Oriented Thin Films of Insoluble Polythiophene Prepared by the Friction Transfer Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
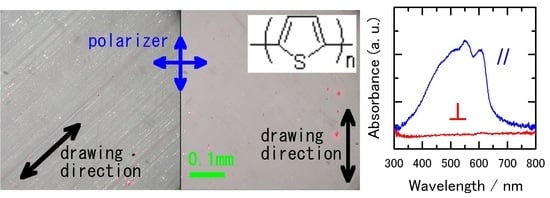
3.1. Surface Morphology
3.2. Molecular Chain Orientation
3.2.1. Polarized UV–Vis Spectroscopy
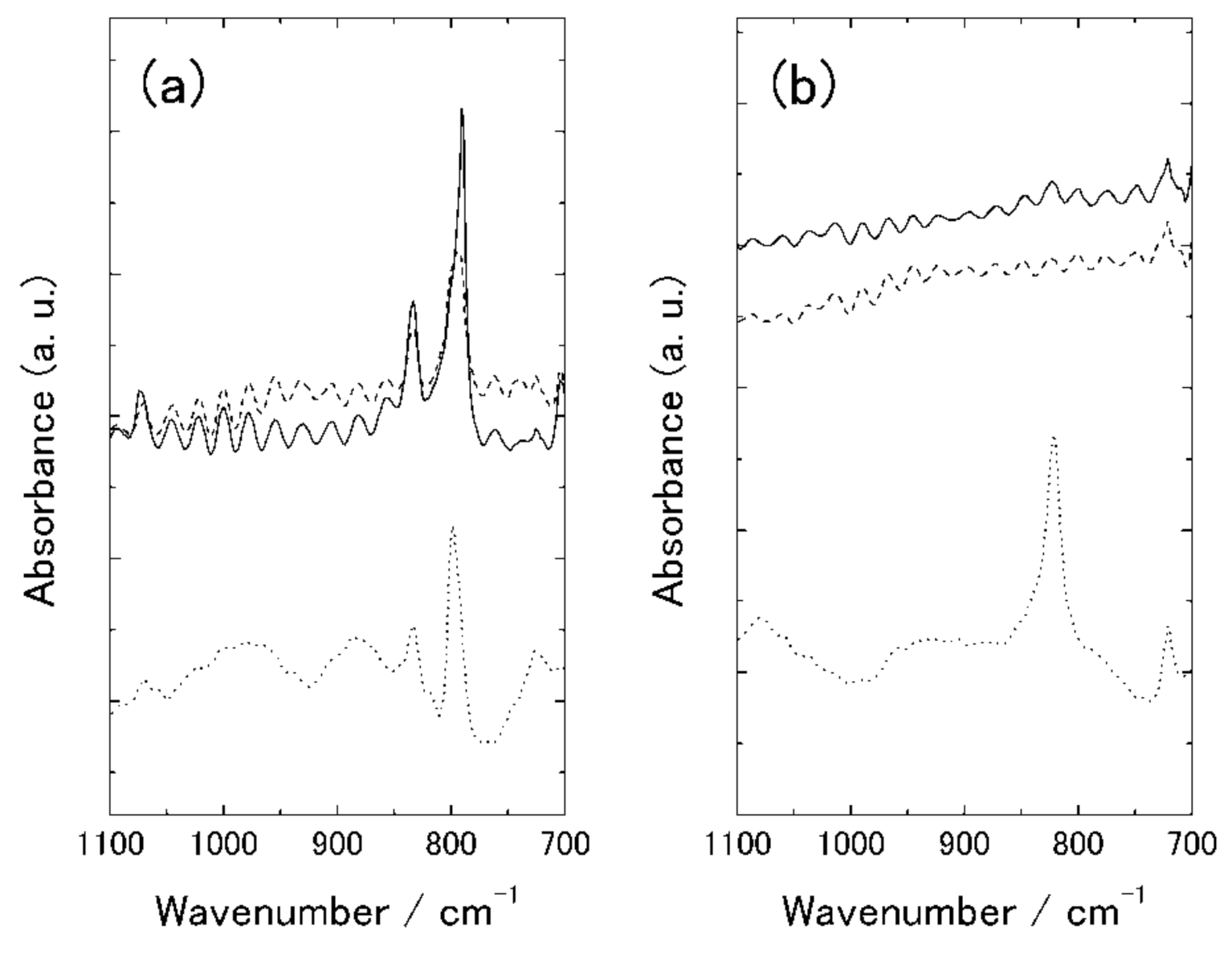
3.2.2. Polarized IR Spectroscopy
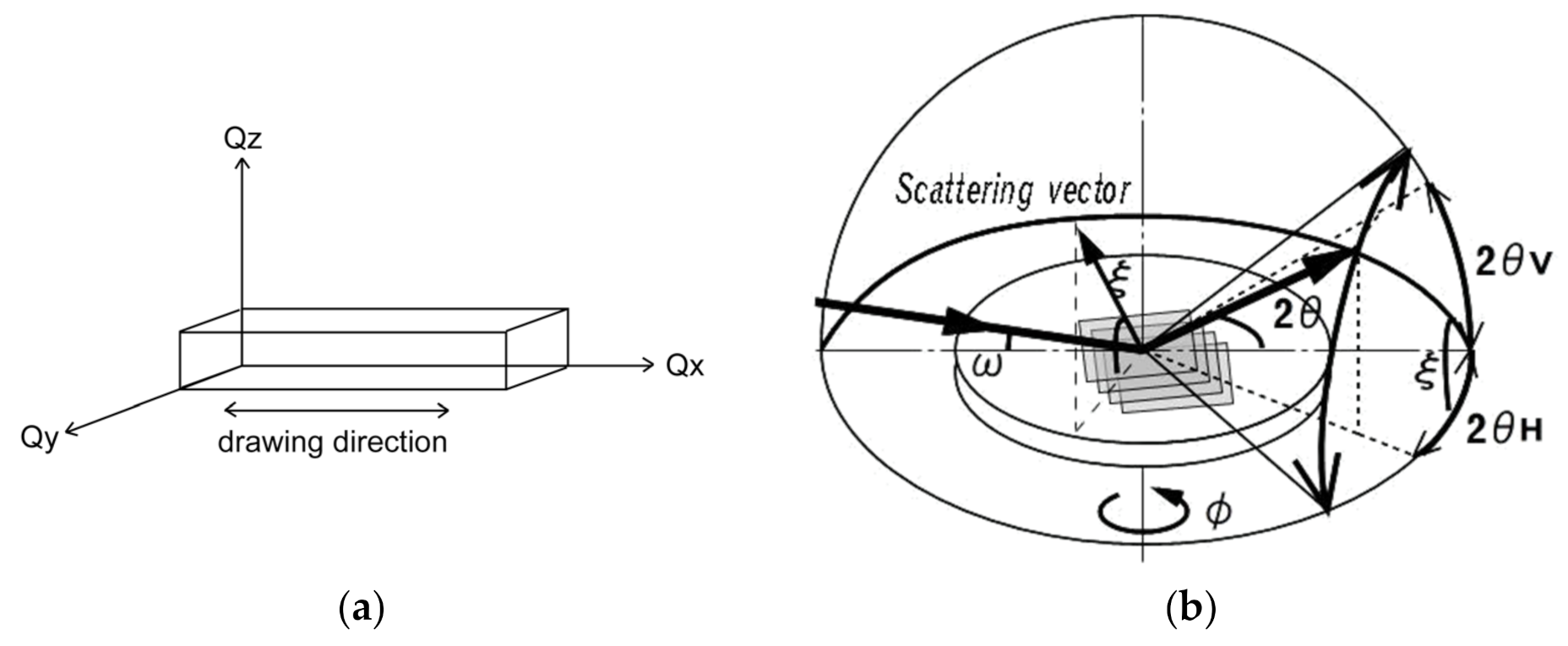
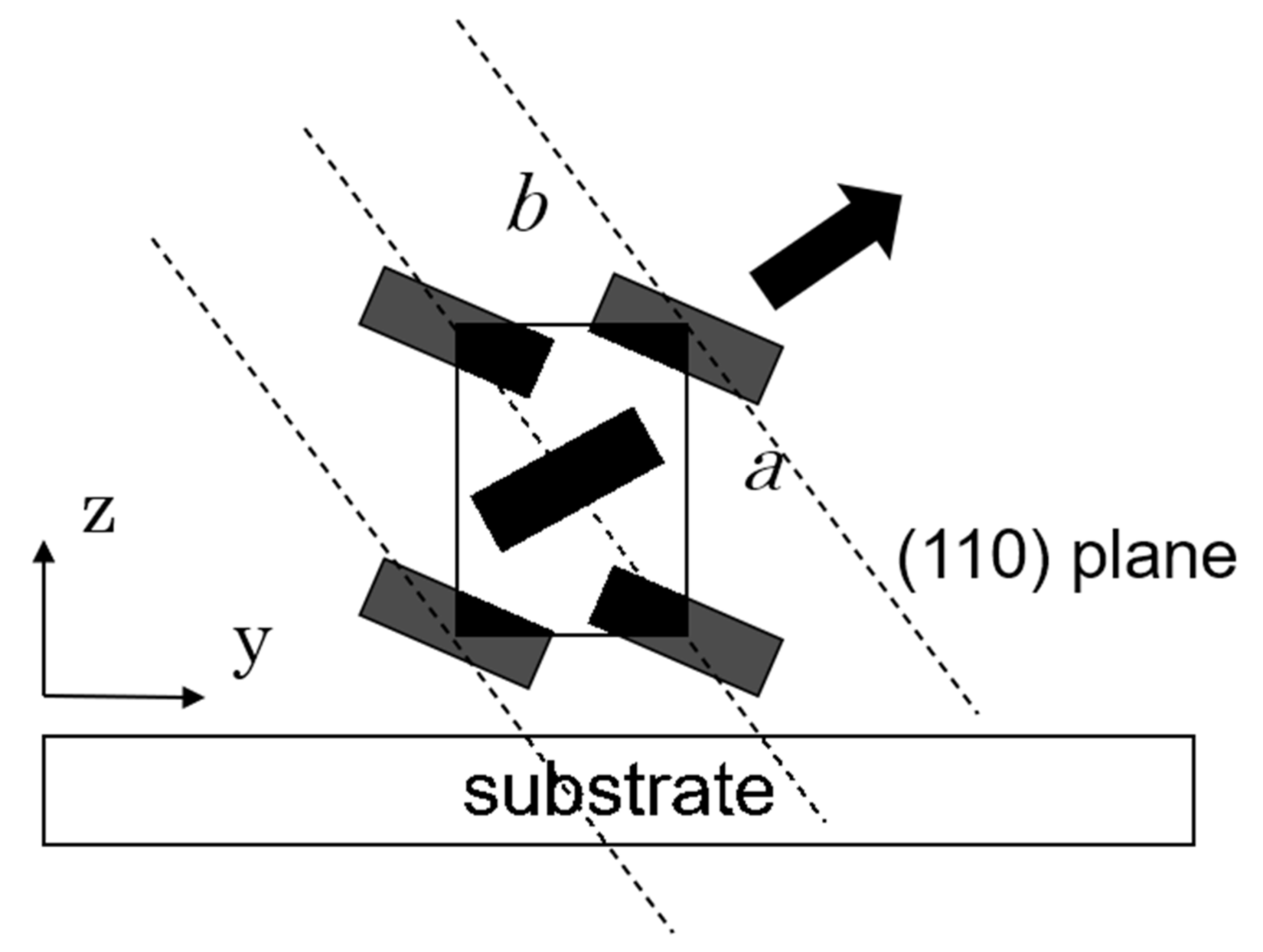
3.2.3. GIXD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epstain, A. Organic Electronic Materials; Farchioni, R., Grosso, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 3–37. [Google Scholar]
- McCullough, R.D. The Chemistry of Conducting Polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Wilson, R.J.; Friend, R.J.; Inbasekaran, M.; Wu, W.W.; Woo, E.P.; Grell, M.; Bradley, D.D.C. Mobility enhancement in conjugated polymer field-effect transistors through chain alignment in a liquid-crystalline phase. Appl. Phys. Lett. 2000, 77, 406–408. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C. Polarized Luminescence from Oriented Molecular Materials. Adv. Mater. 1999, 11, 895–905. [Google Scholar] [CrossRef]
- Whitehead, K.S.; Grell, M.; Bradley, D.D.C.; Jandke, M.; Strohriegl, P. Highly polarized blue electroluminescence from homogeneously aligned films of poly(9,9-dioctylfluorene). Appl. Phys. Lett. 2000, 76, 2946–2948. [Google Scholar] [CrossRef]
- Hagler, T.W.; Pakbaz, K.; Moulton, J.; Wudl, F.; Smith, P.; Heeger, A.J. Highly Ordered Conjugated Polymers in Polyethylene-Orientation by Mesoepitaxy. Polym. Commun. 1991, 32, 339–342. [Google Scholar]
- Jandlke, M.; Strohriegl, P.; Gmeiner, J.; Brutting, W.; Schwoere, M. Polarized Electroluminescence from Rubbing-Aligned Poly(p-phenylenevinylene). Adv. Mater. 1999, 11, 1518–1521. [Google Scholar] [CrossRef]
- Cimrova, V.; Remmers, M.; Neher, D.; Wegner, G. Polarized light emission from LEDs prepared by the Langmuir-Blodgett technique. Adv. Mater. 1996, 8, 146–149. [Google Scholar] [CrossRef]
- Grell, M.; Knoll, W.; Lupo, D.; Meisel, A.; Miteva, T.; Neher, D.; Nothofer, H.-G.; Scherf, U.; Yasuda, A. Blue Polarized Electroluminescence from a Liquid Crystalline Polyfluorene. Adv. Mater. 1999, 11, 671–675. [Google Scholar] [CrossRef]
- Chen, X.L.; Bao, Z.; Sapjeta, B.J.; Lovinger, A.J.; Crone, B. Polarized Electroluminescence from Aligned Chromophores by the Friction Transfer Method. Adv. Mater. 2000, 12, 344–347. [Google Scholar] [CrossRef]
- Amundson, K.R.; Sapjeta, B.J.; Lovinger, A.J.; Bao, Z. An in-plane anisotropic organic semiconductor based upon poly(3-hexyl thiophene). Thin Solid Films 2002, 414, 143–149. [Google Scholar] [CrossRef]
- Makinson, K.R.; Tabor, D. Friction and Transfer of Polytetrafluoroethylene. Nature 1964, 201, 464–466. [Google Scholar] [CrossRef]
- Wittmann, J.C.; Smith, P. Highly oriented thin films of poly(tetrafluoroehylene) as a substrate for a oriented growth of materials. Nature 1991, 352, 414–417. [Google Scholar] [CrossRef]
- Tanigaki, N.; Kyotani, H.; Wada, M.; Kaito, A.; Yoshida, M.; Han, E.-M.; Abe, K.; Yase, K. Oriented thin films of conjugated polymers: Polysilanes and polyphenylenes. Thin Solid Films 1998, 331, 229–238. [Google Scholar] [CrossRef]
- Tanigaki, N.; Yase, K.; Kaito, A.; Ueno, K. Highly oriented films of poly(dimethylsilylene) by friction deposition. Polymer 1995, 36, 2477–2480. [Google Scholar] [CrossRef]
- Tanigaki, N.; Yase, K.; Kaito, A. Oriented Films of Poly(p-Phenylene) by Friction-Deposition and Oriented Growth in Polymerization. Mol. Cryst. Liq. Cryst. 1995, 267, 335–340. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Takashima, W.; Kaneto, K.; Yoshida, Y.; Tanigaki, N.; Yase, K.; Omote, K. Backbone Arrangement in “Friction-Transferred” Regioregular Poly(3-alkylthiophene)s. Macromolecules 2003, 36, 5252–5257. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Misaki, M.; Chikamatsu, M.; Kimura, T.; Yoshida, Y.; Azumi, R.; Tanigaki, N.; Yase, K. Crystal Structure of Friction-Transferred Poly(2,5-dioctyloxy-1,4-phenylenevinylene). J. Phys. Chem. B 2007, 111, 4349–4354. [Google Scholar] [CrossRef] [PubMed]
- Misaki, M.; Ueda, Y.; Nagamatsu, S.; Yoshida, Y.; Tanigaki, N.; Yase, K. Formation of Single-Crystal-like Poly(9,9-dioctylfluorene) Thin Film by the Friction-Transfer Technique with Subsequent Thermal Treatments. Macromolecules 2004, 37, 6926–6931. [Google Scholar] [CrossRef]
- Tanigaki, N.; Heck, C.; Mizokuro, T. Oriented Thin Films of Polyaniline by Friction Transfer Method. Mol. Cryst. Liq. Cryst. 2009, 505, 318–324. [Google Scholar] [CrossRef]
- Tanigaki, N.; Heck, C.; Mizokuro, T.; Shibata, Y.; Miyadera, T.; Koganezawa, T. Oriented Thin Films of the Low-Band-Gap Polymer PTB7 by Friction Transfer Method. Mol. Cryst. Liq. Cryst. 2015, 621, 118–123. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Tanigaki, N.; Yoshida, Y.; Takashima, W.; Yase, K.; Kaneto, K. Effects of molecular alignment on carrier transport in organic transistors. Synth. Met. 2003, 137, 923–924. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Takashima, W.; Kaneto, K.; Tanigaki, N.; Yoshida, Y.; Yase, K. Polymer field-effect transistors by a drawing method. Appl. Phys. Lett. 2004, 84, 4608–4610. [Google Scholar] [CrossRef] [Green Version]
- Misaki, M.; Ueda, Y.; Nagamatsu, S.; Chikamatsu, M.; Yoshida, Y.; Tanigaki, N.; Yase, K. Highly polarized polymer light-emitting diodes utilizing friction-transferred poly(9,9-dioctylfluorene) thin films. Appl. Phys. Lett. 2005, 87, 243503. [Google Scholar] [CrossRef] [Green Version]
- Tanigaki, N.; Kuwajima, S.; Nagamatsu, S.; Yoshida, Y.; Yase, K. Polarization sensitive photoelectic conversion by polymer/titania bilayer. Synth. Met. 2003, 137, 1425–1426. [Google Scholar] [CrossRef]
- Roncali, J. Synthetic Principles for Bandgap Control in Linear π-Conjugated Systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar]
- Kobayashi, M.; Chen, J.; Chung, T.C.; Moraes, F.; Heeger, A.J.; Wudl, F. Synthesis and properties of chemically coupled poly(thiophene). Synth. Met. 1984, 9, 77–86. [Google Scholar] [CrossRef]
- Tsumura, A.; Koezuka, H.; Ando, T. Macromolecular electronic device: Field-effect transistor with a polythiophene thin film. Appl. Phys. Lett. 1986, 49, 1210–1212. [Google Scholar] [CrossRef]
- Wattmann, R.J.; Bargon, J.; Diaz, A.F. Electrochemical studies of some conducting polythiophene films. J. Phys. Chem. 1983, 87, 1459–1463. [Google Scholar] [CrossRef]
- Bazzaoui, E.A.; Lévi, G.; Aeiyach, S.; Aubard, J.; Marsault, J.P.; Lacaze, P.C. SERS Spectra of Polythiophene in Doped and Undoped States. J. Phys. Chem. 1995, 99, 6628–6634. [Google Scholar] [CrossRef]
- Yoshino, K.; Hayashi, S.; Sugimoto, R. Preparation and Properties of Conducting Heterocyclic Polymer Films by Chemical Method. Jpn. J. Appl. Phys. 1984, 23, L899–L900. [Google Scholar] [CrossRef]
- Bjerring, M.; Nielsen, J.S.; Nielsen, N.C.; Krebs, F.C. Polythiophene by Solution Processing. Macromolecules 2007, 40, 6012–6013. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kanbara, T.; Mori, C. Oriented crystalline film of poly(2,5-thinylen) formed by vacuum deposition and its crystal structure. Comparison with a similarly oriented crystalline film of poly(1,4-phenylene). Synth. Met. 1990, 38, 399–402. [Google Scholar] [CrossRef]
- Yoshihiko Tezuka, Yamamoto Tatsuya, Kamikado Yousuke and Hitoshi Tanaka: Partially Interpenetrating Heterojunction on Bilayer Photovoltaic Devices of Electrodeposited Polythiophene/Methanofulleren. Sol. Energy Mater. Sol. Cells 2012, 105, 167–173. [CrossRef]
- Mizokuro, T.; Heck, C.; Tanigaki, N. Orientation of α-Sexithiophene on Friction-Transferred Polythiophene Filma. J. Phys. Chem. B 2012, 116, 189–193. [Google Scholar] [CrossRef]
- Mizokuro, T.; Heck, C.; Tanigaki, N. Orientation of Rod-Shape Molecule, 2,2′-Bis[4-(Trifluoromethyl)Phenyl]-5,5′-Bithiazole in Films Deposited in a Vacuum on Oriented α-Sexithiophene Films. Mol. Cryst. Liq. Cryst. 2015, 621, 156–161. [Google Scholar] [CrossRef]
- Mizokuro, T.; Tanigaki, N.; Miyadera, T.; Shibata, Y.; Koganezawa, T. Organic Photovoltaic Devices Based on Oriented n-Type Molecular Films Deposited on Oriented Polythiophene Films. J. Nanosci. Nanotechnol. 2018, 18, 2702–2710. [Google Scholar] [CrossRef]
- Mizokuro, T.; Takeuchi, K.; Heck, C.; Aota, H.; Tanigaki, N. Orientation management of alpha-sexithiophene layer for the application in organic photovoltaic devices. Org. Electron. 2012, 13, 3130–3137. [Google Scholar] [CrossRef]
- Ishida, K.; Hayashi, K.; Yoshida, Y.; Horiuchi, T.; Matsushige, K. Structural evaluation of epitaxially grown organic evaporated films by total reflection X-ray diffractometer. J. Appl. Phys. 1993, 73, 7338–7343. [Google Scholar] [CrossRef]
- Tanigaki, N.; Yoshida, Y.; Kaito, A.; Yase, K. Total-reflection X-ray diffraction study of friction-transferred poly(tetrafluoroethylene) film. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 432–438. [Google Scholar] [CrossRef]
- Mizokuro, T.; Okamoto, Y.; Heck, C.; Aota, H.; Tanigaki, N. Orientation control of regioregular-poly(3-dodecylthiophene) films formed by the friction-transfer method and the performance of organic photovoltaic devices based on these films. J. Appl. Polym. Sci. 2014, 131, 40136. [Google Scholar] [CrossRef]
- Tanigaki, N.; Nagamatsu, S.; Takashima, W.; Yoshida, Y. Molecular orientation of poly(3-butylthiophene) friction-transferred films. Thin Solid Films 2009, 518, 853–856. [Google Scholar] [CrossRef]
- Derue, G.; Coppée, S.; Gabriel, S.; Surin, M.; Geskin, V.; Monteverde, F.; Leclère, P.; Lazzaroni, R.; Damman, P. Nanorubbing of Polythiophene Surfaces. J. Am. Chem. Soc. 2005, 127, 8018–8019. [Google Scholar] [CrossRef]
- Danno, T.; Kürti, J.; Kuzmany, H. Optical anisotropy and Raman scattering from highly oriented poly(octylthiophene) films. Phys. Rev. B 1991, 43, 4809–4819. [Google Scholar] [CrossRef]
- Bolognesi, A.; Botta, C.; Martinelli, M.; Porzio, W. Polarized photoluminescence and electroluminescence in oriented films of regioregular poly(3-alkylthiophenes). Org. Electron. 2000, 1, 27–32. [Google Scholar] [CrossRef]
- Bolognesi, A.; Bajo, G.; Paloheimo, J.; Ostergard, T.; Stubb, H. Polarized electroluminescence from an oriented poly (3-alkylthiophene) langmuir-blodgett structure. Adv. Matter. 1997, 9, 121–124. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sanechika, K.; Yamamoto, A. Preparation and Characterization of Poly(thienylene)s. Bull. Chem. Soc. Jpn. 1983, 56, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kamijoh, T.; Wataru, I. Preparation and spectroscopic data of 13C-labeled polythiophenes and corresponding iodide monomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1642–1649. [Google Scholar] [CrossRef]
- Winokur, M.J.; Spiegel, D.; Kim, Y.; Hotta, S.; Heeger, A.J. Structural and absorption studies of the thermochromic transition in poly(3-hexylthiophene). Synth. Met. 1989, 28, 419–426. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W.; Herwig, P.; et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- He, J.; Zhou, H.; Wan, F.; Lu, Y.; Xue, G. SERS study of the high quality conducting polythiophene film. Vib. Spectrosc. 2003, 31, 265–269. [Google Scholar] [CrossRef]
- Jin, S.; Xue, G. Interaction between Thiophene and Solvated Lewis Acids and the Low-Potential Electrochemical Deposition of a Highly Anisotropic Conducting Polythiophene Film. Macromolecules 1997, 30, 5753–5757. [Google Scholar] [CrossRef]
- Yamamoto, T.; Morita, A.; Miyazaki, Y.; Maruyama, T.; Wakayama, H.; Zhou, Z.-h.; Nakamura, Y.; Kanbara, T. Preparation of π-conjugated poly(thiophene-2,5-diyl), poly(p-phenylene), and related polymers using zerovalent nickel complexes. Linear structure and properties of the π-conjugated polymers. Macromolecules 1992, 25, 1214–1223. [Google Scholar] [CrossRef]
- Mo, Z.; Lee, K.-B.; Moon, Y.B.; Kobayashi, M.; Heeger, A.J.; Wudl, F. X-ray scattering from poly(thiophene): Crystallinity and crystallographic structure. Macromolecules 1985, 18, 1972–1977. [Google Scholar] [CrossRef]
- Brückner, S.; Porzio, W. The structure of neutral polythiophene. An application of the rietveld method. Makromol. Chem. 1988, 189, 961–967. [Google Scholar] [CrossRef]
- Ferro, D.R.; Porzio, W.; Destri, S.; Ragazzi, M.; Brückner, S. Application of molecular mechanics to refine and understand the crystal structure of polythiophene and its oligomers. Macromol. Theory Simul. 1997, 6, 713–727. [Google Scholar] [CrossRef]
- Misaki, M.; Nagamatsu, S.; Chikamatsu, M.; Yoshida, Y.; Azumi, R.; Tanigaki, N.; Ueda, Y.; Yase, K. Single-Crystal-like Structure of Poly(9,9-dioctylfluorene) Thin Films Evaluated by Synchrotron-Sourced Grazing-Incidence X-ray Diffraction. Polym. J. 2007, 39, 1306–1311. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanigaki, N.; Takechi, C.; Nagamatsu, S.; Mizokuro, T.; Yoshida, Y. Oriented Thin Films of Insoluble Polythiophene Prepared by the Friction Transfer Technique. Polymers 2021, 13, 2393. https://doi.org/10.3390/polym13152393
Tanigaki N, Takechi C, Nagamatsu S, Mizokuro T, Yoshida Y. Oriented Thin Films of Insoluble Polythiophene Prepared by the Friction Transfer Technique. Polymers. 2021; 13(15):2393. https://doi.org/10.3390/polym13152393
Chicago/Turabian StyleTanigaki, Nobutaka, Chikayo Takechi, Shuichi Nagamatsu, Toshiko Mizokuro, and Yuji Yoshida. 2021. "Oriented Thin Films of Insoluble Polythiophene Prepared by the Friction Transfer Technique" Polymers 13, no. 15: 2393. https://doi.org/10.3390/polym13152393
APA StyleTanigaki, N., Takechi, C., Nagamatsu, S., Mizokuro, T., & Yoshida, Y. (2021). Oriented Thin Films of Insoluble Polythiophene Prepared by the Friction Transfer Technique. Polymers, 13(15), 2393. https://doi.org/10.3390/polym13152393