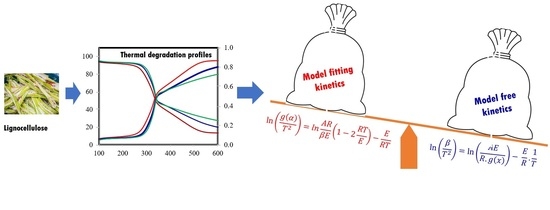
A Comparative Study on Suitability of Model-Free and Model-Fitting Kinetic Methods to Non-Isothermal Degradation of Lignocellulosic Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Kinetic Modelling
3.1. Model-Fitting Approach
3.1.1. Arrhenius Model
3.1.2. Coats-Redfern Model
3.2. Model-Free Approach
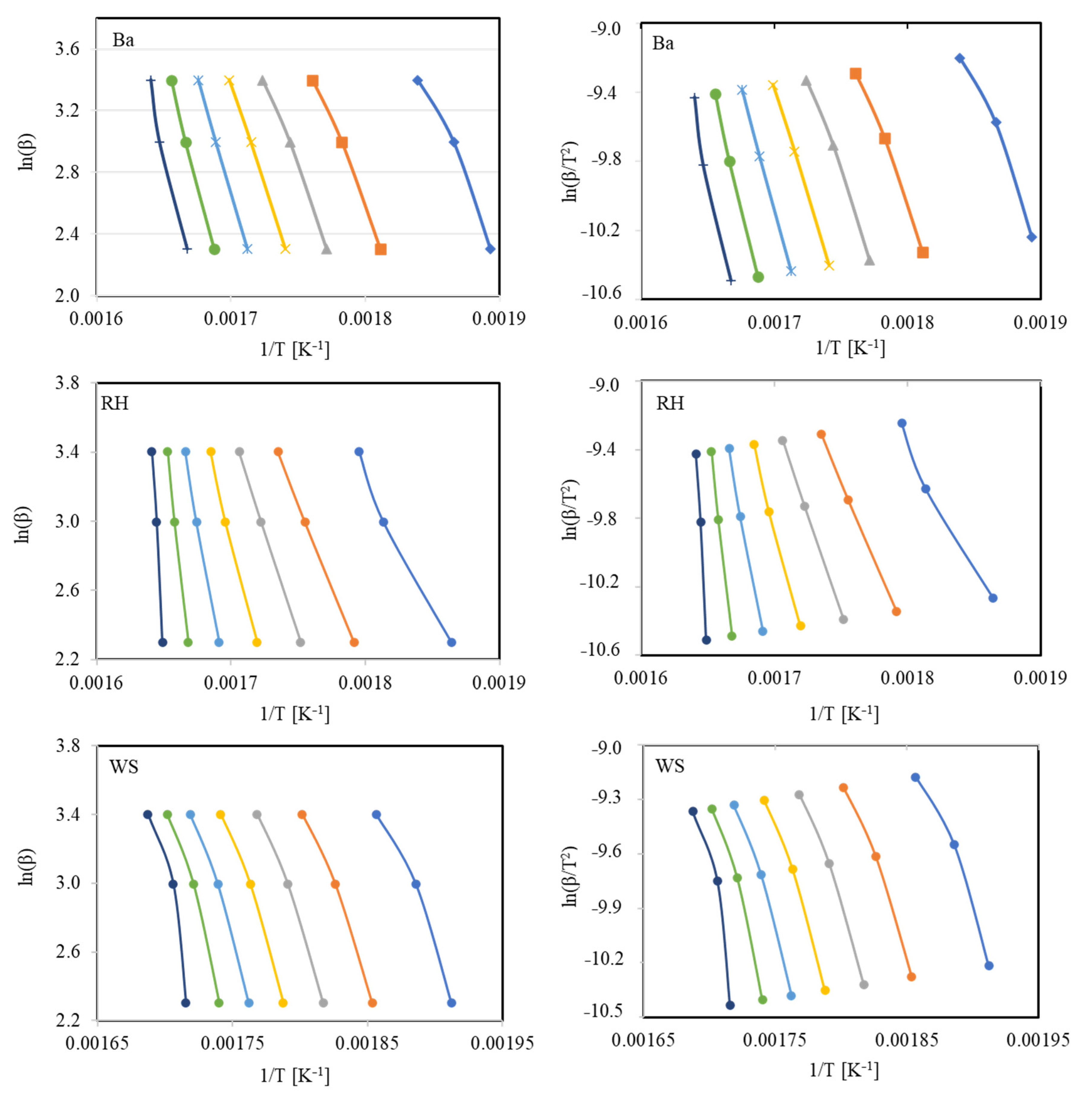
3.2.1. Flynn–Wall–Ozawa (FWO) Method
3.2.2. Kissinger–Akahira–Sunose (KAS) Method
4. Results and Discussion
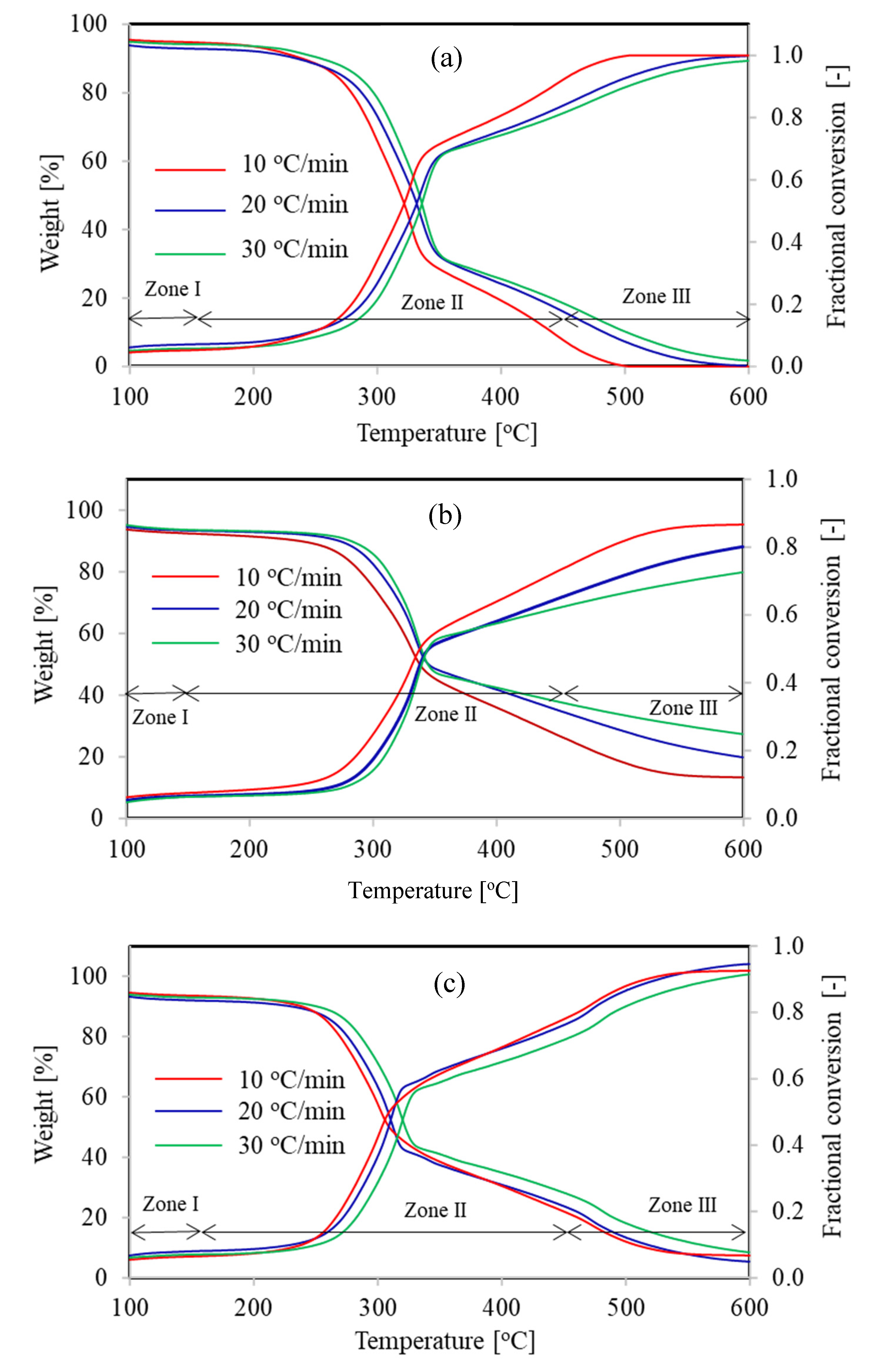
4.1. Thermal Decomposition Behavior of Lignocellulosic Fuels
4.2. Comparative Kinetic Analysis of Lignocellulosic Fuels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panos, E.; Densing, M.; Volkart, K. Access to electricity in the World Energy Council’s global energy scenarios: An outlook for developing regions until 2030. Energy Strategy Rev. 2016, 9, 28–49. [Google Scholar] [CrossRef] [Green Version]
- Casillas, C.E.; Kammen, D.M. The energy-poverty-climate nexus. Science 2010, 330, 1181–1182. [Google Scholar] [CrossRef]
- Machineni, L. Lignocellulosic biofuel production: Review of alternatives. Biomass Convers. Biorefin. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Varma, V.S.; Muthusamy, S.; Kumar, G. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production—A review. Biomass Convers. Biorefin. 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Berger, A.; Zobel, N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel 2014, 123, 230–240. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic study and thermal decomposition behavior of lignite coal. Int. J. Chem. Eng. 2015, 2015, 481739. [Google Scholar] [CrossRef] [Green Version]
- Czajka, K.; Kisiela, A.; Moroń, W.; Ferens, W.; Rybak, W. Pyrolysis of solid fuels: Thermochemical behaviour, kinetics and compensation effect. Fuel Process. Technol. 2016, 142, 42–53. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Yusup, S.; Rashid, M.; Raza, A. Comparative effect of ionic liquids pretreatment on thermogravimetric kinetics of crude oil palm biomass for possible sustainable exploitation. J. Mol. Liq. 2019, 282, 88–96. [Google Scholar] [CrossRef]
- Lynam, J.G.; Chow, G.I.; Hyland, P.L.; Coronella, C.J. Corn stover pretreatment by ionic liquid and glycerol mixtures with their density, viscosity, and thermogravimetric properties. ACS Sustain. Chem. Eng. 2016, 4, 3786–3793. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Yusup, S. Effect of ionic liquids pretreatment on thermal degradation kinetics of agro-industrial waste reinforced thermoplastic starch composites. J. Mol. Liq. 2017, 247, 164–170. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, P.; Minu, K. Thermogravimetric analysis and kinetic modelling studies of selected agro-residues and biodiesel industry wastes for pyrolytic conversion to bio-oil. Biomass Convers. Biorefin. 2014, 4, 259–268. [Google Scholar] [CrossRef]
- Amer, M.; Nour, M.; Ahmed, M.; El-Sharkawy, I.; Ookawara, S.; Nada, S.; Elwardany, A. Kinetics and physical analyses for pyrolyzed Egyptian agricultural and woody biomasses: Effect of microwave drying. Biomass Convers. Biorefin. 2020, 1–14. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Pradhan, R.R.; Garnaik, P.P.; Regmi, B.; Dash, B.; Dutta, A. Pyrolysis kinetics of Sal (Shorea robusta) seeds. Biomass Convers. Biorefin. 2017, 7, 237–246. [Google Scholar] [CrossRef]
- Radhakumari, M.; Prakash, D.J.; Satyavathi, B. Pyrolysis characteristics and kinetics of algal biomass using tga analysis based on ICTAC recommendations. Biomass Convers. Biorefin. 2016, 6, 189–195. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Mokaya, R. Valorisation of adzuki bean waste to biofuel precursors via pyrolysis: Kinetics, product distribution and characterisation. Biomass Convers. Biorefin. 2018, 8, 699–710. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process. Technol. 2015, 130, 147–154. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Montiano, M.; Díaz-Faes, E.; Barriocanal, C. Kinetics of co-pyrolysis of sawdust, coal and tar. Bioresour. Technol. 2016, 205, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Hamid, M.K.A.; Isha, R.; Hasbullah, H.; Ali, R.R. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2017, 167, 218–228. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, X.; Zhang, J.; Zhong, M.; Zhang, G.; Wang, Y.; Xu, G. Isothermal differential characteristics of gas–solid reaction in micro-fluidized bed reactor. Fuel 2013, 103, 29–36. [Google Scholar] [CrossRef]
- Tran, K.-Q.; Bach, Q.-V.; Trinh, T.T.; Seisenbaeva, G. Non-isothermal pyrolysis of torrefied stump–A comparative kinetic evaluation. Appl. Energy 2014, 136, 759–766. [Google Scholar] [CrossRef]
- Oza, S.; Ning, H.; Ferguson, I.; Lu, N. Effect of surface treatment on thermal stability of the hemp-PLA composites: Correlation of activation energy with thermal degradation. Compos. Part B Eng. 2014, 67, 227–232. [Google Scholar] [CrossRef]
- Doyle, C.D. Series approximations to the equation of thermogravimetric data. Nature 1965, 207, 290–291. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Wang, L. Thermal decomposition of rape straw: Pyrolysis modeling and kinetic study via particle swarm optimization. Energy Convers. Manag. 2017, 146, 124–133. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, H.; Zheng, C. Thermogravimetric analysis—Fourier transform infrared analysis of palm oil waste pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.W. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013, 15, 1331–1340. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, S.K. Determination of kinetic parameters of biomass samples using thermogravimetric analysis. Environ. Prog. Sustain. Energy 2014, 33, 256–266. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Effective activation energies of lignocellulosic biomass pyrolysis. Energy Fuels 2014, 28, 3916–3923. [Google Scholar] [CrossRef]
- De Godois Baroni, É.; Tannous, K.; Rueda-Ordonez, Y.J.; Tinoco-Navarro, L.K. The applicability of isoconversional models in estimating the kinetic parameters of biomass pyrolysis. J. Therm. Anal. Calorim. 2016, 123, 909–917. [Google Scholar] [CrossRef]
- De Britto, D.; Assis, O.B. Thermal degradation of carboxymethylcellulose in different salty forms. Thermochim. Acta 2009, 494, 115–122. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340, 53–68. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Agrawal, R.K. Compensation effect in the pyrolysis of cellulosic materials. Thermochim. Acta 1985, 90, 347–351. [Google Scholar] [CrossRef]
- Narayan, R.; Antal, M.J. Thermal lag, fusion, and the compensation effect during biomass pyrolysis. Ind. Eng. Chem. Res. 1996, 35, 1711. [Google Scholar] [CrossRef]
- Pokol, G.; Várhegyi, G.; Dollimore, D. Kinetic aspects of thermal analysis. Crit. Rev. Anal. Chem. 2008, 1988, 65–93. [Google Scholar] [CrossRef]
- Janković, B.; Adnađević, B.; Jovanović, J. Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: Thermogravimetric analysis. Thermochim. Acta 2007, 452, 106–115. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Holstein, A.; Bassilakis, R.; Wójtowicz, M.A.; Serio, M.A. Kinetics of methane and tar evolution during coal pyrolysis. Proc. Combust. Inst. 2005, 30, 2177–2185. [Google Scholar] [CrossRef]
| Mechanism | Abbreviation | ||
|---|---|---|---|
| Power law | P2 | ||
| Power law | P3 | ||
| Power law | P4 | ||
| Avarami-Eroféve | A2 | ||
| Avarami-Eroféve | A3 | ||
| Avarami-Eroféve | A4 | ||
| Contracting Sphere | R2 | ||
| Contracting Cylinder | R3 | ||
| One Dimensional Diffusion | D1 | ||
| Two Dimensional Diffusion | D2 | ||
| Three Dimensional Diffusion–Jander | D3 | ||
| Three-Dimensional Diffusion-GB | D4 | ||
| First Order | F1 | ||
| Second Order | F2 | ||
| Third Order | F3 |
| Bagasse | Rice Husk | Wheat Straw | ||
|---|---|---|---|---|
| Proximate Analysis (%) | MC | 7.00 | 7.94 | 6.40 |
| VM | 75.00 | 56.19 | 71.34 | |
| FC | 10.00 | 11.75 | 9.67 | |
| Ash | 8.00 | 24.13 | 12.59 | |
| C-S Analysis (%) | C | 43.55 | 34.35 | 43.50 |
| S | 0 | 0.28 | 0 |
| Heating Rate | 10 °C/min | 20 °C/min | 30 °C/min | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone | I | II | III | I | II | III | I | II | III |
| Bagasse | 40–114 | 114–362 | 362–600 | 40–123 | 123–376 | 376–600 | 40–130 | 130–385 | 385–600 |
| Rice Husk | 40–119 | 119–365 | 365–560 | 40–128 | 128–374 | 374–568 | 40–147 | 147–387 | 387–576 |
| Wheat Straw | 40–117 | 117–355 | 355–561 | 40–127 | 127–365 | 365–589 | 40–140 | 140–374 | 374–698 |
| Biomass | Arrhenius Model | Coats Redfern Model | % Difference | ||||
|---|---|---|---|---|---|---|---|
| Mechanism | (kJ/mol) | R2 | Mechanism | (kJ/mol) | R2 | ||
| Bagasse | D2 | 92.73 | 0.92 | D1 | 89.41 | 0.96 | 3.65 |
| Rice husk | D2 | 77.18 | 0.84 | D1 | 71.49 | 0.89 | 7.65 |
| Wheat straw | D2 | 86.22 | 0.89 | D1 | 85.05 | 0.94 | 1.36 |
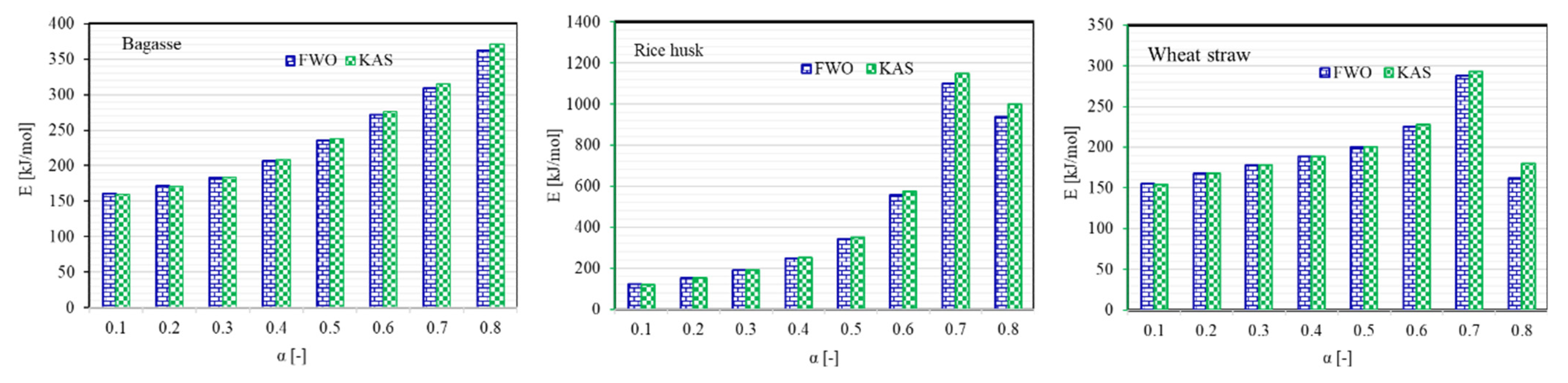
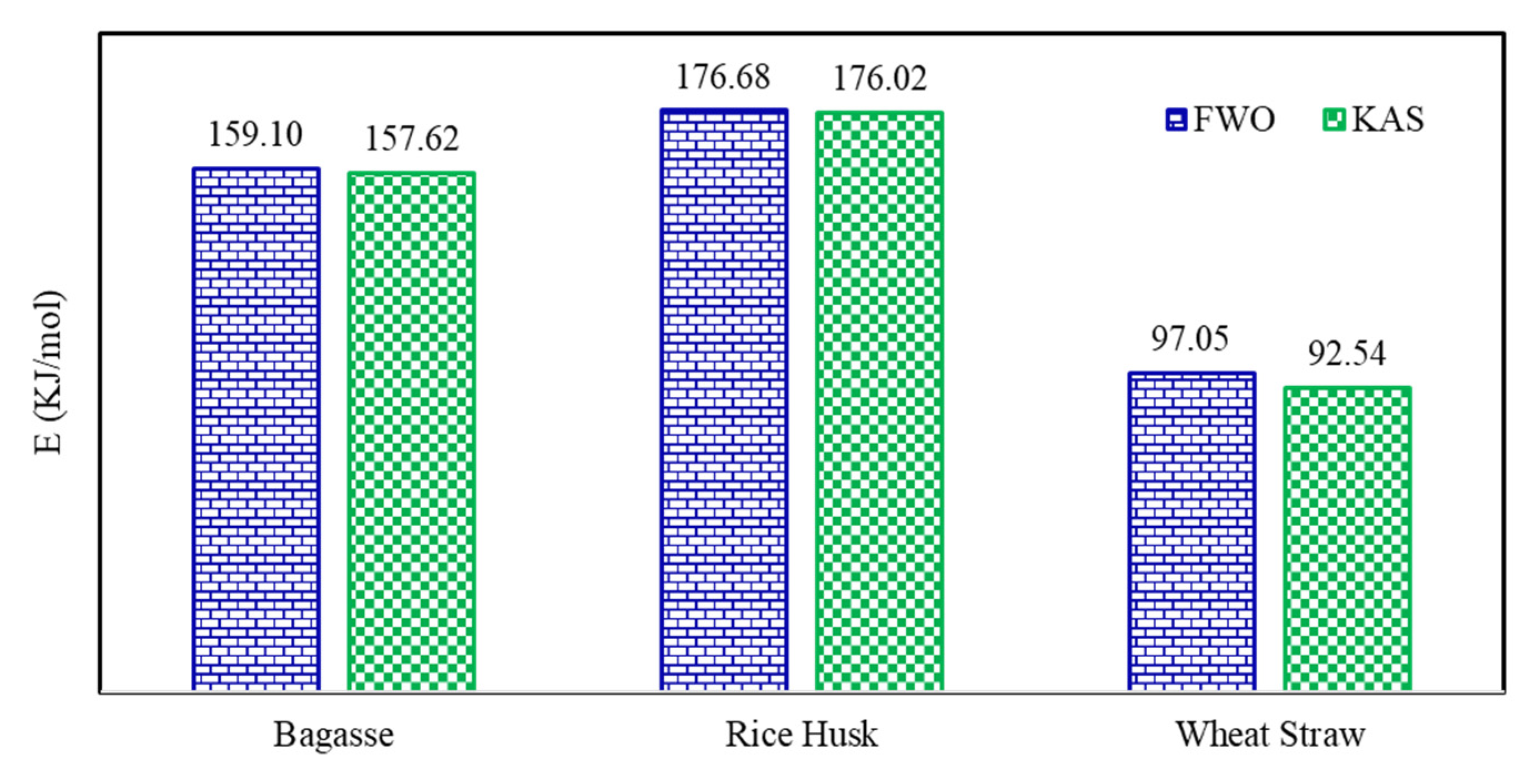
| Biomass | FWO Model | KAS Model | % Difference * | |||
|---|---|---|---|---|---|---|
| (kJ/mol) | R2 | (kJ/mol) | R2 | |||
| Bagasse | 0.10 | 160.21 | 0.98 | 159.63 | 0.98 | 0.36 |
| 0.20 | 171.81 | 0.99 | 171.43 | 0.99 | 0.22 | |
| 0.30 | 183.48 | 1.00 | 183.50 | 0.99 | 0.01 | |
| 0.40 | 206.82 | 1.00 | 207.91 | 1.00 | 0.53 | |
| 0.50 | 234.95 | 1.00 | 237.35 | 1.00 | 1.02 | |
| 0.60 | 271.66 | 1.00 | 275.84 | 1.00 | 1.53 | |
| 0.70 | 309.07 | 0.98 | 315.10 | 0.98 | 1.93 | |
| 0.80 | 362.33 | 0.96 | 371.02 | 0.96 | 2.37 | |
| Rice husk | 0.10 | 122.42 | 0.99 | 119.70 | 0.98 | 2.25 |
| 0.20 | 153.62 | 1.00 | 152.19 | 1.00 | 0.94 | |
| 0.30 | 190.22 | 1.00 | 190.50 | 1.00 | 0.15 | |
| 0.40 | 247.91 | 1.00 | 251.04 | 1.00 | 1.25 | |
| 0.50 | 342.65 | 1.00 | 350.57 | 1.00 | 2.28 | |
| 0.60 | 555.63 | 1.00 | 574.51 | 1.00 | 3.34 | |
| 0.70 | 1100.40 | 0.99 | 1147.51 | 0.99 | 4.19 | |
| 0.80 | 938.95 | 0.72 | 998.00 | 0.73 | 6.10 | |
| Wheat straw | 0.10 | 155.04 | 0.97 | 154.28 | 0.96 | 0.49 |
| 0.20 | 168.28 | 0.99 | 167.93 | 0.98 | 0.21 | |
| 0.30 | 177.89 | 0.99 | 177.87 | 0.99 | 0.01 | |
| 0.40 | 188.17 | 0.99 | 188.54 | 0.99 | 0.20 | |
| 0.50 | 199.99 | 0.99 | 200.84 | 0.98 | 0.42 | |
| 0.60 | 225.45 | 0.98 | 227.51 | 0.97 | 0.91 | |
| 0.70 | 287.77 | 0.89 | 292.95 | 0.88 | 1.78 | |
| 0.80 | −161.73 | 0.22 | −180.06 | 0.24 | 10.73 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, H.; Shakeel, A.; Abdullah, A.; Khan, M.I.; Moniruzzaman, M. A Comparative Study on Suitability of Model-Free and Model-Fitting Kinetic Methods to Non-Isothermal Degradation of Lignocellulosic Materials. Polymers 2021, 13, 2504. https://doi.org/10.3390/polym13152504
Mahmood H, Shakeel A, Abdullah A, Khan MI, Moniruzzaman M. A Comparative Study on Suitability of Model-Free and Model-Fitting Kinetic Methods to Non-Isothermal Degradation of Lignocellulosic Materials. Polymers. 2021; 13(15):2504. https://doi.org/10.3390/polym13152504
Chicago/Turabian StyleMahmood, Hamayoun, Ahmad Shakeel, Ammar Abdullah, Muhammad Ilyas Khan, and Muhammad Moniruzzaman. 2021. "A Comparative Study on Suitability of Model-Free and Model-Fitting Kinetic Methods to Non-Isothermal Degradation of Lignocellulosic Materials" Polymers 13, no. 15: 2504. https://doi.org/10.3390/polym13152504
APA StyleMahmood, H., Shakeel, A., Abdullah, A., Khan, M. I., & Moniruzzaman, M. (2021). A Comparative Study on Suitability of Model-Free and Model-Fitting Kinetic Methods to Non-Isothermal Degradation of Lignocellulosic Materials. Polymers, 13(15), 2504. https://doi.org/10.3390/polym13152504