1. Introduction
Carbon fibers (CFs) are carbon-like yarn fabrics with impressive mechanical performance and myriad functional properties. CFs have a tremendous strength, low density, stiffness, and remarkable electrical and thermal conductivity. Furthermore, they are fire-retardant and chemically stable [
1,
2]. With comparison to metals such as steel, CFs have higher unique modulus and strength. These fibers comprise at least 92 (wt %) of carbon content [
3] acquired from the pyrolysis of stabilized precursor fibers above (200–400 °C) in air with the aid of an oxidation approach. Then, in an inert atmosphere, the infusible stabilized fibers are carbonized at a temperature of about 1000 °C to extract gases and other non-carbon elements [
4]. The high mechanical efficiency makes CFs desirable for using in composite manufacturing in the form of woven textiles as well as straight or chopped fibers [
5].
Presently, CFs are also applied to rockets, golf club shafts, medicine, and in the automobile industry [
5]. The polyacrylonitrile (PAN) is currently the main precursor for processing CFs, and it accounts about half of the cost of production [
4,
6]. Petroleum pitch and regenerated cellulose (rayon) are alternative precursors. The key obstacle of using these precursors is the high cost of production that restricts delivery despite the developing request; additionally, the nitrile groups in PAN generate harmful by-products [
7]. The environmental interest and related costs associated with this method can be resolved by using bio-based precursors in particular [
8].
One of the most polluting resources is organic waste from agro-industries. Clean technology can be incorporated to play down organic wastes via recycling [
8]. In particular, renewable raw materials consisting of biopolymers or biogenic polymers are interesting sources for handling CFs. These biogenic sources are accessible in plants that are comprised from cellulose, hemicellulose, and lignin. Analysis projects have been undertaken worldwide to investigate biogenic-based CFs [
9].
Lignin is the largest source of biomass with an aromatic function and a carbon content material of more than 10%. In view of lignin’s low price and carbon-building chemical structure [
10], it can be considered the most fascinating sustainable precursor for the development of CFs [
11]. The amount of lignin varies between plant types. For lignocellulosic materials including banana bunch, palm fronds, bagasse, etc., there is a wide range of diversity wherein their chemical behavior will be different primarily depending on original source and extraction technique [
12]. The exceptional popular delignification techniques for isolating lignin are the kraft and organosolv approaches [
13]. The organosolv method involves the addition of aqueous organic solvents such as ethanol, formic acid, acetic acid, and ethylene glycol to the biomass under specific temperature and pressure conditions [
14]. In general, this process could virtually fractionate the three biomass components in one stage to complete the lignin and biorefinery concept standards.
The electrospinning process has picked up much consideration in the view that it can be an adaptable method that proceeds the production of nano and submicron fibers from biopolymer solution [
15,
16]. Nevertheless, it is important to investigate the need to generate fibers based on new lignocellulosic biomass in the application of this method [
17]. However, this may be conditioned by the lack of solvents that are capable of deconstructing the fibers to create arrangements for their components (cellulose, hemicellulose, and lignin) that have affordable electrospinning properties at the same time [
18]. Lignin is a large-scale sustainable source of aromatic functionalities that promote its remarkable properties, but the electrospinning of its solutions is impeded by its complex nonlinear structure. In addition to the number of possible modifications owing to its chemical structure, blending lignin with other bio-based polymers has been of great interest due to its excessive accessibility, biodegradability, and strong mechanical properties [
19]. Several research studies have focused on the combination of lignin into polyethylene terephthalate (PET), polylactic acid (PLA), and polyvinyl alcohol (PVA) to form bioplastics [
20]. PET is one of the most common recycled, new, and reused polymers [
21]. It has been widely used as an additive to manufacture low-cost ultrafine fibers for the electrospinning technique [
22]. Plasticizers may be associated with another polymer by replacing polymer interactions [
20]. This phenomenon strengthened the mobility and flexibility of the polymer by promoting the lowering of intermolecular forces, glass transition temperature (
Tg), and handling temperature of the blends. PLA is a biopolymer obtained from corn starch and sugarcane [
23]. It is a highly brittle polymer with very low toughness [
24]. Therefore, several studies are trying to explore new formulations i.e., evaporation, copolymerization, and blending to resolve this constrain [
25]. Poly(hydroxybutyrate) (PHB) [
26], oligomeric acid (OLA), and acetyl tributyl citrate (ATBC), which impart chain mobility, are popular plasticizers for PLA, resulting in highly accelerated ductile properties [
27,
28].
Various studies have focused on the conversion of precursor fibers to CFs through the preservation of fibrous morphology by the process of carbonization. Throughout the stabilization process, the fibers can soften and melt together when the temperature increases above the
Tg of the precursor fibers [
11]. Iodine has been investigated as a treatment to improve the stabilization process of various materials [
29]. Iodine is well known to make charge transfer complexes (CTCs) with electron-rich particles such as those with lone pairs and aromatic rings within the form of polyiodides [
30]. Consequently, this promotes improvements of dehydrogenation and speeds up the thermostabilization process.
New surface modification technologies such as conducting polymer coating, gold spraying coating, metal oxide coating, etc., [
31] have been developed for lignin-based fibers with adjustable physicochemical properties that have infinite applications in science and technology including energy storage, sensors, drug delivery, and adsorption [
32]. By the in situ oxidative chemical polymerization of aniline monomer in the presence of fiber using a suitable oxidant, the conducting polymer coating of hydrophilic polymers can simply be achieved. Natural fibers including kenaf, jute, mango, and coconut fibers coated with polyaniline have validated properties in various fields [
33]. Therefore, polyaniline is an appropriate prospect for natural fiber coating to meet the necessity of continuous disposable technology.
Industrial wastewater containing both inorganic and organic contaminants really impacts biodiversity, the ecological environment, and the ocean framework’s characteristic activities. Amongst these contaminants, one such pollutant is synthetic dye (methylene blue, MB), which is considered to be the foremost common and harmful water contaminant [
34]. It is one of the greatest prevalent used substances for dyeing cotton, silk, and wool [
35]. Physical adsorption has received attention due to its straightforward treatment technique and persistent efficiency as well as low cost amongst various water purification techniques [
36]. Due to the excessive cost production of high quantities of sludge and difficulties in regeneration, the cost of water treatment has risen [
37]. Natural fibers, carbon fibers, and material-based polymer composites may be impressive waste water adsorbents due to their low price, eco-friendliness, and highly stable viability in traditionally organic solvents [
38].
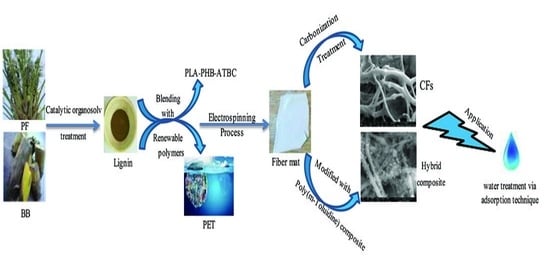
The feasibility of isolating lignin from palm fronds and banana bunches utilizing organosolv treatment with acetic acid and formic acid as solvents and H2SO4 as a catalyst was investigated in this research. Throughout the electrospinning process, electrospun nanofibers were derived by combining lignin with either recycled polymer such as PET or biodegradable polymers such as a plasticized blend of PLA and PHB using various lignin loads. Iodine treatment of biopolymer-based fibers was carried out to enhance and hasten the thermostabilization stage. The fibers were further either carbonized at 500 °C to prepare carbon nanofibers or coated with poly(m-toluidine). The adsorption of methylene blue dye as a working pattern on tested materials was addressed.
2. Materials and Methods
2.1. Materials
In the maritime sub-tropical district (Nile Delta), palm fronds (PF) and banana bunch (BB) feed stocks were collected from neighborhood lands. The raw materials were initially rinsed regularly with distilled water to remove impurities and dust, followed by drying at room temperature. The dried samples were chopped and sieved to mesh sizes ranging from 30 to 50 mm.
All chemicals were of analytical grade and used as received without further purification unless otherwise stated. Formic acid (85%), acetic acid (85%), sulfuric acid (98%), 2,2,2-triflouro ethanol (TFE), dichloromethane (DCM), and 1,1,1,3,3,3-hexaflouro-2-propanol (HFIP) were purchased from Sigma-Aldrich (Weinheim, Germany). Polylactic acid blended with polyhydroxy butyrate plasticized with acetyl tributyl citrate (PLA-PHB-ATBC) pellets were obtained from Panara, s.r.o. (Nitra, Slovakia), while polyethylene terephthalate (PET) was received as red bottle scraps from Baldovská water (Baldovce, Slovakia). Ammonium peroxydisulfate ((NH4)2S2O8) (APS), hydrochloric acid (HCl), and iodine crystals were purchased from Fisher-Scientific (Waltham, MA, USA) as the reinforcement materials used in this study. Monomer (m-toluidine; 2-methyl aniline) purchased from Merck company (Burlington, MA, USA) was vacuum distilled before employing. Methylene blue dye (MB) was attained from RIEDEL-DE HAEN AG company (Berlin, Germany) with an assay 96%.
2.2. Delignification Process
The delignification process was carried out in a one-liter glass reaction vessel fitted with reflux condensers by heating the lignin source in 0.2% (
v/
v) H
2SO
4 as a promoter at 100 °C (heated by an electrical mantle) under atmospheric pressure for 2 h. Eventually, with 10 g of PF or BB, a mixture of formic acid/acetic acid with a constant (
v/
v) ratio of 70:30 was assorted to provide a 10:1 liquid-to-solid ratio. In order to precipitate the soluble lignin, the cooked liquors were first concentrated under vacuum to at least one-half of the initial volume applying a rotary evaporator. Then, the concentrated liquors were blended with five volumes of water and settled at room temperature for 1 h. The precipitated lignin was subsequently extracted by filtration and dried up to a constant mass at 90 °C. These samples were designated as organosolv palm frond lignin (OPFL) and organosolv banana bunch lignin (OBBL), respectively.
Scheme 1 illustrates steps for the delignification process.
2.3. Chemical Analysis of Extracted Lignin
The content of ash was gravimetrically measured according to the TAPPI test method T-413 [
39], while the content of dry and moisture matter was assessed using the standard official analysis methods of the Association of Official Analytical Chemists (AO-AC, 1990) [
40].
Crude protein (CP) is an evaluation of the total protein based on a laboratory N2 analysis, which can be used to measure the total protein content in a matter through multiplying the nitrogen figure by 6.25. This is based on the premise that N
2 is derived from protein containing 16% N
2 (AO-AC 1984) [
41].
The molecular formulas of both extracted lignin samples OPFL and OBBL were predicted by the percentages of C, H, N, and O resulted from elemental analysis [
41]. In addition, their chemical composition is reflected in
Table 1.
2.4. Processing of Electrospun Polymer Solutions
2.4.1. OPFL/PLA Polymer Blend
PLA-PHB-ATBC pellets were dissolved in a single solvent system (TFE) at various concentrations (20, 30, 40, and 50% (wt/v)) embedded under magnetic stirring in water bath sonication for 24 h to eliminate particle agglomeration. All of the above-mentioned solutions have been subjected to the process of electrospinning. The optimum concentration was achieved at 30% (wt/v), which created entangled chains that were stretched during spinning, and strengthened PLA thin bead-free fibers were formed.
OPFL (0.67 g) was subsequently submerged in 10 mL of TFE at a concentration of 6.7% (wt/v) (lignin to TFE). Ultimately, PLA-PHB-ATBC solution was added to the OPFL dispersion and mixed together for 1 h either in a water bath sonicator or using a finger sonicator (UP 400S with 30% amplitude and 0.5 cycle) in an ice bath under constant stirring. The required total polymer concentration of 36.7% (wt/v) was achieved by this method. The sample is listed as OPFL/PLA/36.7%.
2.4.2. OBBL/PET Polymer Blend
PET origin was extracted from waste plastic bottles crushed in a knife mill and dissolved in a binary solvent mixture composed of HFIP/DCM with ratio of (2:1) for two separate concentrations of PET (9 and 20% (wt/v)). These polymer solutions have been subjected to the process of electrospinning.
In two glass vials containing HFIP (6.7 mL), two sections of weighted OBBL (0.45 gm) were dissolved and processed for 10 min in a high-speed planetary mixer until lignin was fully dissolved. Subsequently, PET was introduced and homogenized for a further 10 min with two separate concentrations of (9 and 20% (wt/v)). After that, 3.3 mL of DCM was added to each glass vial solution to obtain a HFIP/DCM ratio of 2:1. Then, the vials were placed in an ice bath, and finger sonication was applied for 1 h. The resulted solutions were fully homogeneous and assigned as OBBL/PET/13.5% and OBBL/PET/24.5% with the total polymer concentrations of 13.5 and 24.5% (wt/v), respectively.
2.5. Electrospun Fiber Mats Preparation
There are four main components of the primary electrospinning apparatus: a high-voltage source (Spellman SL-150W, Bochum, Germany) that produces an electrical field between a positive-charged syringe needle and a grounded collector, 21-gauge (0.8 mm) stainless steel needle (B.Braun, Bratislava, Slovakia) with a flat end where the charged solution is pressured to stretch under its electrostatic forces, a syringe pump model NE-1000 (Era Pump System, Inc., Farmingdale, NY, USA) (5 mL), and a grounded pattern to deposit the fabricated fibers. The power supply is connected to the metallic needle by electrical wires, and there is a fairly short distance (15 cm) between the syringe tube and the target, as shown in
Figure 1. The jet would be elongated by electrostatic repulsion as the solvent evaporates while electrospinning. This is followed by the thinning process that leads to the development of a micro-to-nano scale uniform fiber, which can be gathered in different orientations to create some specialized structures with distinct composition and mechanical properties. Within the unit, the temperature and relative humidity were 25 °C and 50%, respectively.
To reduce the impact of applied shear while spinning, all the checked mixed samples were held at least one hour prior to spinning. The fibers were collected on a stationary flat plate covered with aluminum foil. The syringe pump was working at a flow rate of 0.5 mL/h supplied to the spinneret by the polymer solution. For PET/9%, PET/20%, OBBL/PET/13.5%, and OBBL/PET/24.5%, the running voltage was 15 kV, while for PLA-PHB-ATBC at 20, 30, 40, and 50% (wt/v) and for OPFL/PLA/36.7%, the running voltages were 16.5 kV and 19.5 kV, respectively.
2.6. Handling Fibers with Iodine
The as-spun fibers (OBBL/PET/13.5%, OBBL/PET/24.5%, and OPFL/PLA/36.7%) were enclosed in a porcelain crucible and placed in a sealed glass jar containing iodine crystals. The iodine jar was kept in an air oven for 15 min at 100 °C. The system was eventually cooled down to room temperature before the fibers were detached [
43]. The iodinated fiber mats have been converted into a dark brown color. They are denoted as I-OBBL/PET/13.5%, I-OBBL/PET/24.5%, and I-OPFL/PLA/36.7%.
2.7. Thermo-Stabilization and Carbonization Treatment
In a muffle furnace (Shimadzu GAS CHROMATOGRAPH (GC-14A) apparatus (used as an air oven)), the stabilization of non-iodinated (OPFL/PLA/36.7%) fiber mats was carried out heating from room temperature to 120, 140, and 160 °C at a heating rate 1 °C/min and isothermally maintained for 30 min for each temperature under a steady air flow during the entire phase. For non-iodinated (OBBL/PET/13.5% and OBBL/PET/24.5%) fiber mats, the same procedure was replicated and performed at 230, 250, and 270 °C. Then, the temperature was isothermally maintained for 1 h. The iodinated fibers were also supplied with the optimum temperature that was achieved to stabilize non-iodinated fiber mat samples.
In a tubular furnace, the stabilized (iodinated and non-iodinated fiber mats were mounted on a porcelain boat and carbonized. Throughout the carbonization phase, the heating rate was 3 °C/min. In order to preserve an inert environment, fibers were isothermally kept at 500 °C for 15 min underneath a nitrogen flow rate of 0.5 standard cubic feet per hour; then, they were naturally cooled down to room temperature.
2.8. Preparation of Hybrid Composite Materials
Via the in situ oxidative chemical polymerization of m-toluidine, dry as-spun fiber mats with a length of 30 mm and width of 10 mm were drawn up. In 25 mL of 0.4 M ammonium peroxydisulphate (APS, oxidant), 0.25 g of each (OBBL/PET/13.5%, OBBL/PET/24.5%, and OPFL/PLA/36.7%) nanofibers was dispersed and sonicated for half an hour. At room temperature, the polymerization process was initiated by drop-wise addition of m-toluidine (monomer) and HCl (dopant) to the above-mentioned solution for an additional 1 h under continuous sonication to obtain 0.1 M concentration of both. The weight ratio of fibers to monomer (0.5:1) was held constant in 50 mL of total reaction solution [
44]. Then, the received composites were filtered and washed with repeatedly distilled water to remove oligomeric and non-treated monomers; then, they were dried at 80 °C in an air oven and labeled as OBBL/PET/13.5%/P-mTol, OBBL/PET/24.5%/P-mTol, and OPFL/PLA/36.7%/P-mTol.
2.9. Characterization
Elemental mapping observation of the generated samples at distinct stages was identified by using (Automatic Vario El Elementar, Device, Germany). The major elemental composition is C, H, N, and O contents. The morphology of the fabricated samples was monitored with scanning electron microscope (SEM) using QUANTA FEG 250 ESEM (Japan). A transmission electron microscope JEOL 1200FX (Tokyo, Japan) operated at 80 kV was used. Surface functional groups were analyzed through Fourier transform infrared using a KBr pellet approach on an FT-IR NICOLET 8700 spectrometer (Thermo Scientific, Loughborough, UK) in the spectral range of 400–4000 cm−1 along with four resolutions averaged over 40 scans. Thermogravimetric analysis (TGA) was performed to analyze the thermal decomposition behavior employing a Perkin Elmer 7 series Thermal Analysis (Perkin Elmer, USA). Samples were preheated in the nitrogen flow (200 mL/min) at 25 °C for 5 min and then heated up to 1000 °C at a heating rate of 5 °C/min. The specific surface area (Brunauer–Emmett–Teller (BET) method) and pore characteristics were evaluated by N2 adsorption–desorption at 77K with a surface area analyzer model (Quanta Chrome instruments, NOVA Automated GAS Sorption System Version 1.12, USA).
2.10. Liquid Phase Adsorption Characteristics
The purpose of this study is to investigate the feasibility of the treatment of aqueous solutions contaminated with methylene blue dye using as-spun fiber mats, CFs and hybrid composites. Consequently, by dissolving 50 mg of MB dye in 1 L of distilled water, a stock solution was prepared. Then, 10 mg of adsorbents at neutral pH (6.5) was administrated with MB dye (10 mL) without any external adjustment [
45]. To achieve equilibrium, each sample was held in a rotary shaker at 180 rpm for 24 h at room temperature. Preliminary tests showed that a time of 24 h was sufficient to achieve conditions of equilibrium. The UV-visible absorption spectra of the supernatant solution were analyzed using a UV-visible spectrophotometer (Type UV-2401PC) in a 1 cm quartz cuvette to track the characteristic absorption peaks of MB at a wavelength of 664 nm.
The amount of adsorption at equilibrium (
qe, (mg/g)) was calculated using the following equation:
where
Co and
Ce (mg/L) are the initial and equilibrium liquid phase concentrations,
V (L) is the volume of the equilibrium solution, and
m (g
) is the mass of the adsorbent.
The percentage dye removal from the aqueous solution was determined according to the following equation:
where
R is the removal efficiency of the dye.
2.11. Mechanical Characteristics
Uniaxial tensile tests using a universal (LR10K; L1oyd Instruments, Fareham, UK) machine were conducted on as-spun fiber mats (OPFL/PLA/36.7%, OBBL/PET/13.5%, and OBBL/PET/24.5%) and their composites (OPFL/PLA/36.7%/P-mTol, OBBL/PET/13.5%/P-mTol, and OBBL/PET/24.5%/P-mTol). The load cell capacity used was 5 N. The tensile testing of tested samples was performed for 5 days in a humid environment. The fiber mats (30 mm length × 10 mm width × 0.2 mm thickness) were adhered to a paper mounting tab using an epoxy adhesive with a gauge length of 20 mm and tensile speed of 2 mm/min. When the sample was tightly grasped and cut away from both sides of the tab, the tensile test began. The average value of the tensile strength, Young’s modulus, and fraction strain was calculated for at least 3 samples [
46].