Temperate UV-Accelerated Weathering Cycle Combined with HT-GPC Analysis and Drop Point Testing for Determining the Environmental Instability of Polyethylene Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Temperate Accelerated Laboratory Weathering
2.3. Outdoor Exposure of Films
2.4. Infrared Analysis and FTIR Spectroscopic Mapping
2.5. Molecular Weight Analysis
2.6. Drop Point Test
3. Results
3.1. Outdoor Exposure Sites
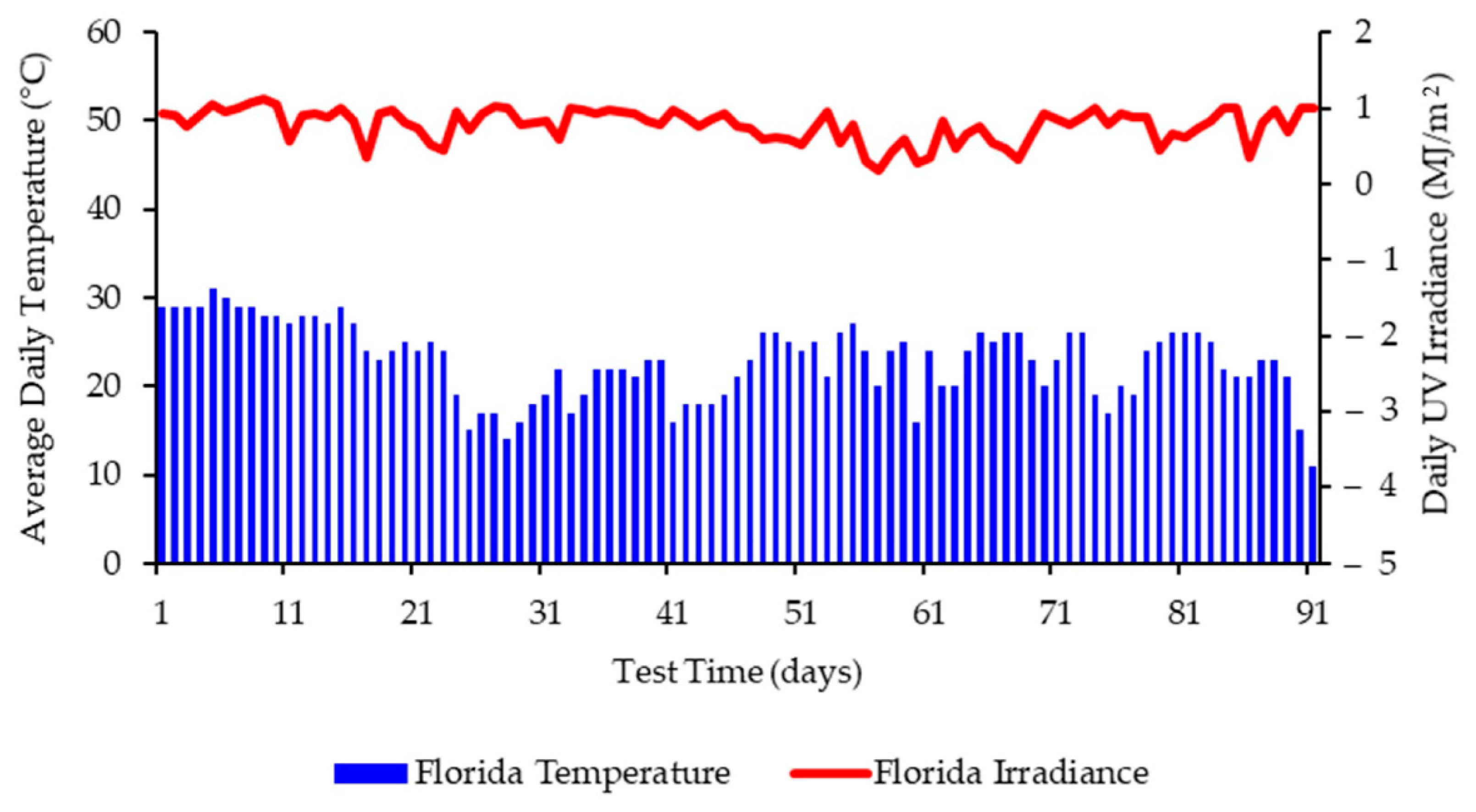
3.2. Weathering Data and Conditions for Exposure in France
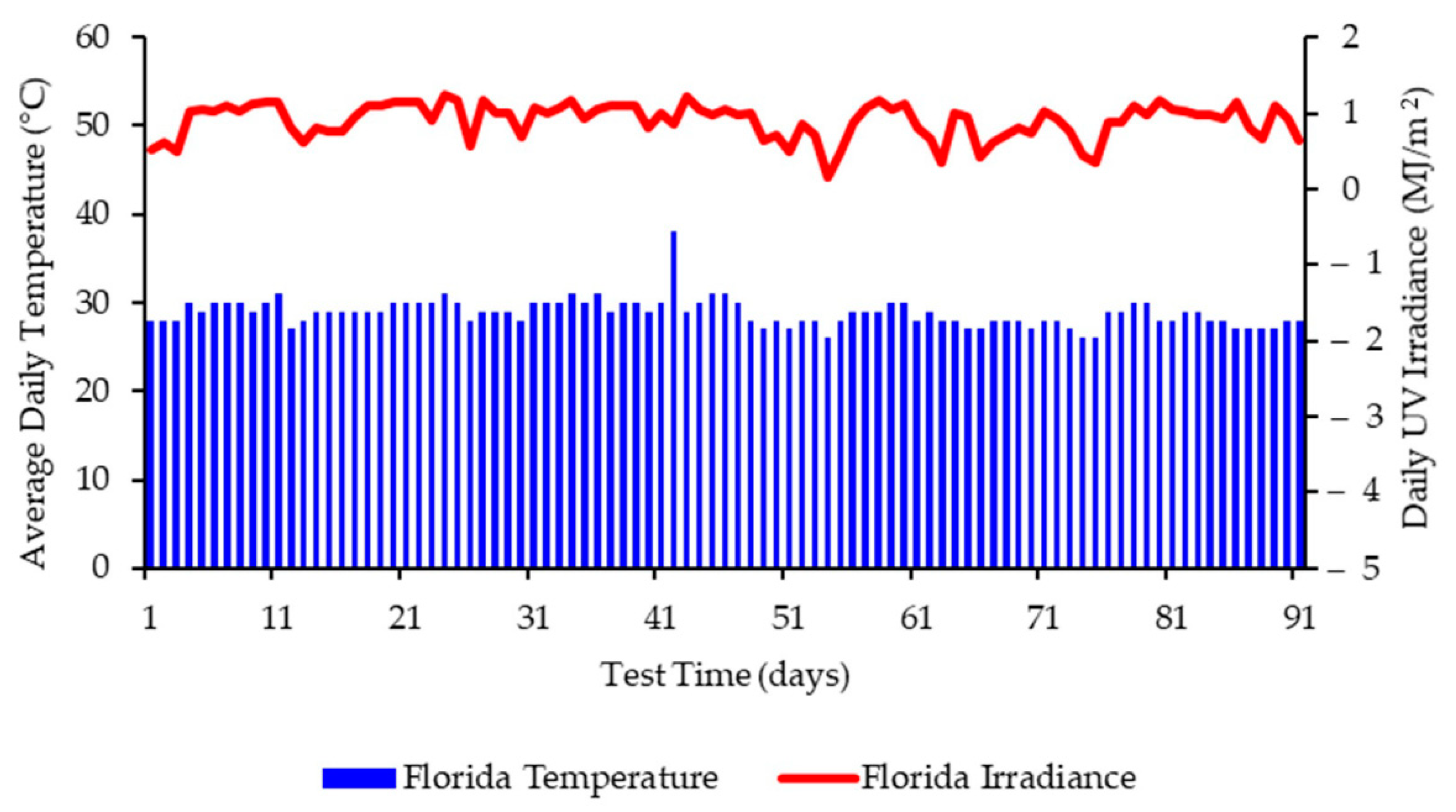
3.3. Weathering Data and Conditions for Exposure in Florida
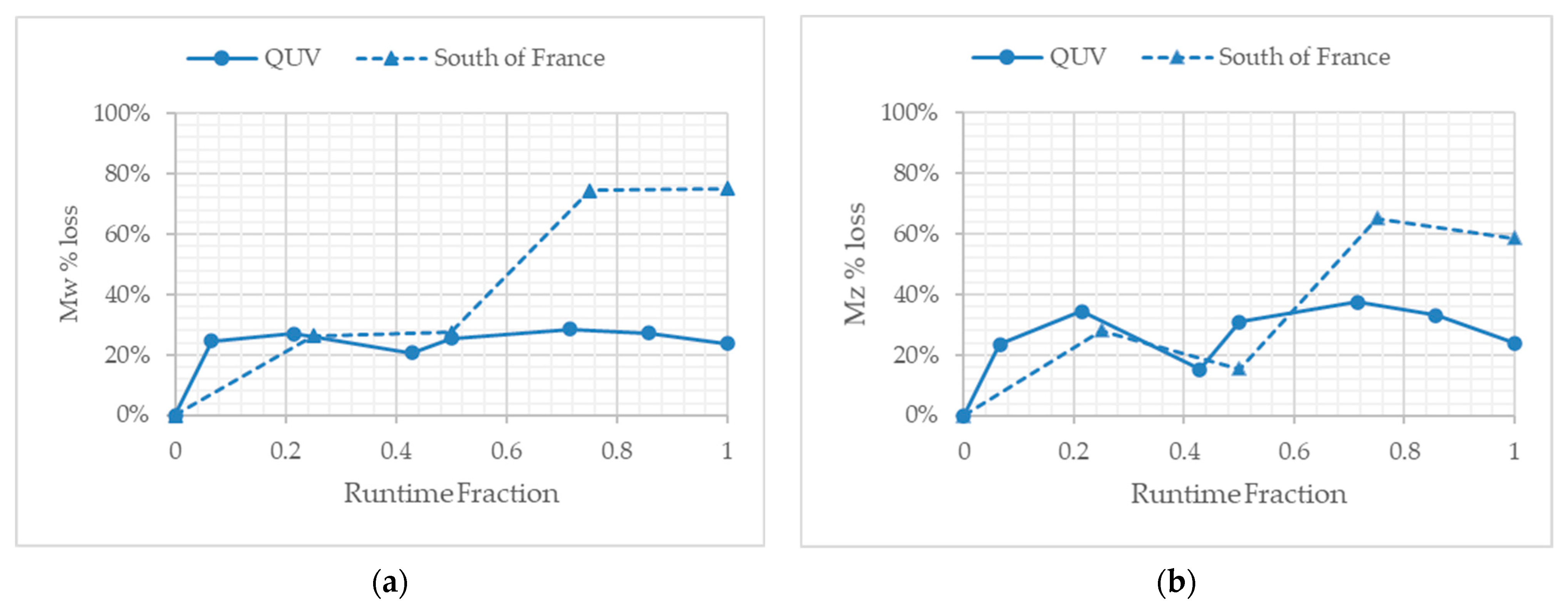
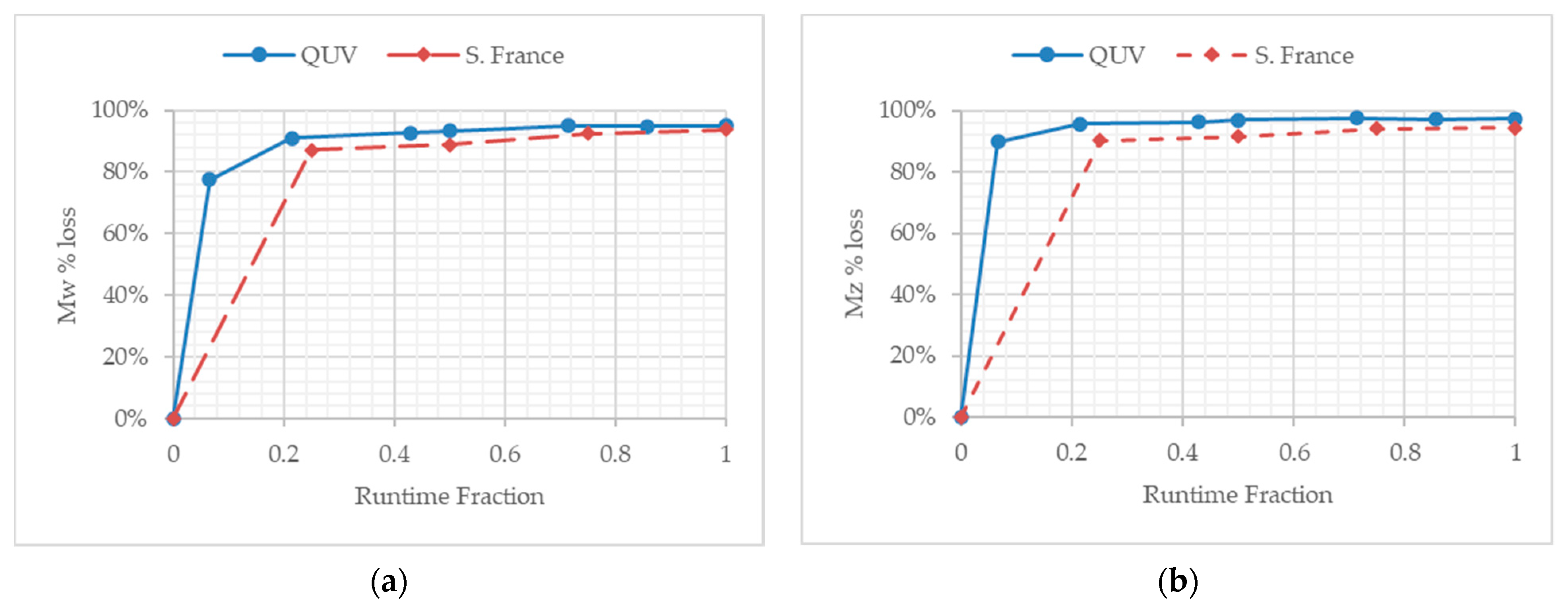
3.4. Molecular Weight Changes during Temperate Laboratory Exposure and under Outdoor Exposure in France
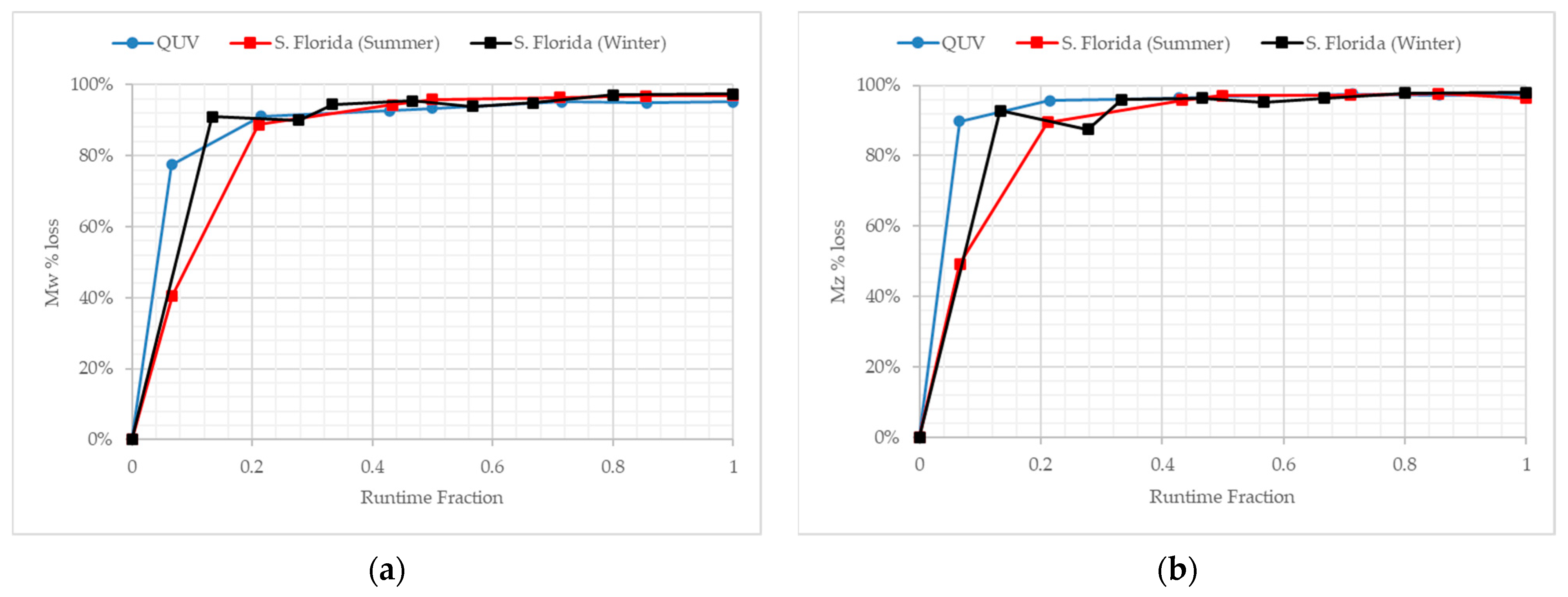
3.5. Molecular Weight Changes during Outdoor Exposure in Florida at Different Times of the Calendar Year
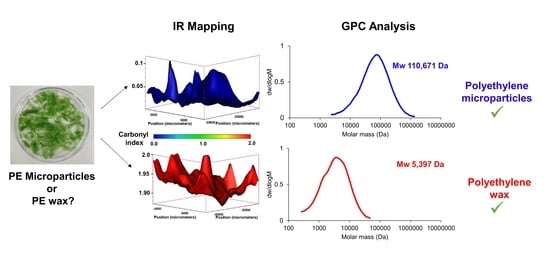
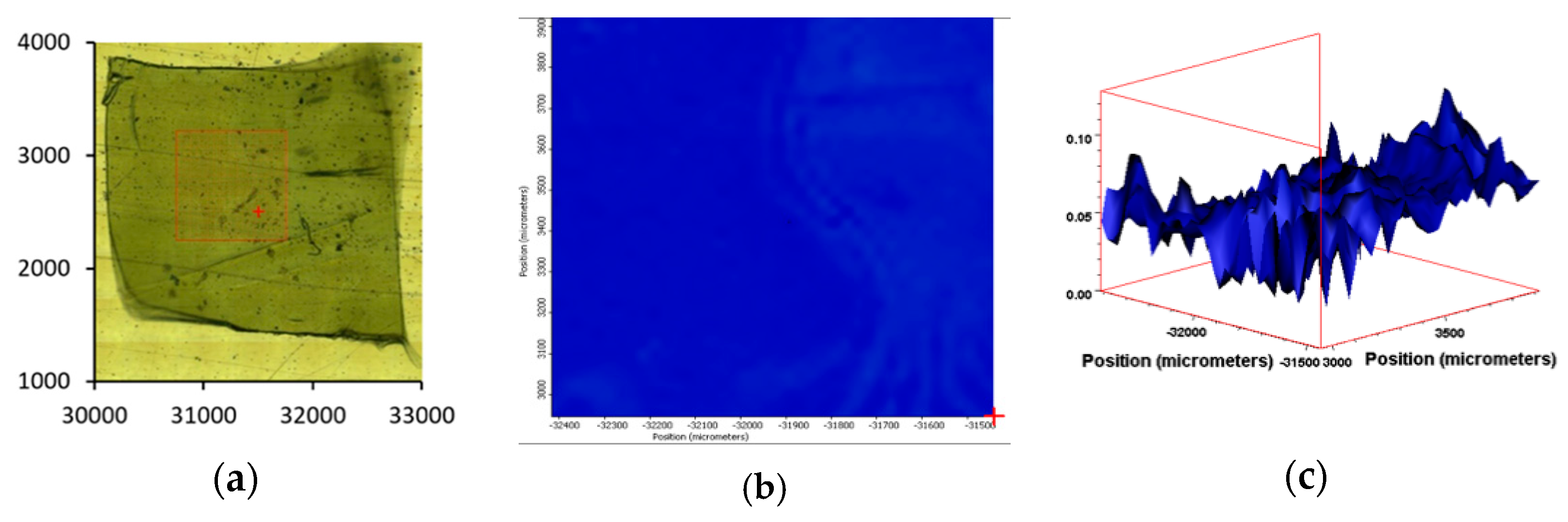
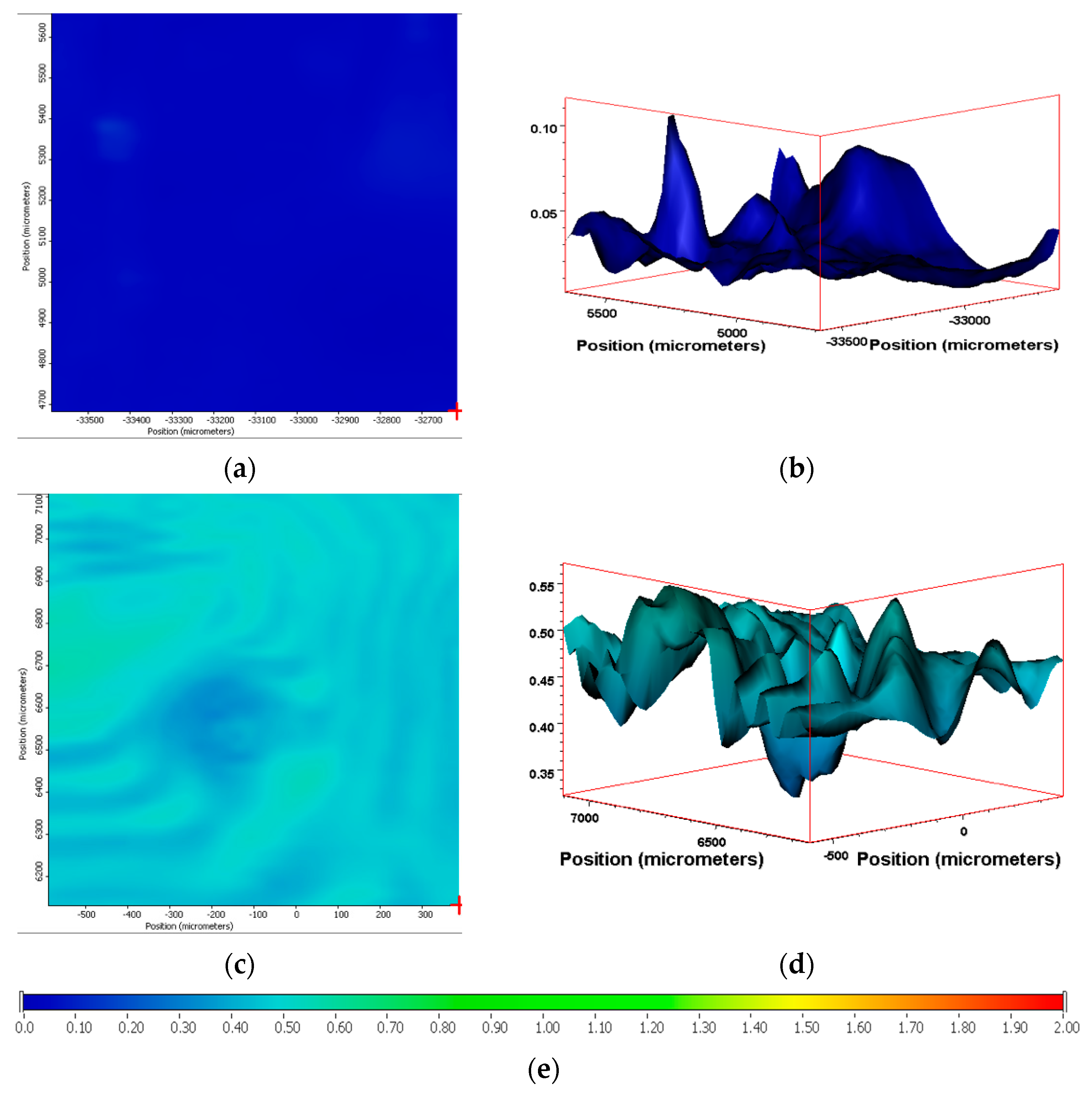
3.6. IR Mapping Images of the Surfaces of the Films during Exposure
3.7. Drop Tests for Polyethylene Waxes
4. Discussion
4.1. Comparison of Exposure Sites
4.2. Weathering Leading to Microparticles of Polyethylene or Polyethylene Waxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freedonia. Available online: https://www.freedoniagroup.com/industry-study/world-polyethylene-3210.htm (accessed on 9 June 2021).
- Plasticker. Available online: https://plasticker.de/preise/pms_en.php?kat=Mahlgut&aog=A&show=ok&make=ok (accessed on 9 June 2021).
- Britannica. Available online: https://www.britannica.com/science/polyethylene (accessed on 9 June 2021).
- British Plastics Federation—Plastipedia: The Web’s Largest Plastics Encyclopedia. Available online: https://www.bpf.co.uk/plastipedia/default.aspx (accessed on 9 June 2021).
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoellein, T. The global odyssey of plastic pollution. Science 2020, 368, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Ocean Conservancy. Available online: https://oceanconservancy.org/blog/2020/09/08/food-wrappers-now-1-item-found-icc/ok (accessed on 9 June 2021).
- White, C.C.; White, K.M.; Pickett, J.E. Service Life Prediction of Polymers and Plastics Exposed to Outdoor Weathering; William Andrew Publishing: Norwich, NY, USA, 2017. [Google Scholar]
- Albihn, P. The 5-Year Accelerated Aging Project for Thermoset and Thermoplastic Elastomeric Materials: A Service Life Prediction Tool. In Elastomers and Components; Woodhead Publishing: Cambridge, UK, 2006; Chapter 1; pp. 3–25. [Google Scholar]
- Qin, J.; Jiang, J.; Tao, Y.; Zhao, S.; Zeng, W.; Shi, Y.; Lu, T.; Guo, L.; Wang, S.; Zhang, X.; et al. Sunlight Tracking and Concentrated Accelerated Weathering Test Applied in Weatherability Evaluation and Service Life Prediction of Polymeric Materials. A review. Polym. Test. 2021, 93, 106940. [Google Scholar] [CrossRef]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J.P. Polyethylene Terephtalate Degradation under Natural and Accelerated Weathering Conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.-F.; Inoue, C. Changes during the Weathering of Polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Ghosh, D.; Khastgir, D. Degradation and Stability of Polymeric High-Voltage Insulators and Prediction of Their Service Life through Environmental and Accelerated Aging processes. ACS Omega 2018, 3, 11317–11330. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Yang, J.; Kong, M.; Yang, H.; Zhao, J.; Li, G. Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study. Polym. Degrad. Stab. 2015, 112, 145–159. [Google Scholar] [CrossRef]
- Shimizu, K.; Tokuta, Y.; Oishi, A.; Kuriyama, T.; Kunioka, M. Weatherability of Polypropylene by Accelerated Weathering Tests and Outdoor Exposure Tests in Japan. J. Polym. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- ASTM D4329-13, Standard Practice for Fluorescent Ultraviolet (UV) Lamp Apparatus Exposure of Plastics; ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–6. Available online: https://www.astm.org/Standards/D4329.htm (accessed on 9 June 2021). [CrossRef]
- ISO 4892-3:2016, Plastics—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps; International Organization for Standardization: Geneva, Switzerland, 2016.
- ASTM G154-16, Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Nonmetallic Material; ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–11. Available online: https://www.astm.org/Standards/G154 (accessed on 9 June 2021). [CrossRef]
- Hill, G.; Moreira, C.; Huynh, F.; Trufasila, A.; Ly, F.; Lloyd, R.; Sawal, H.; Wallis, C. Correlation of a temperate UV-weathering cycle to outdoor exposure for the determination of the environmental instability of polyethylene films using HT-GPC analysis. Polymers 2021, 13, 591. [Google Scholar] [CrossRef]
- ASTM D1435-20, Standard Practice for Outdoor Weathering of Plastics; ASTM International: West Conshohocken, PA, USA, 2020; Available online: https://www.astm.org/Standards/D1435.htm (accessed on 9 June 2021). [CrossRef]
- ISO/IEC 17025:2017, General Requirements for the Competence of Testing and Calibration Laboratories; Organization for Standardization: Geneva, Switzerland, 2017.
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymer 2020, 20, 369–381. [Google Scholar] [CrossRef]
- ASTM D6474-20, Standard Test Method for Determining Molecular Weight Distribution and Molecular Weight Averages of Polyolefins by High Temperature Gel Permeation Chromatography; ASTM International: West Conshohocken, PA, USA, 2020; Available online: https://www.astm.org/Standards/D6474.htm (accessed on 9 June 2021). [CrossRef]
- ASTM D127-19, Standard Test Method for Drop Melting Point of Petroleum Wax, Including Petrolatum; ASTM International: West Conshohocken, PA, USA, 2019; Available online: www.astm.org (accessed on 9 June 2021). [CrossRef]
- Weber, G.V. Wax Characterization by Instrumental Analysis; University of Stellenbosch: Stellenbosch, South Africa, 2000. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.L.; Wang, S.-Q.; Bhargava, R. Peer Reviewed: FTIR Images. A new technique produces images worth a thousand spectra. Anal. Chem. 2001, 73, 360–369. [Google Scholar] [CrossRef][Green Version]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- ASTM D1986-14(2021), Standard Test Method for Determining the Apparent Viscosity of Polyethylene Wax; ASTM International: West Conshohocken, PA, USA, 2021; Available online: https://www.astm.org/Standards/D1986.htm (accessed on 9 June 2021). [CrossRef]
- ASTM D3954-15, Standard Test Method for Dropping Point of Waxes; ASTM International: West Conshohocken, PA, USA, 2015; Available online: https://www.astm.org/Standards/D3954.htm (accessed on 9 June 2021). [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á; Irigoien, X.; Duarte, C.M. Plastic accumulation in the mediterranean sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; A Van Franeker, J.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.M.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, S. Characterization of lubricant polyethylene waxes. Eur. J. Tech. 2020, 10, 489–500. [Google Scholar] [CrossRef]
- Yetgin, S.; Gonen, M.; Savrik, S.A.; Balkose, D. Imidic Polymers and Green Polymer Chemistry; Apple Academic Press: Palm Bay, FL, USA, 2021. [Google Scholar]

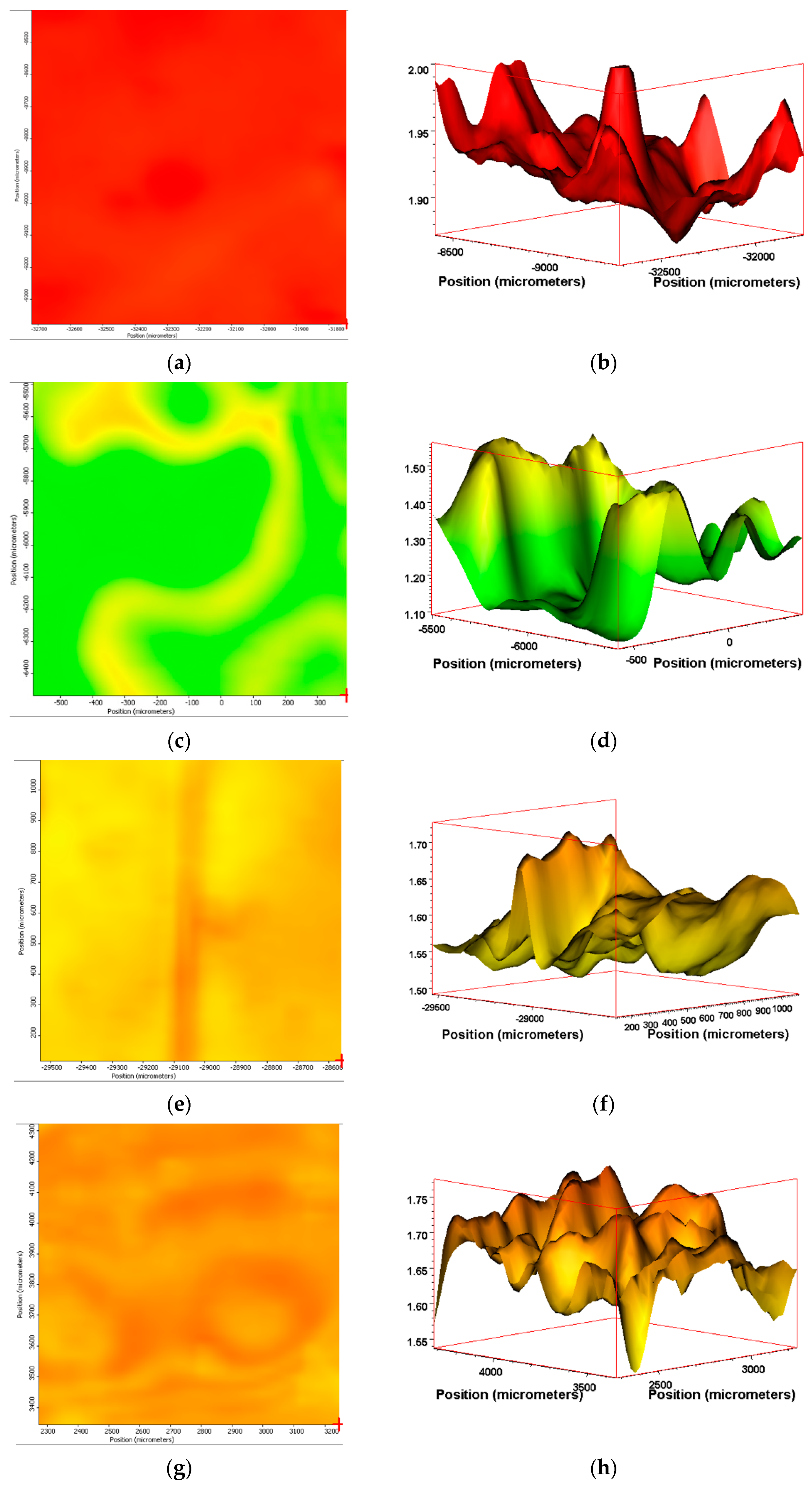
| Sample Name | Description of Weathering Exposure |
|---|---|
| PE-01 | Standard PE film weathered under temperate UV-accelerated laboratory conditions. |
| PE-02 | Enhanced PE film containing the biotransformation technology weathered under temperate UV-accelerated laboratory conditions. |
| PE-03 | Standard PE film weathered under outdoor exposure at Sanary Sur Mer, France between September and December 2020. |
| PE-04 | Enhanced PE film containing the biotransformation technology weathered under outdoor exposure at Sanary Sur Mer, France between September and December 2020. |
| PE-05 | Enhanced PE film containing the biotransformation technology weathered under outdoor exposure at Homestead, Florida between July and October 2020. |
| PE-06 | Enhanced PE film containing the biotransformation technology weathered under outdoor exposure at Homestead, Florida between October 2019 and January 2020. |
| Total No. of Days of Exposure (Days/Hours) | Runtime Fraction | PE-01 | PE-02 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw (Da) | % Mw Loss | Mz (Da) | % Mz Loss | Mw (Da) | % Mw Loss | Mz (Da) | % Mz Loss | ||
| 0 d | 0 | 110,018 | / | 251,393 | / | 151,588 | / | 459,400 | / |
| 0.92 d (22 h) | 0.067 | 109,470 | 28% | 281,419 | 24% | 24,746 | 78% | 47,063 | 90% |
| 3 d (72 h) | 0.21 | 105,799 | 30% | 241,274 | 34% | 9815 | 91% | 19,873 | 96% |
| 6 d (144 h) | 0.43 | 114,936 | 24% | 311,659 | 15% | 8099 | 93% | 16,581 | 96% |
| 7 d (168 h) | 0.5 | 107,947 | 29% | 254,525 | 31% | 7301 | 93% | 14,260 | 97% |
| 10 d (240 h) | 0.71 | 103,704 | 32% | 230,252 | 37% | 5397 | 95% | 11,052 | 98% |
| 12 d (288 h) | 0.86 | 105,529 | 30% | 246,260 | 33% | 5663 | 95% | 12,884 | 97% |
| 14 d (336 h) | 1 | 110,671 | 27% | 279,801 | 24% | 5397 | 95% | 11,929 | 97% |
| Total No. of Days of Exposure (Days/Hours) | Runtime Fraction | PE-03 | PE-04 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw (Da) | % Mw Loss | Mz (Da) | % Mz Loss | Mw (Da) | % Mw Loss | Mz (Da) | % Mz Loss | ||
| 0 d | 0 | 110,018 | / | 251,393 | / | 151,588 | 0% | 459,400 | / |
| 30 d (720 h) | 0.25 | 81,032 | 26% | 180,593 | 28% | 19,494 | 87% | 44,833 | 90% |
| 60 d (1440 h) | 0.5 | 79,746 | 28% | 212,682 | 15% | 16,779 | 89% | 38,360 | 92% |
| 90 (2160 h) | 0.75 | 37,029 | 66% | 87,500 | 65% | 11,566 | 92% | 26,857 | 94% |
| 120 (2880 h) | 1 | 36,198 | 67% | 104,311 | 59% | 9532 | 94% | 25,834 | 94% |
| Total No. of Days of Exposure (Days/Hours) | Runtime Fraction | PE-05 | PE-06 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw | % Mw Loss | Mz | % Mz Loss | Time | Runtime Fraction | Mw | % Mw Loss | Mz | % Mz Loss | ||
| 0 d | 0 | 151,588 | / | 459,400 | / | 0 d | 0 | 151,588 | / | 459,400 | / |
| 6 d (144 h) | 0.078 | 90,062 | 41% | 232,866 | 49% | 12 d (288 h) | 0.13 | 13,575 | 91% | 32,942 | 93% |
| 19 d (456 h) | 0.21 | 17,068 | 89% | 48,171 | 90% | 25 d (600 h) | 0.28 | 15,234 | 90% | 57,343 | 88% |
| 39 d (936 h) | 0.43 | 8574 | 94% | 19,450 | 96% | 30 d (720 h) | 0.33 | 8318 | 95% | 18,668 | 96% |
| 45 d (1080 h) | 0.50 | 6241 | 96% | 13,190 | 97% | 42 d (1008 h) | 0.47 | 6907 | 95% | 16,550 | 96% |
| 64 d (1536 h) | 0.71 | 5529 | 97% | 12,690 | 97% | 60 d (1440 h) | 0.67 | 7676 | 95% | 16,912 | 96% |
| 77 d (1848 h) | 0.86 | 4850 | 97% | 10,955 | 98% | 72 d (1728 h) | 0.80 | 4475 | 97% | 10,325 | 98% |
| 90 d (2160 h) | 1 | 4694 | 97% | 16,885 | 96% | 90 d (2160 h) | 1 | 3936 | 97% | 9179 | 98% |
| Sample | Weathering Protocol | Temperature of Dropping Point | Visual Observation at Drop Temperature (Optical Zoom 2×; Aspect Ratio 1:1; Images Cropped to Show Area of Thermometer Bulb 1 cm by 0.5 cm at Dropping Point) |
|---|---|---|---|
| PE-01 | 14 days Temperate UV-accelerated weathering | No drop |  |
| PE-02 | 14 days Temperate UV-accelerated weathering | 114 °C | 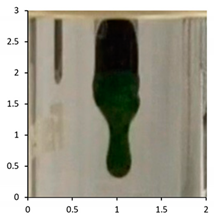 |
| PE-03 | 120 days Outdoor exposure—France | No drop | 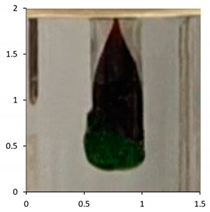 |
| PE-04 | 120 days Outdoor exposure—France | 114 °C | 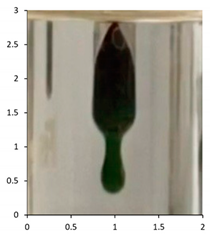 |
| PE-05 | 90 days Outdoor exposure—Florida (Summer) | 118 °C | 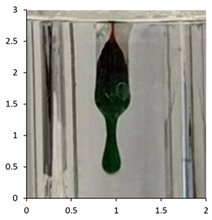 |
| PE-06 | 90 days Outdoor exposure—Florida (Winter) | 116 °C | 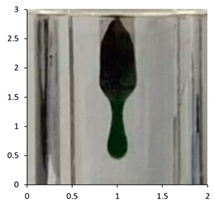 |
| Homestead, Florida [a] | Sanary Sur Mer, France [b] | |
|---|---|---|
| Average daily temperature during testing exposure period | 26 °C | 15 °C |
| Average daily humidity during testing exposure period | 84% | 70% |
| Total rainfall during testing exposure period | 323 mm | 217 mm |
| Comparable global cities [c] | Mumbai, India | Casablanca, Morocco |
| Mombasa Kenya | Istanbul, Turkey | |
| Bangkok, India | Tel Aviv, Israel |
| PE-03 |  |  |  |
| Day 0 Dropping point—above 140 | Day 60 Dropping point—above 140 | Day 120 Dropping point—above 140 | |
| PE-04 |  |  |  |
| Day 0 Dropping point—above 140 | Day 60 Dropping point—116 | Day 120 Dropping point—114 | |
| PE-05 |  |  |  |
| Day 0 Dropping point—above 140 | Day 45 Dropping point—above 113 | Day 90 Dropping point—118 | |
| PE-06 |  |  |  |
| Day 0 Dropping point—above 140 | Day 60 Dropping point—112 | Day 90 Dropping point—116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, C.; Lloyd, R.; Hill, G.; Huynh, F.; Trufasila, A.; Ly, F.; Sawal, H.; Wallis, C. Temperate UV-Accelerated Weathering Cycle Combined with HT-GPC Analysis and Drop Point Testing for Determining the Environmental Instability of Polyethylene Films. Polymers 2021, 13, 2373. https://doi.org/10.3390/polym13142373
Moreira C, Lloyd R, Hill G, Huynh F, Trufasila A, Ly F, Sawal H, Wallis C. Temperate UV-Accelerated Weathering Cycle Combined with HT-GPC Analysis and Drop Point Testing for Determining the Environmental Instability of Polyethylene Films. Polymers. 2021; 13(14):2373. https://doi.org/10.3390/polym13142373
Chicago/Turabian StyleMoreira, Celine, Richard Lloyd, Gavin Hill, Florence Huynh, Ana Trufasila, Faith Ly, Hasan Sawal, and Christopher Wallis. 2021. "Temperate UV-Accelerated Weathering Cycle Combined with HT-GPC Analysis and Drop Point Testing for Determining the Environmental Instability of Polyethylene Films" Polymers 13, no. 14: 2373. https://doi.org/10.3390/polym13142373
APA StyleMoreira, C., Lloyd, R., Hill, G., Huynh, F., Trufasila, A., Ly, F., Sawal, H., & Wallis, C. (2021). Temperate UV-Accelerated Weathering Cycle Combined with HT-GPC Analysis and Drop Point Testing for Determining the Environmental Instability of Polyethylene Films. Polymers, 13(14), 2373. https://doi.org/10.3390/polym13142373