder before friction test | 4.425 |
| water with powder after friction test | 0.463 |
| Solution Environment | Corrosion Current Density(μA/cm2) |
|---|---|
| seawater | 6.342 |
| seawater after friction test | 3.286 |
| seawater with powder before friction test | 10.420 |
| seawater with powder after friction test | 3.229 |
| water | 0.704 |
| water after friction test | 1.728 |
| water with powder before friction test | 1.709 |
| water with powder after friction test | 1.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yang, T.; Zhan, S.; Jia, D.; Ma, L.; Ma, S.; Duan, H. Effect of Powder on Tribological and Electrochemical Properties of Nylon 66 and Ultra-High Molecular Weight Polyethylene in Water and Seawater Environments. Polymers 2021, 13, 2874. https://doi.org/10.3390/polym13172874
Xu W, Yang T, Zhan S, Jia D, Ma L, Ma S, Duan H. Effect of Powder on Tribological and Electrochemical Properties of Nylon 66 and Ultra-High Molecular Weight Polyethylene in Water and Seawater Environments. Polymers. 2021; 13(17):2874. https://doi.org/10.3390/polym13172874
Chicago/Turabian StyleXu, Wanxing, Tian Yang, Shengpeng Zhan, Dan Jia, Lixin Ma, Saisai Ma, and Haitao Duan. 2021. "Effect of Powder on Tribological and Electrochemical Properties of Nylon 66 and Ultra-High Molecular Weight Polyethylene in Water and Seawater Environments" Polymers 13, no. 17: 2874. https://doi.org/10.3390/polym13172874
APA StyleXu, W., Yang, T., Zhan, S., Jia, D., Ma, L., Ma, S., & Duan, H. (2021). Effect of Powder on Tribological and Electrochemical Properties of Nylon 66 and Ultra-High Molecular Weight Polyethylene in Water and Seawater Environments. Polymers, 13(17), 2874. https://doi.org/10.3390/polym13172874







