Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Ethical Issues
2.3. Eligible Criteria
2.4. Article Selection and Data Extraction
3. Results
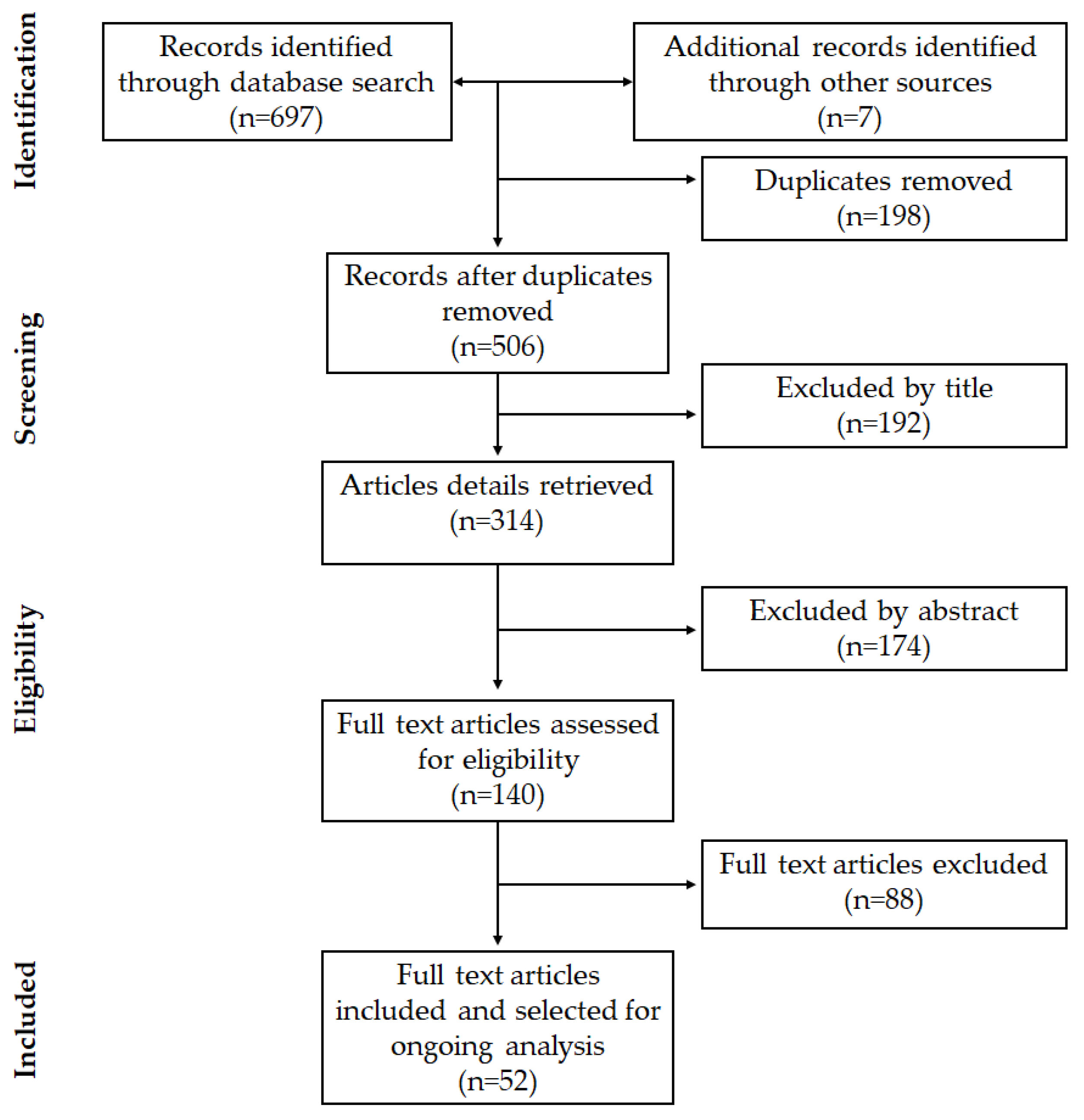
3.1. Study Selection
3.2. Characteristics of the Selected Studies
4. Discussion
4.1. Polymeric Nerve Guide Conduits
4.2. Morphological Characteristics of NGCs
4.2.1. Scaffold Diameter/Adjustment
4.2.2. Scaffold Wall Thickness
4.2.3. Porosity of the Scaffold
4.2.4. Pore Size
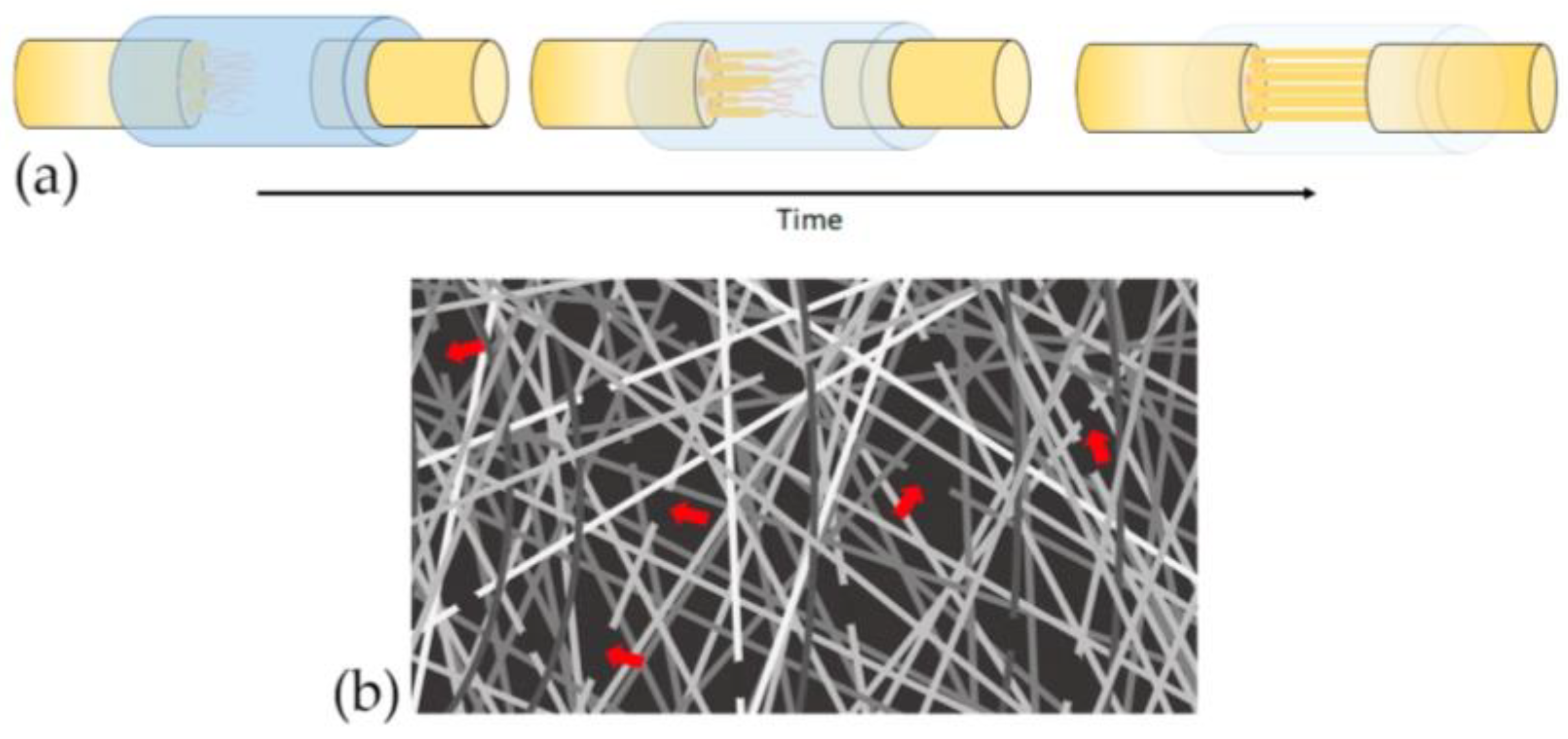
4.2.5. Distribution and Orientation of Scaffold Pores
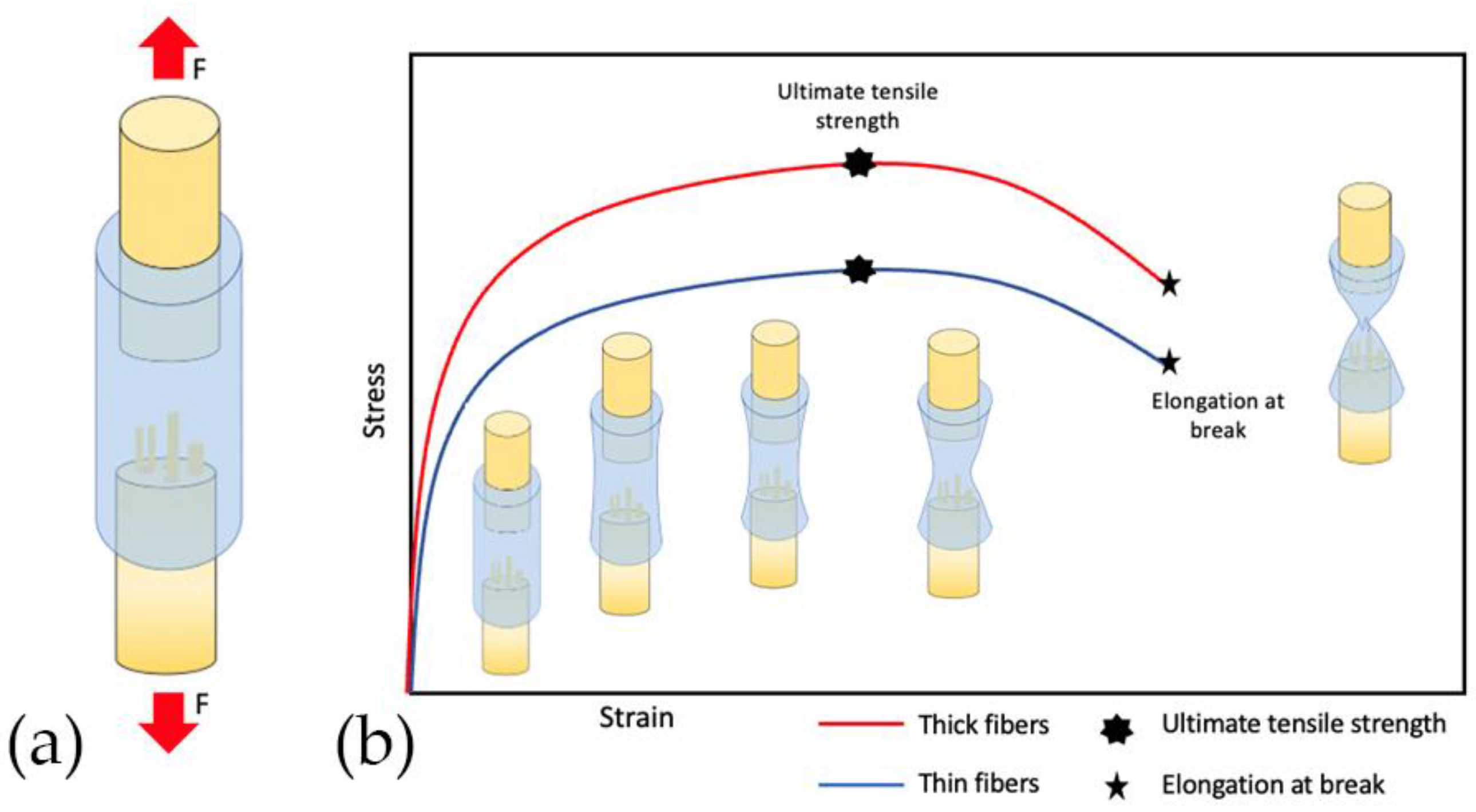
4.2.6. Diameter of Polymer Fiber
4.2.7. Alignment of the Polymer Fibers
4.3. Biodegradable Properties
4.4. Mechanical Properties
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NGC | Nerve Guide Conduits |
| ECM | Extracellular matrix |
| CNS | Central Nervous System |
| SCs | Schwann cells |
| UTS | Ultimate Tensile Strength |
| EM | Elastic modulus |
| EB | Elongation at break |
| NTFs | Neurotrophic factors |
References
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Mi, R.; Hoke, A.; Chew, S.Y. Nanofibrous nerve conduit-enhanced peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Satish, A.; Korrapati, P.S. Strategic design of peptide-decorated aligned nanofibers impregnated with triiodothyronine for neural regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Meng, H.Y.; Chang, B.; Liu, G.B.; Cheng, X.Q.; Tang, H.; Wang, Y.; Peng, J.; Zhao, Q.; Lu, S.B. Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage. Neural Regen. Res. 2019, 14, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Fabrication of Nerve Growth Factor Encapsulated Aligned Poly (ε-Caprolactone) Nanofibers and Their Assessment as a Potential Neural Tissue Engineering Scaffold. Polymers 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Namgung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 1601–1609. [Google Scholar] [CrossRef]
- Yao, L.; de Ruiter, G.C.; Wang, H.; Knight, A.M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Pandit, A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010, 5789–5797. [Google Scholar] [CrossRef]
- Panahi-Joo, Y.; Karkhaneh, A.; Nourinia, A.; Abd-Emami, B.; Negahdari, B.; Renaud, P.; Bonakdar, S. Design and fabrication of a nanofibrous polycaprolactone tubular nerve guide for peripheral nerve tissue engineering using a two-pole electrospinning system. Biomed. Mater. 2016, 11, 025017. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Hsu, S.H.; Yen, H.J.; Chang, H.; Hsu, S.K. Effects of unidirectional permeability in asymmetric poly (DL-lactic acid-co-glycolic acid) conduits on peripheral nerve regeneration: An in vitro and in vivo study. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2007, 83, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Rao, K.M.; Kamal, T.; Alghyamah, A.A.A.; Jan, I.F.; Bano, B.; Khan, N.; Amjid, A.M.; Soon, H.S.; et al. Advances in the Scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A Technical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Aldini, N.; Fini, M.; Giardino, R. Preparation of a new nerve guide from a poly(L-lactide-co-6-caprolactone). Biomaterials 1994, 15, 189–193. [Google Scholar] [CrossRef]
- Den Dunnen, W.F.A.; Van Der Lei, B.; Robinson, P.H.; Holwerda, A.; Pennings, A.J.; Schakenraad, J.M. Biological performance of a degradable poly (lactic acid-ε-caprolactone) nerve guide: Influence of tube dimensions. J. Biomed. Mater. Res. 1995, 29, 757–766. [Google Scholar] [CrossRef]
- Rutkowski, G.E.; Heath, C.A. Development of a bioartificial nerve graft. I. Design based on a reaction-diffusion model. Biotechnol. Prog. 2002, 18, 362–372. [Google Scholar] [CrossRef]
- Mobasseri, A.; Faroni, A.; Minogue, B.M.; Downes, S.; Terenghi, G.; Reid, A.J. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng. Part A 2015, 21, 1152–1162. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Geetha, B.R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Hu, Y.; Wang, S.; Zhang, J.; Liu, X.; Gou, Z.; Cheng, H.; Liu, Q.; Zhang, Q.; You, S.; et al. A 3D-engineered porous conduit for peripheral nerve repair. Sci. Rep. 2017, 7, 46038. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, J.; Mallu, S.; Yan, W.; Little, B. Consequences of oversizing: Nerve-to-nerve tube diameter mismatch. J. Bone. J. Sur. Am. 2014, 96, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Den Dunnen, W.F.; Meek, M.F.; Robinson, P.H.; Schakernraad, J.M. Peripheral nerve regeneration through P(DLLA-epsilon-CL) nerve guides. J. Mater. Sci. Mater. Med. 1998, 9, 811–814. [Google Scholar] [CrossRef]
- Rutkowski, G.E.; Heath, C.A. Development of a bioartificial nerve graft. II. Nerve regeneration in vitro. Biotechnol. Prog. 2002, 18, 373–379. [Google Scholar] [CrossRef]
- Jenq, C.B.; Coggeshall, R.E. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res. 1987, 408, 239–242. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, S.H. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials 2006, 27, 1035–1042. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; de Ruiter, G.C.W.; Wolfs, J.F.C.; Pêgo, A.P.; van den Berg, R.J.; Feirabend, H.K.P.; Malessy, M.J.A.; Lakke, E.A.J.F. Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. A 2007, 80, 965–982. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, J.H. Fabrication and characterization of hydrophilized porous PLGA nerve guide conduits by a modified immersion precipitation method. J. Biomed. Mater. Res. A 2007, 80, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Lin, Y.C.; Oyster, N.M.; Marra, K.G. Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomater. 2009, 5, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, J.R.; Kwon, G.B.; Namgung, U.; Song, K.S.; Lee, J.H. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng. Part. C Methods 2013, 19, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Oh, S.H.; An, H.Y.; Kim, Y.M.; Lee, J.H.; Lim, J.Y. Functional regeneration of recurrent laryngeal nerve injury during thyroid surgery using an asymmetrically porous nerve guide conduit in an animal model. Thyroid 2014, 24, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, F.; Zamanian, A.; Nojehdehian, H. Effects of pore orientation on in-vitro properties of retinoic acid-loaded PLGA/gelatin scaffolds for artificial peripheral nerve application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, L.; Shi, X.; Xia, B.; Liu, Z.; Zhu, S.; Yang, Y.; Ma, T.; Cheng, P.; Luo, K.; et al. A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo. Acta Biomater. 2018, 68, 223–236. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Zhang, S.; Thaharah, S.; Sriram, G.; Lu, W.F.; Fuh, J. Electrohydrodynamic Jet 3D Printed Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Polymers 2018, 10, 753. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, K.M.; Hix, J.; Shapiro, E.M.; Sakamoto, J. The mechanics of scaling-up multichannel scaffold technology for clinical nerve repair. J. Mech. Behav. Biomed. Mater. 2019, 91, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Wen, X.; Tresco, P.A. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J. Biomed. Mater. Res. A 2006, 76, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta. Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef]
- Daud, M.F.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 620–631. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Zhao, Q.; Li, X.; Xu, F.; Yao, X.; Wang, M. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; O’Brien, N.; Windebank, A.; Pandit, A. Orienting neurite growth in electrospun fibrous neural conduits. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Yong, T.; Teo, W.E.; Chan, C.K.; Puhaindran, M.E.; Tan, T.C.; Lim, A.; Lim, B.H.; Ramakrishna, S. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J. Neural. Eng. 2010, 7, 046003. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Patel, S.; Kurpinski, K.; Diao, E.; Bao, X.; Kwong, G.; Young, W.L.; Li, S. Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration. Tissue Eng. Part. C Methods 2011, 17, 705–715. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Bhattarai, N.; Zhang, M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85, 149–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Zhao, B.; Qi, H.X.; Peng, J.; Zhang, L.; Xu, W.J.; Hu, P.; Lu, S.B. Biocompatibility evaluation of electrospun aligned poly (propylene carbonate) nanofibrous scaffolds with peripheral nerve tissues and cells in vitro. Chin. Med. J. 2011, 124, 2361–2366. [Google Scholar] [PubMed]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. A 2012, 100, 406–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kijeńska, E.; Prabhakaran, M.P.; Swieszkowski, W.; Kurzydlowski, K.J.; Ramakrishna, S. Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1093–1102. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Nasr-Esfahani, M.H.; Sadri, S.; Hilderink, J.; Van Apeldoorn, A.; Van Blitterswijk, C.A.; Moroni, L. Fabrication, characterization and cellular compatibility of poly (hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS ONE 2013, 8, e57157. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Huang, C.; Zhu, Y.; Fan, C.; Ke, Q. Fabrication of seamless electrospun collagen/PLGA conduits whose walls comprise highly longitudinal aligned nanofibers for nerve regeneration. J. Biomed. Nanotechnol. 2013, 9, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Liu, W.; Jesuraj, N.; Li, X.; Hunter, D.; Xia, Y. Nerve Guidance Conduits Based on Double-Layered Scaffolds of Electrospun Nanofibers for Repairing the Peripheral Nervous System. ACS Appl. Mater. Interfaces 2014, 6, 9472–9480. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Kuppuswamy, A.A.; Sethuraman, S.; Subramanian, A. Topographic Cue from Electrospun Scaffolds Regulate Myelin-Related Gene Expressions in Schwann Cells. J. Biomed. Nanotechnol. 2015, 11, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, F.; Wang, C.; Xia, Z.; Mo, X.; Fan, C. The role of an aligned nanofiber conduit in the management of painful neuromas in rat sciatic nerves. Ann. Plast. Surg. 2015, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Gum tragacanth/poly(l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016, 140, 104–112. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, H.X.; Ortiz, L.S.; Xiao, Z.D.; Huang, N.P. Laminin-modified and aligned poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide nanofibrous nerve conduits promote peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e627–e636. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. In vitro evaluation of gelatin and chitosan electrospun fibres as an artificial guide in peripheral nerve repair: A comparative study. J. Tissue Eng. Regen. Med. 2018, 12, e679–e694. [Google Scholar] [CrossRef]
- Karimi, A.; Karbasi, S.; Razavi, S.; Zargar, E.N. Poly(hydroxybutyrate)/chitosan Aligned Electrospun Scaffold as a Novel Substrate for Nerve Tissue Engineering. Adv. Biomed. Res. 2018, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, W.; Zhang, K.; Qiu, S.; Xu, J.; Wang, C.; Chai, Y. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta. Biomater. 2019, 83, 291–301. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, Z.; Chen, F.; Wang, X.; Ren, M.; Zhao, Y.; Wu, P.; He, X.; Chen, P.; Chen, Y. Aligned soy protein isolate-modified poly (L-lactic acid) nanofibrous conduits enhanced peripheral nerve regeneration. J. Neural. Eng. 2020, 17, 036003. [Google Scholar] [CrossRef]
- Pateman, C.J.; Harding, A.J.; Glen, A.; Taylor, C.S.; Christmas, C.R.; Robinson, P.P.; Rimmer, S.; Boissonade, F.M.; Claeyssens, F.; Haycock, J.W. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015, 49, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2018, 4, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Kemp, G.J.; Frostick, S.P. Neurotrophins, Neurones and Peripheral Nerve Regeneration. J. Hand Surg. 1998, 23, 433–437. [Google Scholar] [CrossRef]
- Sultana, T.; Amirian, J.; Park, C.; Lee, S.J.; Lee, B.T. Preparation and characterization of polycaprolactone-polyethylene glycol methyl ether and polycaprolactone-chitosan electrospun mats potential for vascular tissue engineering. J. Biomater. Appl. 2017, 32, 648–662. [Google Scholar] [CrossRef]
- Gomes, L.E.; Dalmarco, E.M.; André, E.S. The brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, and induced nitric oxide synthase expressions after low-level laser therapy in an axonotmesis experimental model. Photomed. Laser Surg. 2012, 30, 642–647. [Google Scholar] [CrossRef]
- Santos, D.; Giudetti, G.; Micera, S.; Navarro, X.; Del Valle, J. Focal release of neurotrophic factors by biodegradable microspheres enhance motor and sensory axonal regeneration in vitro and in vivo. Brain Res. 2016, 1636, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Prabhakaran, M.P.; Hu, J.; Chen, M.; Besenbacher, F.; Ramakrishna, S. Synergistic effect of topography, surface chemistry and conductivity of the electrospun nanofibrous scaffold on cellular response of PC12 cells. Colloids Surf. B Biointerfaces 2016, 145, 420–429. [Google Scholar] [CrossRef]
- Kim, Y.T.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 2008, 29, 3117–3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Study | Type of Study | NGC Material | Morphological Parameter | Main Outcome |
|---|---|---|---|---|
| Den Dunnen et al., 1995 [14] | In vivo Sciatic nerve/rats | Lactic acid-caprolactone | Tube dimensions (Diameters) NGC Wall thicknesses | NGC diameters influenced biodegradation, foreign body reaction, and nerve regeneration. |
| Isaacs et al., 2014 [21] | In vivo Sciatic nerve/female Sprague-Dawley (SD) rats | Collagen (type I) | Tube dimensions (Diameters) | NGC diameters influenced tube collapse, nerve regeneration, and decreased muscle reinnervation. |
| Study | Type of Study | Material | Morphological Parameter | Main Outcome |
|---|---|---|---|---|
| Perego et al., 1994 [13] | In vitro | Poly(l-lactide-co-6-caprolactone) | Wall thicknesses | Wall thicknesses influenced values of tensile strength, percent elongation at break, and elastic modulus. |
| Den Dunnen et al., 1998 [22] | In vivo Sciatic nerve/rat | Copolymer of DLlactide and e-caprolacone | Internal diameter Wall thicknesses | Evaluated the biodegradation, collapsed, nerve regeneration. |
| Rutkowski et al., 2002 [15] | In vitro: Schwann Cell (SC) Cultures. Dorsal root ganglia (DRG) of SD rats. | Poly-d, l-lactide | The computer model predicts the wall thickness, porosity, and SC seeding density needed to maximize the axon extension rate while ensuring that sufficient nutrients to the neurons. | Transport of nutrients, nerve growth factor, oxygen, glucose, and nerve regeneration. |
| Rutkowski et al., 2002 [23] | In vitro: SC Cultures. DRG of SD rats. | Poly-d, l-lactide with SC | Porosity Wall thicknesses | Wall thickness and porosities influenced the number of nutrients and growth factors made available to the neural tissue. Higher porosities: more growth factors diffused out of the conduit. Low porosities: competition for nutrients. |
| Mobasseri et al., 2015 [16] | In vitro: stem cells differentiated to Schwann cell-like cells. In vivo: SD rat sciatic nerve injury. | Poly ε-caprolactone (PCL) and polylactic acid (PLA) | Wall thicknesses | Increasing the thickness of the wall increased stiffness and limited the permeability of the canal, so it did not show any positive effect on the biological response of the regenerating nerve. |
| Study | Type of Study | Material | Fabrication Technique | Morphological Parameter | Main Outcome |
|---|---|---|---|---|---|
| Jenq et al., 1987 [24] | In vivo: sciatic nerve of SD rats | Silicone | Not reported | Porosity | The regenerating axons crossed much longer gaps if the tube was permeable. |
| Chang et al., 2006 [25] | In vivo: sciatic nerve defects in SD rats. | Poly(dl-lactic acid-co-glycolic acid) (PLGA) | Immersion precipitation method | Conduits with asymmetric porosity (The external surface of the NGC has a larger pore size than the lumen surface). | Asymmetric PLGA NGC showed a stable supporting structure, inhibiting exogenous cell invasion during the regeneration, higher regenerated axons at the mid-conduit, and distal nerve site. Asymmetric structure in the NGC wall enhanced the removal of the blockage of the waste drain from the inner inflamed wound in the early stage. |
| Vleggeert-Lankamp et al., 2007 [26] | In vivo: sciatic nerve of Wistar rat | Poly-(ε-caprolactone) | NaCl as porosifying agent preparing porous structures | Size of pores. Conduits with asymmetric porosity. | The pore size of the outer and inner layers influenced tissue bridge formation, myelination, nerve regeneration, electrophysiological response rate, and muscle reinnervation. |
| Oh et al., 2007 [27] | Pre-Experimental Study of Biomaterials Development (BD) | Poly(lactic-co-glycolic acid) PLGA and Pluronic F127 | Modified immersion precipitation | Conduits with asymmetric porosity—nanopores on the inner surface and micropores on the outer surface. | Asymmetric NGC influenced optimal mechanical properties and hydrophilicity and affected nutrient permeability. |
| Chan et al., 2007 [11] | In vitro: SC and FibroblastsIn vivo: sciatic nerve of SD rats | Poly(DL-lactic acid-co-glycolic acid) (PLGA) | Immersion–precipitation phase inversion using a casting process | Asymmetric poreswith high, medium, and low porosity. | Asymmetric porosity NGC influenced the nutrients and oxygen permeation, and proliferation of SC. Also prevented fibrous scar tissue invasion. Unidirectional permeability NGC showed more myelin fibers than the high bidirectional patency NGC. |
| Oh et al., 2008 [8] | In vivo: Sciatic nerve of SD rats | Poly(lactic-co-glycolic acid) (PLGA) and Pluronic F127 | Modified immersion precipitation method | Conduits with asymmetric porosity. | Asymmetric porosity NGC influenced the infiltration of fibrous tissue, neurotrophic factors, and nutrients. Allowing vascular growth for effective delivery of nutrients and oxygen, resulting in rapid and continuous axonal growth. |
| Kokai et al., 2009 [28] | Pre-Experimental Study of BD | Poli (caprolactona) (PCL) | Dip-coating/salt-leaching technique | Wall thickness Porosity Pore size | The wall thickness influences permeability molecules as lysozyme and glucose. The porosity determines the interconnected through-pores for transluminal flow and solute diffusion. |
| Oh et al., 2013 [29] | In vivo: Sciatic nerve of rats | Polycaprolactone (PCL)/Pluronic F127 | Immersion precipitation method | Asymmetric pores | Nerve fibers regenerated along the longitudinal direction through the NGC with a nano-porous inner surface, while they were grown toward the porous wall of the NGC with a micro-porous inner surface. |
| Choi et al., 2014 [30] | In vivo: Recurrent laryngeal nerve of rabbits | Polycaprolactone (PCL)/Pluronic F127 | Immersion precipitation method | Conduits with asymmetric porosity | Asymmetrically porous PCL/F127 NGC tubes facilitated nerve regeneration compared with nonporous silicone tubes. |
| Kim et al., 2016 [2] | In vitro: PC12 and S42 cells | Poly lactic-co-glycolic acid (PLGA) and polyurethane (PU) | Electrospinning | Alignment of fibers Pore size | The alignment of fibers affects the porosity and pores diameter. The pore diameter in the aligned nanofibrous mat was 3× larger and the porosity of the aligned nanofibrous scaffolds was higher. Additionally, alignment nanofibers influence cells proliferation and migration |
| Ghorbani et al., 2017 [31] | In vitro: L929 fibroblast cells | Poly (lactic-co-glycolic acid) (PLGA) | Freeze-drying and freeze-cast molding method | Porosity Pore size | Randomly oriented pore (freeze-dried) and interconnected pore (freeze-cast) scaffolds mimic ECM to support cellular adhesion and migration. Different scaffold manufacturing processes affect their properties by altering the microstructure of pores. |
| Huang et al., 2018 [32] | In vitro: DRG cells cultures In vivo: not reported | Poly(ε-caprolactone) (PCL) sheaths and collagen-chitosan (O-CCH) filler. | Electrospinning | Pore size Wall thickness | NGC pore size influenced the fibroblast invasion and the mechanical strength. Porosity influences nerve regeneration and functional recovery. |
| Vijayavenkataraman et al., 2018 [33] | In vitro: PC12 cells | Poli (ε-caprolactona) (PCL) | Electrohydrodynamic jet 3D printing (EHD-jetting) | Pore size Porosities | Pore size and porosity significantly influenced mechanical properties and scaffold degradation. |
| Pawelec et al., 2019 [34] | Pre-Experimental Study of BD | Poly(lactide co-glycolide) (PLGA) Poly(caprolactone) (PCL) | Polymer + salt slurry | Porosity Wall thickness | Porosity and biomaterials influenced the compliance and bending stiffness. Porous PLGA scaffolds were stiffer than porous PCL, fracturing in bend tests. PCL showed high compliance without signs of deformation/kinking after bending. |
| Study | Study Type | Material | Fabrication Technique | Morphological Parameter | Main Outcome |
|---|---|---|---|---|---|
| Yang et al., 2005 [35] | In vitro: Neural stem cells (NSCs) | Poly(l-lactic acid) (PLLA) | Electrospinning | Aligned fibers and diameters of scaffold fibers | The alignment of the scaffold fibers influenced cell orientation. SC differentiation rate was affected by the diameter of the scaffold fiber. |
| Wen et al., 2006 [36] | In vitro: DRG explants | Poly(acrylonitrile-co-vinyl chloride) (PAN-PVC) | Wet-phase inversion process | Diameters of scaffold fibers | The diameter of scaffold fibers influenced alignment, the outgrowth of neurite, and SC migration. |
| Wang et al., 2010 [37] | In vitro: DRG | Poly-l-lactic acid (PLLA) | Electrospinning | Diameters of scaffold fibers in aligned fibers | Diameter of scaffold fibers influenced neurite extension and SC migration. |
| Daud et al., 2012 [38] | In vitro: I. neuronal or primary SC cultures alone; II. neuronal and primary SC in co-culture; III. isolated DRG cultures, containing both neuronal and SC. | Polycaprolatone | Electrospinning | Diameters of scaffold fibers | The diameter of scaffold fibers influenced neurite extension or SC migration. |
| Jiang et al., 2014 [3] | In vivo: adult female SD rats | Poly (ε-caprolactone) (PCL) | Electrospinning | Diameter scaffold of fibers | The diameter of the scaffold fibers affected the number of recovered myelinated axons and the thickness of myelin sheaths. Nanofiber conduits possessed a smaller pore size compared to microfiber conduits. |
| Gnavi et al., 2015 [39] | In vitro: Explant cultures of SC and DRG Ex vivo: SC | Gelatin | Electrospinning | Diameter of scaffold fibers | The diameters of fibers: influenced SC migration, proliferation and adhesion, and axonal outgrowth; affected actin cytoskeleton organization; and influenced migration rate, motility, and axonal density. |
| Hu et al., 2016 [7] | In vitro: PC12 cells | Poly(ε-caprolactone) (PCL)-Nerve Growth Factor (NGF) and Bovine Serum Albumin (BSA) | Emulsion electrospinning technique | Aligned fibers and diameters of scaffold fibers | Aligned scaffold nanofibers presented similar diameters to randomly aligned nanofibers, but the aligned nanofibers were uniform. The diameter of fibers influences the neurite length. |
| Liu et al., 2018 [40] | In vitro: Rat adrenal pheochromocytoma (PC12) cell line | poly(d, l-lactic acid) (PDLLA) and poly(lactic-co-glycolic acid) (PLGA) | Dual-source dual-power electrospinning (DSDP-ES) | Diameter of scaffold fibers | The diameter of scaffold fibers influences the ultimate tensile strength, elastic modulus, and elongation at break. |
| Lizarraga et al., 2019 [5] | In vitro: NG108-15 neuronal cells and Schwann cells | Poly(3-hydroxybutyrate) P(3HB)poly(3-hydroxyoctanoate) P(3HO) 25:75 % P(3HO)/P(3HB) blend (PHA blend) | Electrospinning | Diameter of scaffold fibers | A direct correlation between fiber diameter and neuronal growth and differentiation was noted. |
| Study | Type of Study | Material | Fabrication Technique | Main Outcome |
|---|---|---|---|---|
| Yao et al., 2009 [41] | In vitro: PC12 cells | Polycaprolactone (PCL) | Electrospinning | Aligned scaffold fibers influenced cell orientation and elongation independent of fiber diameter. |
| Koh et al., 2010 [42] | In vivo: Sciatic nerve of Wistar rats | Poly(l-lactic acid) (PLLA) and laminin–PLLA | Electrospinning | Alignment of the scaffold fibers influenced neuronal growth through injury, and sensory and motor functional recoveries. |
| Zhu et al., 2011 [43] | Tensile testing and In vitro degradation In vivo: sciatic nerve of adult female Lewis rats | poly(l-lactide-co-caprolactone), poly(propylene glycol) and sodium acetate | Electrospinning | The alignment of scaffold fibers influenced the neural tissue regeneration, mechanical properties of NGC, and the amplitude and conduction velocity of compound muscle action potential (CMAP). |
| Subramanian et al., 2011 [44] | In vitro: SC | Poly(lactide-co-glycolide) (PLGA) | Electrospinning | The alignment of the scaffold fibers influenced biodegradation and porosity rate, tensile strength, and longitudinal elasticity. This alignment assisted the direction of SC and influenced its proliferation. |
| Cooper et al., 2011 [45] | In vitro: SC; PC-12 cells | Chitosan–polycaprolactone (chitosan–PCL) | Electrospinning | Alignment of the scaffold fibers influenced the environment for nerve cell proliferation, neurite extension, phenotype, and morphology cell. Additionally, elicit chemical and topographical cues for the modulation of neuritogenesis. |
| Wang et al., 2011 [46] | In vitro: DRG from SD rats | Poly(propylene carbonate) (PPC) | Electrospinning | Alignment of the scaffold fibers determines growth orientation neurite and SC migration. Additionally, alignment affected the speed of neurite growth and distances of SC migration. |
| Neal et al., 2012 [47] | In vitro: PC-12 cells, DRG In vivo: Rat tibial nerve | Laminin and laminin–polycaprolactone (PCL) | Electrospinning | The alignment of scaffold fibers influenced retrograde nerve conduction speed, motor, and sensory function. |
| Kijeńska et al., 2012 [48] | In vitro: C17.2 nerve stem cells | Poly(l-lactic acid)-co-poly(ε-caprolactone) or P(LLA-CL), collagen I and collagen III | Electrospinning | Aligned scaffold fibers affected the average tensile strength, cell growth, and cell proliferation. |
| Masaeli et al., 2013 [49] | In vitro: SC | Poly (3-hydroxy-butyrate) (PHB) & Poly (3-hydroxy butyrate-co-3- hydroxyvalerate) (PHBV) | Electrospinning | Aligned scaffold fibers affected average tensile strength and elongation percentage nanofiber scaffolds. Additionally, the morphology of SC oriented along the fiber direction was affected. |
| Ouyang et al., 2013 [50] | In vitro: SC of rat | Collagen type I and poly(lactic-co-glycolic acid) (PLGA) | Electrospinning | Aligned scaffold fibers favored motor function, nerve conduction, markers expressions, SC morphology, axon regeneration, and myelination. |
| Xie et al., 2014 [51] | In vitro: DRG, SCin vivo: Sciatic nerve of rats | Poly(ε-caprolactone) (PCL) | Electrospinning | Bilayer NGC (random and aligned nanofibers layers) are more tear-resistant in surgical procedures due to isotropic mechanical properties. It was unclear if random nanofibers could interfere with the aligned nanofibers in the extension pattern of the neurites and interfere in nerve regeneration. |
| Radhakrishnan et al., 2015 [52] | In vitro: SC | Poly(lactide-co-glycolide) | Electrospinning | Aligned scaffold fibers favored the adhesion, proliferation, and morphology of cells. Additionally, the expression of myelination markers and maturation of SC was regulated. |
| Yan et al., 2015 [53] | In vivo: Sciatic nerve of adult SD rats | Poly(l-lactic acid-co-e-caprolactone) | Electrospinning | Alignment of the scaffold fibers influenced cell growth orientation, myelination, and neuropathic pain post-injury. |
| Gnavi et al., 2015 [54] | In vitro: Primary cultures, SC line RT4-D6P2T, and cell line 50B11. | Gelatin | Electrospinning | The alignment of the scaffold fibers influenced adhesion and proliferation cell. |
| Panahi-Joo et al., 2016 [10] | In vitro: PC12, SH-SY5Y and C6 cell culture | Polycaprolactone (PCL) | Electrospinning dual pole: fibers alignedConventional electro-spinning: semi-aligned and random fibers. | The alignment of the scaffold fibers influenced topographical guidance, growth, elongation, and migration of cells. The tensile strength and degree of crystallinity of the fibers were also influenced. |
| Ranjbar-Mohammadi et al., 2016 [55] | In vitro: PC12 cells | Poly(l-lactic acid) and gum tragacanth (PLLA/GT 100:0, 75:25, 50:50). | Electrospinning | Aligned scaffold fibers favored cell orientation and elongation. |
| Zhang et al., 2018 [56] | In vivo: Sciatic nerve, Adult Sprague–Dawley rats In vitro: SC | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) & polyethylene oxide (PEO): PHBVPEO | Electrospinning | The alignment of the scaffold fibers influenced cell orientation and elongation. |
| Gnavi et al., 2018 [57] | In vitro: RT4-D6P2T SC line and DRG neuronal 50B11 cell line cultures and DRG explants | Gelatin (GL) and Chitosan (CTS) | Electrospinning | Aligned scaffold fibers favored cell adhesion and proliferation rates and filopodia formation. |
| Karimi et al., 2018 [58] | Pre-Experimental Study of BD | Poly(3-hydroxybutyrate) (PHB) solutions with chitosan (15%, 20%) | Electrospinning | The alignment of the scaffold fibers influenced scaffold morphology, hydrophilicity, and mechanical properties. |
| Quan et al., 2019 [6] | In vitro: SC, PC12 cells and DRG In vivo: Sciatic nerve of adult female rats | Polycaprolactone and 2% chitosan | Electrospinning | The alignment of the scaffold fibers influenced the expression of ATF3 and caspase-3 at the regenerative matrix and distal nerve segment. Thus, influencing nerve function, muscle mass, and recovery of distal nerve ultrastructure. |
| Yachao Jia et al., 2019 [59] | In vivo: Rat sciatic nerve In vitro: SC line RSC96 | Poly(l-lactic acid-co-ε-caprolactone) | Electrospinning | Aligned scaffold fibers influenced macrophage elongation along the fibers, and induced the development of M2 macrophage type (pro-healing) or pro-inflammatory M1 type. Additionally, the proliferation and migration of SC were affected. |
| Zhang et al., 2020 [60] | In vitro: RSC96 and PC-12 cell lines In vivo: Sciatic nerve of rats | Poly(l-lactic acid) (PLLA)/soy protein isolate (SPI) | Electrospinning | Alignment of the scaffold fibers was determinant in the regeneration and functional reconstruction of the injured nerve. |
| ↑ Fiber diameter | ↓ Biodegradability |
| ↑ Porosity | ↑ Biodegradability |
| ↑ Pore size | ↑ Biodegradability |
| Random Fibers | ↑ Biodegradability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apablaza, J.A.; Lezcano, M.F.; Lopez Marquez, A.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers 2021, 13, 2563. https://doi.org/10.3390/polym13152563
Apablaza JA, Lezcano MF, Lopez Marquez A, Godoy Sánchez K, Oporto GH, Dias FJ. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers. 2021; 13(15):2563. https://doi.org/10.3390/polym13152563
Chicago/Turabian StyleApablaza, Josefa Alarcón, María Florencia Lezcano, Alex Lopez Marquez, Karina Godoy Sánchez, Gonzalo H. Oporto, and Fernando José Dias. 2021. "Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review" Polymers 13, no. 15: 2563. https://doi.org/10.3390/polym13152563
APA StyleApablaza, J. A., Lezcano, M. F., Lopez Marquez, A., Godoy Sánchez, K., Oporto, G. H., & Dias, F. J. (2021). Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers, 13(15), 2563. https://doi.org/10.3390/polym13152563







