Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Grafted Pullulan (BMO-PUL) Derivatives
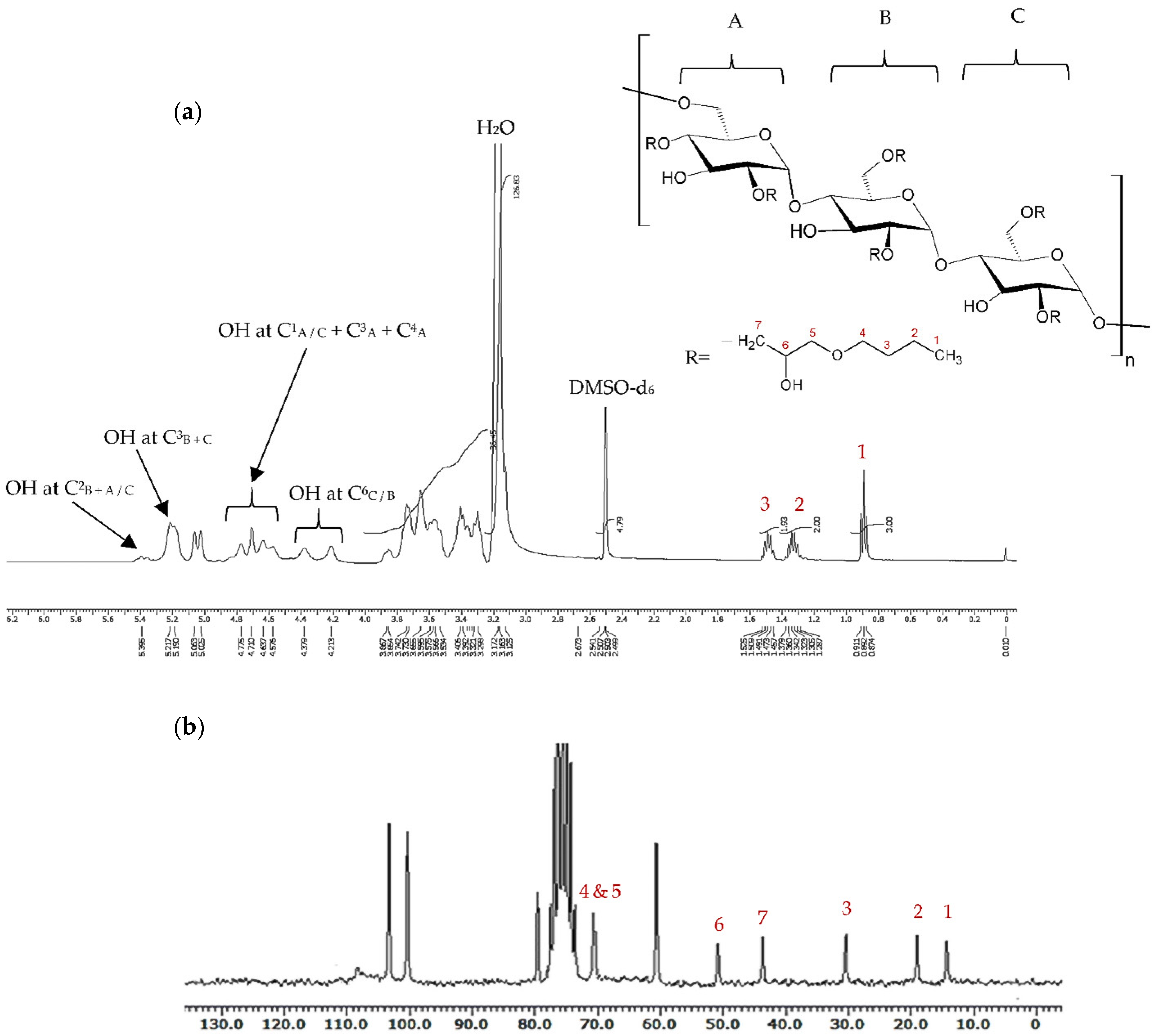
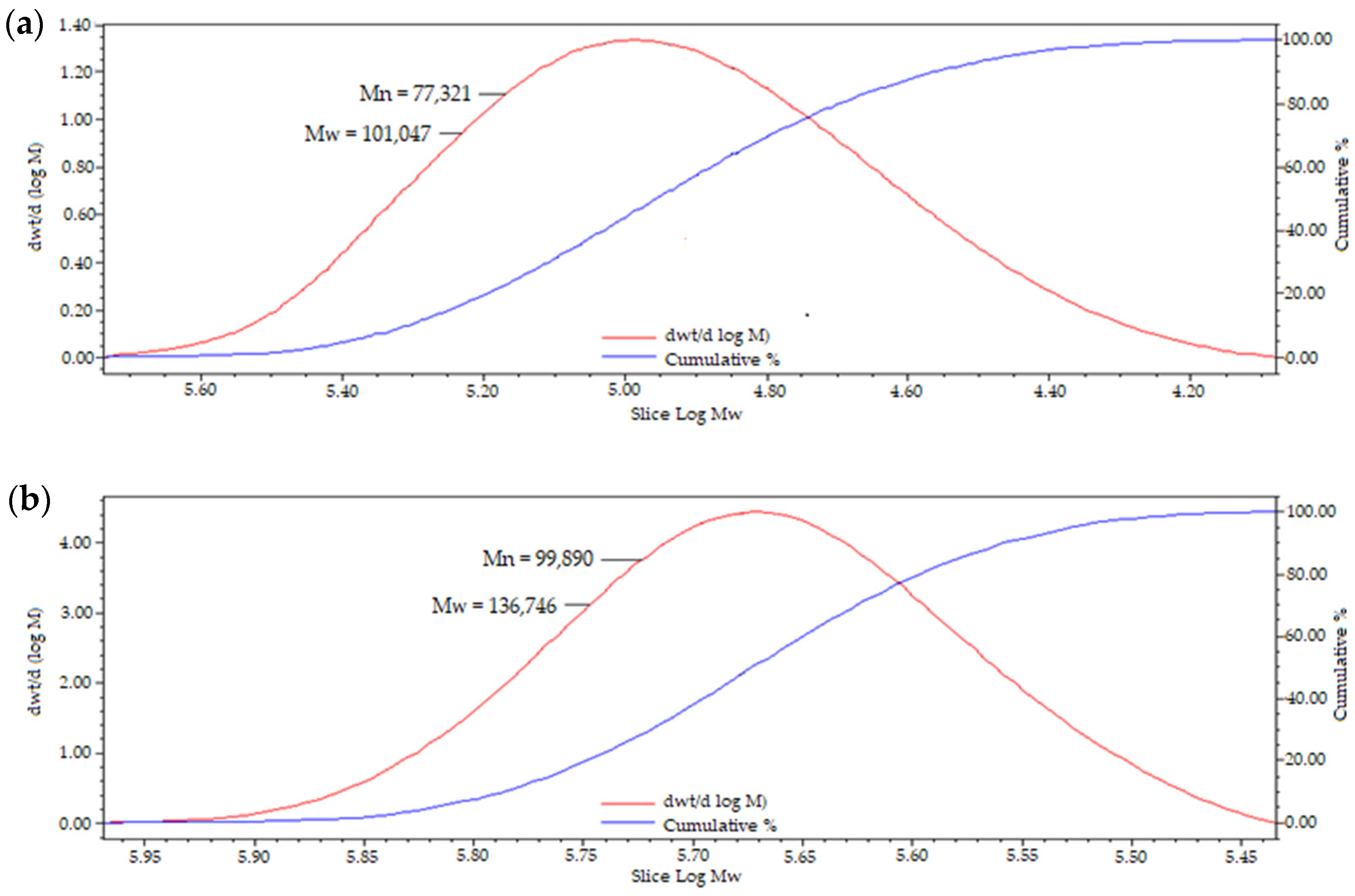
2.3. Characterisation of Grafted Pullulan (BMO-PUL) Derivatives
2.4. Formulation of Nanoparticles from Grafted Pullulan (BMO-PUL)
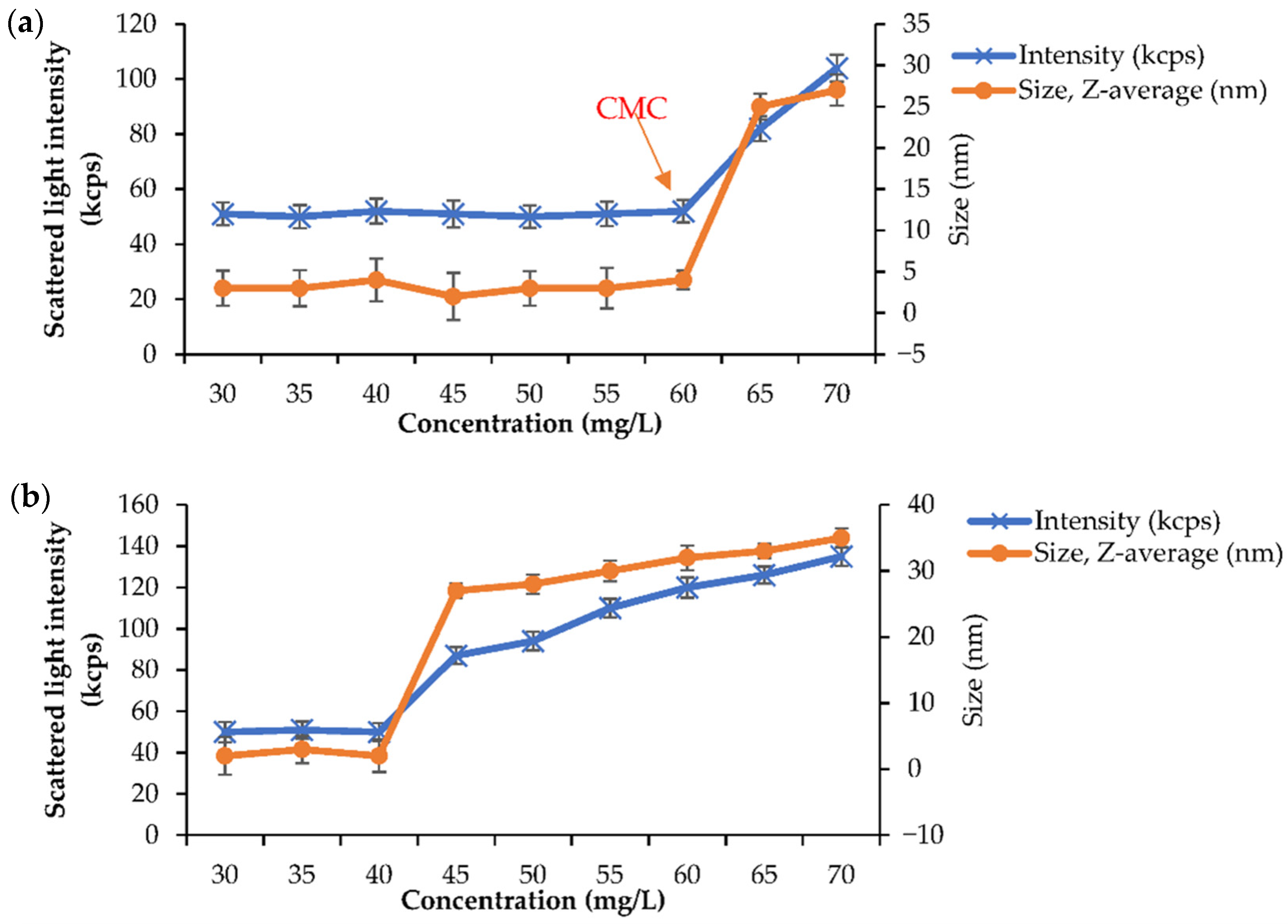
2.5. Physical Characterisation of Grafted Pullulan (BMO-PUL) Nanoparticles
2.6. Loading and Release Studies
2.7. Stability Studies
2.8. Cell Culture
2.9. Cytotoxicity Assay
2.10. Permeability Studies across HaCaT Cell Monolayers
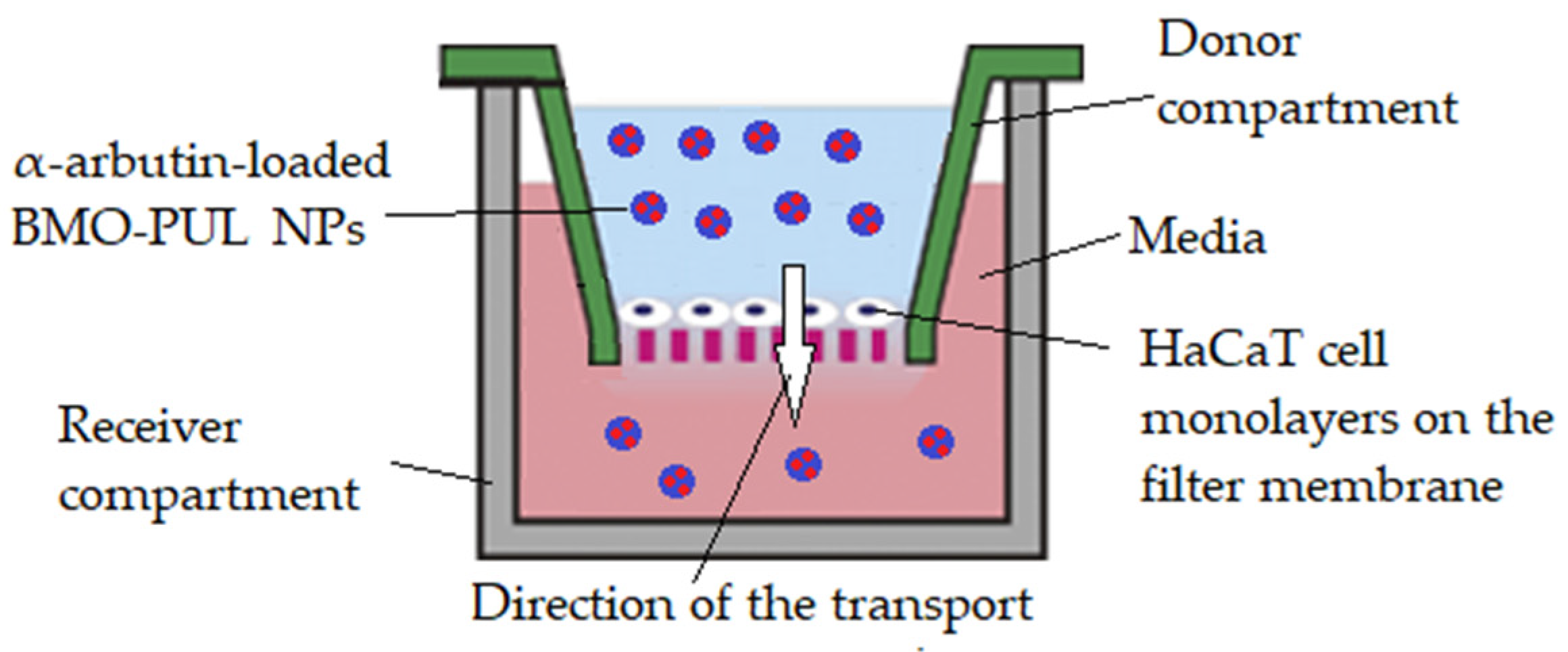
2.11. Data Statistical Analysis
3. Results and Discussion
4. Future Directions and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayumi, N.S.; Sahudin, S.; Hussain, Z.; Hussain, M.; Samah, N.H.A. Polymeric nanoparticles for topical delivery of alpha and beta arbutin: Preparation and characterization. Drug Deliv. Transl. Res. 2019, 9, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Nair, S.S. Chitosan-based transdermal drug delivery systems to overcome skin barrier functions. J. Drug Deliv. Ther. 2019, 9, 266–270. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Mishra, D.K.; Pandey, V.; Maheshwari, R.; Ghode, P.; Tekade, R.K. Cutaneous and Transdermal Drug Delivery: Techniques and Delivery Systems. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 595–650. [Google Scholar]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef]
- Nanda, A.; Nanda, S.; Nguyen, T.A.; Slimani, Y.; Rajendran, S. Nanocosmetics: Fundamentals, Applications and Toxicity; Micro and Nano Technologies: Jaipur, India, 2020; ISBN 0128222867. [Google Scholar]
- Li, H.; Xue, Y.; Jia, B.; Bai, Y.; Zuo, Y.; Wang, S.; Zhao, Y.; Yang, W.; Tang, H. The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydr. Polym. 2018, 188, 92–100. [Google Scholar] [CrossRef]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.-S.; Kumar, G.; Benelli, G.; Ghodake, G.S.; Jiang, Y.Y.; Kim, D.S.; Saratale, G.D. Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus × clementina peel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells. Environ. Sci. Pollut. Res. 2018, 25, 10250–10263. [Google Scholar] [CrossRef]
- Al-Owasi, Y.; Elshafie, A.; Sivakumar, N.; Al-Bahry, S. Synthesis of Pullulan-Mediated Silver Nanoparticles (AgNPs) and their Antimicrobial Activities. Sultan Qaboos Univ. J. Sci. 2019, 24, 88–94. [Google Scholar] [CrossRef]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef]
- Ganduri, V.; Mangamuri, U.; Muvva, V.; Poda, S. Pullulan-Stabilized Silver Nanoparticles -Their Synthesis, Characterization and Application as Bactericidal Agents. J. Appl. Pharm. Sci. 2016, 6, 027–037. [Google Scholar] [CrossRef]
- Dionísio, M.; Cordeiro, C.; Remuñán-López, C.; Seijo, B.; da Costa, A.M.R.; Grenha, A. Pullulan-based nanoparticles as carriers for transmucosal protein delivery. Eur. J. Pharm. Sci. 2013, 50, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Lalatsa, A.; Górecki, D.C.; Barbu, E. Engineering butylglyceryl-modified polysaccharides towards nanomedicines for brain drug delivery. Carbohydr. Polym. 2020, 236, 116060. [Google Scholar] [CrossRef]
- Yuan, H.; Zhong, W.; Wang, R.; Zhou, P.; Nie, Y.; Hu, W.; Tao, X.; Yang, P. Preparation of Cholesteryl-Modified Aminated Pullulan Nanoparticles to Evaluate Nanoparticle of Hydrophobic Degree on Drug Release and Cytotoxicity. J. Nanomater. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.-S.; Lee, G.-Y.; Kim, J.-K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Akagi, T.; Watanabe, K.; Kim, H.; Akashi, M. Stabilization of Polyion Complex Nanoparticles Composed of Poly(amino acid) Using Hydrophobic Interactions. Langmuir 2010, 26, 2406–2413. [Google Scholar] [CrossRef]
- Tao, X.; Jin, S.; Wu, D.; Ling, K.; Yuan, L.; Lin, P.; Xie, Y.; Yang, X. Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles. Nanomaterials 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Su, H.; Jin, R.; Li, D.; Wu, C.; Jiang, X.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes. Sci. Bull. 2015, 60, 1272–1280. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Korshunova, G.A.; Antonenko, Y.N. Effect of Alkyl Chain Length on Translocation of Rhodamine B n-Alkyl Esters across Lipid Membranes. Biophys. J. 2018, 115, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2015, 15, 237–250. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Noor, N.S.M.; Sahudin, S.; Lazim, A.M.; Tan, S.F.; Sarker, M.Z.I. Investigations of amphiphilic butylglyceryl-functionalized dextran nanoparticles for topical delivery. J. Appl. Polym. Sci. 2021, 138, 50235. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2020, 21, 25. [Google Scholar] [CrossRef]
- Won, J.; Park, J.W. Improvement of arbutin trans-epidermal delivery using radiofrequency microporation. Trop. J. Pharm. Res. 2014, 13, 1775. [Google Scholar] [CrossRef][Green Version]
- Easmin, S.; Sarker, M.Z.I.; Ghafoor, K.; Ferdosh, S.; Jaffri, J.M.; Akanda, M.J.H.; Al-Juhaimi, F.Y.; Bostanudin, F.M.; Khatib, A. Extraction of α-glucosidase inhibitory compounds from Phaleria macrocarpa fruit flesh using solvent, sonication, and subcritical carbon dioxide soxhlet methods. J. Food Biochem. 2017, 41, e12399. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of α-arbutin loaded dissolving and hydrogel forming microneedles. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Xu, K.; Bessarab, D.; Obaje, J.; Xu, C. Arbutin encapsulated micelles improved transdermal delivery and suppression of cellular melanin production. BMC Res. Notes 2016, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Bostanudin, M.F.; Arafat, M.; Sarfraz, M.; Górecki, D.C.; Barbu, E. Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization. Polymers 2019, 11, 789. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Kracht, J.-K.; Harder, L.E.; Rudnik, K.; Lindner, G.; Schimek, K.; Marx, U.; Pörtner, R. A Method for Determination and Simulation of Permeability and Diffusion in a 3D Tissue Model in a Membrane Insert System for Multi-well Plates. J. Vis. Exp. 2018, 132, e56412. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Abbas, K.; Jantan, I.; Bukhari, S.N.A. Polysaccharide-based materials in macromolecular prodrug design and development. Int. Mater. Rev. 2017, 62, 1–21. [Google Scholar] [CrossRef]
- Culica, M.E.; Kasperczyk, K.; Baron, R.I.; Biliuta, G.; Macsim, A.M.; Lazea-Stoyanova, A.; Orlinska, B.; Coseri, S. Recyclable Polymer-Supported N-Hydroxyphthalimide Catalysts for Selective Oxidation of Pullulan. Materials 2019, 12, 3585. [Google Scholar] [CrossRef]
- Pereira, J.M. Synthesis of New Pullulan Derivatives for Drug Delivery. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2013. [Google Scholar]
- Cumpstey, I. Chemical modification of polysaccharides. Int. Sch. Res. Not. 2013, 2013, 417672. [Google Scholar] [CrossRef]
- Caine, D. Potassium tert-Butoxide. Encycl. Reag. Org. Synth. 2001. [Google Scholar] [CrossRef]
- Li, W.; Zhou, P.; Li, G.; Lin, L.; Feng, X. Catalytic Asymmetric Halohydroxylation of α,β-Unsaturated Ketones with Water as the Nucleophile. Adv. Synth. Catal. 2020, 362, 1982–1987. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gulcan, H.O.; Saber-Samandari, S.; Gazi, M. Efficient Removal of Anionic and Cationic Dyes from an Aqueous Solution Using Pullulan-graft-Polyacrylamide Porous Hydrogel. Water Air Soil Pollut. 2014, 225, 1–14. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ji, F.; Bao, Y.; Wang, J.; Wang, X.; Guo, L.; Li, Y. New bifunctional-pullulan-based micelles with good biocompatibility for efficient co-delivery of cancer-suppressing p53 gene and doxorubicin to cancer cells. RSC Adv. 2015, 5, 94719–94731. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Xing, F.; Han, N. Effect of a healing agent on the curing reaction kinetics and its mechanism in a self- healing system. Appl. Sci. 2018, 8, 2241. [Google Scholar] [CrossRef]
- Panda, H. Epoxy Resins Technology Handbook (Manufacturing Process, Synthesis, Epoxy Resin Adhesives and Epoxy Coatings); Asia Pacific Business Press Inc.: New Delhi, India, 2019; ISBN 8178331829. [Google Scholar]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, H.; Liu, Y.; Wang, Y.; Li, H.; Yu, J. The use of of inulin, maltitol and lecithin as fat replacers and plasticizers in a model reduced-fat mozzarella cheese-like product. J. Sci. Food Agric. 2019, 99, 5586–5593. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Richel, A.; Blecker, C.; Boufi, S.; Attia, H.; Garna, H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019, 135, 647–658. [Google Scholar] [CrossRef]
- Bizot, H.; Le-Bail, P.; Leroux, B.; Davy, J.; Roger, P.; Buleon, A. Calorimetric evaluation of the glass transition in hydrated, linear and branched polyanhydroglucose compounds. Carbohydr. Polym. 1997, 32, 33–50. [Google Scholar] [CrossRef]
- Roos, Y.H.; Drusch, S. Phase Transitions in Foods; Academic Press: Cambridge, MA, USA, 2015; ISBN 0124079229. [Google Scholar]
- Karakus, S.; Tan, E.; Ilgar, M.; Kahyaoglu, I.M.; Şahin, Y.M.; Mansuroglu, D.S.; Ismik, D.; Tasaltin, N.; Kilislioglu, A. Preparation, Characterization, and Swelling Behavior of PEGylated Guar Gum @ Ag Nanoparticles. In Sonochemical Reactions; IntechOpen: London, UK, 2019. [Google Scholar]
- Lapčík, L., Jr.; Benešová, K.; Lapčík, L.; De Smedt, S.; Lapčíková, B. Chemical modification of hyaluronic acid: Alkylation. Int. J. Polym. Anal. Charact. 2010, 15, 486–496. [Google Scholar] [CrossRef]
- Chen, N.-X.; Zhang, J.-H. The role of hydrogen-bonding interaction in poly(vinyl alcohol)/poly(acrylic acid) blending solutions and their films. Chin. J. Polym. Sci. 2010, 28, 903–911. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, G.-Y.; Jeong, Y.-I.; Kang, M.-S.; Oh, J.-S.; Song, C.-E.; Lee, H.C. Paclitaxel-incorporated nanoparticles of hydrophobized polysaccharide and their antitumor activity. Int. J. Pharm. 2012, 433, 121–128. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. micelle Determination of critical concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Shah, M.; Choi, M.H.; Yoon, S.C. Amphiphilic polymeric nanoparticles for drug delivery: Synthesis and characterization. In Proceedings of the ICONN 2010—2010 International Conference on Nanoscience and Nanotechnology, Sydney, NSW, Australia, 22–26 February 2010. [Google Scholar]
- Akagi, T.; Baba, M.; Akashi, M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: Potential applications. Polymer 2007, 48, 6729–6747. [Google Scholar] [CrossRef]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of Hydrophilic and Lipophilic Drugs in PLGA Nanoparticles by the Nanoprecipitation Method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef]
- Liao, A.-H.; Ma, W.-C.; Wang, C.-H.; Yeh, M.-K. Penetration depth, concentration and efficiency of transdermal α-arbutin delivery after ultrasound treatment with albumin-shelled microbubbles in mice. Drug Deliv. 2014, 23, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Avonto, C.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Quantitative Determination of α-Arbutin, β-Arbutin, Kojic Acid, Nicotinamide, Hydroquinone, Resorcinol, 4-Methoxyphenol, 4-Ethoxyphenol, and Ascorbic Acid from Skin Whitening Products by HPLC-UV. J. AOAC Int. 2015, 98, 5–12. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical modeling of drug release from swellable polymeric nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP)—Chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1430–1434. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Jafari, A.; Javadian, S. Temperature effect on performance of nanoparticle/surfactant flooding in enhanced heavy oil recovery. Pet. Sci. 2019, 16, 1387–1402. [Google Scholar] [CrossRef]
- Alameda, J.P.; Navarro, M.; Ramírez, Á.; Page, A.; Suárez-Cabrera, C.; Moreno-Maldonado, R.; Paramio, J.M.; del Carmen Fariña, M.; Del Río, M.; Fernández-Aceñero, M.J.; et al. IKKα regulates the stratification and differentiation of the epidermis: Implications for skin cancer development. Oncotarget 2016, 7, 76779. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.N.; Sahudin, S.; Lazim, A.M.; Azfaralariff, A. Formulation and In-Vitro Characterisation of Cross-linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Vavrova, K.; Zbytovska, J.; Hrabálek, A. Amphiphilic Transdermal Permeation Enhancers: Structure-Activity Relationships. Curr. Med. Chem. 2005, 12, 2273–2291. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Quan, P.; Wan, X.; Liu, C.; Liu, X.; Fang, L. Mechanistic insights of the enhancement effect of sorbitan monooleate on olanzapine transdermal patch both in release and percutaneous absorption processes. Eur. J. Pharm. Sci. 2017, 107, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, L.; Gong, K.; Zhi, X.; Guan, Y.; Cai, W. Identification of the Constituents of Percutaneous Absorption from Duhaldea nervosa Based on UHPLC-Q-Exactive Orbitrap MS and Microdialysis Technique. Int. J. Anal. Chem. 2019, 2019, 8328942. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD: Paris, France, 2004; pp. 1–31. [Google Scholar]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 3662532700. [Google Scholar]









| Material (DG %) | H2O Evaporation | Decomposition | Tg (°C) | ||
|---|---|---|---|---|---|
| DTG Peak (°C) | Mass Loss (%) | DTG Peak (°C) | Mass Loss (%) | ||
| Native pullulan | 80.9 ± 4.4 | 6.52 ± 1.9 | 288.5 ± 3.2 | 77.17 ± 6.8 | 21.3 ± 0.8 |
| BMO-PUL (5.8%) | 102.3 ± 4.8 | 5.32 ± 1.4 | 340.5 ± 4.9 | 78.02 ± 4.1 | 24.9 ± 0.7 |
| BMO-PUL (12.6%) | 104.5 ± 7.7 | 3.59 ± 0.9 | 349.2 ± 5.2 | 85.50 ± 5.9 | 26.7 ± 1.1 |
| BMO-PUL (33.8%) | 108.8 ± 5.1 | 2.93 ± 1.0 | 358.8 ± 3.6 | 88.67 ± 7.8 | 30.1 ± 1.2 |
| Material | Mn ± SD | Mw ± SD | DI ± SD |
|---|---|---|---|
| PUL (Mw 100 kDa) | 77,121 ± 828 | 101,191 ± 923 | 1.31 ± 1.11 |
| BMO-PUL (DG 12.6%) | 86,236 ± 970 | 117,007 ± 911 | 1.36 ± 0.94 |
| BMO-PUL (DG 33.8%) | 99,991 ± 829 | 136,526 ± 931 | 1.37 ± 1.12 |
| Material | Viscosity η (cp) |
|---|---|
| Pullulan | 1.82 ± 0.1 |
| BMO-PUL (DG 12.6%) | 1.58 ± 0.1 |
| BMO-PUL (DG 33.8%) | 1.41 ± 0.1 |
| DG (%) | Polymer Conc. (mg/mL) | Diameter (nm) | DI | ζ Potential (mV) |
|---|---|---|---|---|
| 12.6 | 1 | 143 ± 12 | 0.18 ± 0.07 | −25.5 ± 3.0 |
| 5 | 155 ± 16 | 0.17 ± 0.09 | −23.3 ± 2.3 | |
| 10 | 182 ± 18 | 0.12 ± 0.06 | −28.9 ± 4.1 | |
| 33.8 | 1 | 136 ± 15 | 0.19 ± 0.05 | −25.7 ± 1.9 |
| 5 | 145 ± 19 | 0.12 ± 0.11 | −23.7 ± 2.3 | |
| 10 | 173 ± 18 | 0.13 ± 0.09 | −34.2 ± 4.2 | |
| 47.4 | 1 | 125 ± 13 | 0.14 ± 0.11 | −26.7 ± 5.2 |
| 5 | 139 ± 12 | 0.18 ± 0.04 | −28.6 ± 3.2 | |
| 10 | 158 ± 19 | 0.20 ± 0.07 | −29.3 ± 4.1 |
| pH Medium | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | Hixson-Crowell |
|---|---|---|---|---|---|
| 5 | 0.7986 | 0.9804 | 0.9489 | 0.3864 | 0.9384 |
| 7 | 0.8746 | 0.9829 | 0.9737 | 0.45 | 0.9641 |
| Material | Papp 5 h (cm/s) |
|---|---|
| Free α-arbutin | (1.29 ± 0.26) × 10−6 |
| α-arbutin-loaded BMO-PUL NPs | (5.55 ± 0.14) × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostanudin, M.F.; Barbu, E.; Liew, K.B. Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation. Polymers 2021, 13, 2852. https://doi.org/10.3390/polym13172852
Bostanudin MF, Barbu E, Liew KB. Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation. Polymers. 2021; 13(17):2852. https://doi.org/10.3390/polym13172852
Chicago/Turabian StyleBostanudin, Mohammad F., Eugen Barbu, and Kai Bin Liew. 2021. "Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation" Polymers 13, no. 17: 2852. https://doi.org/10.3390/polym13172852
APA StyleBostanudin, M. F., Barbu, E., & Liew, K. B. (2021). Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation. Polymers, 13(17), 2852. https://doi.org/10.3390/polym13172852







