Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
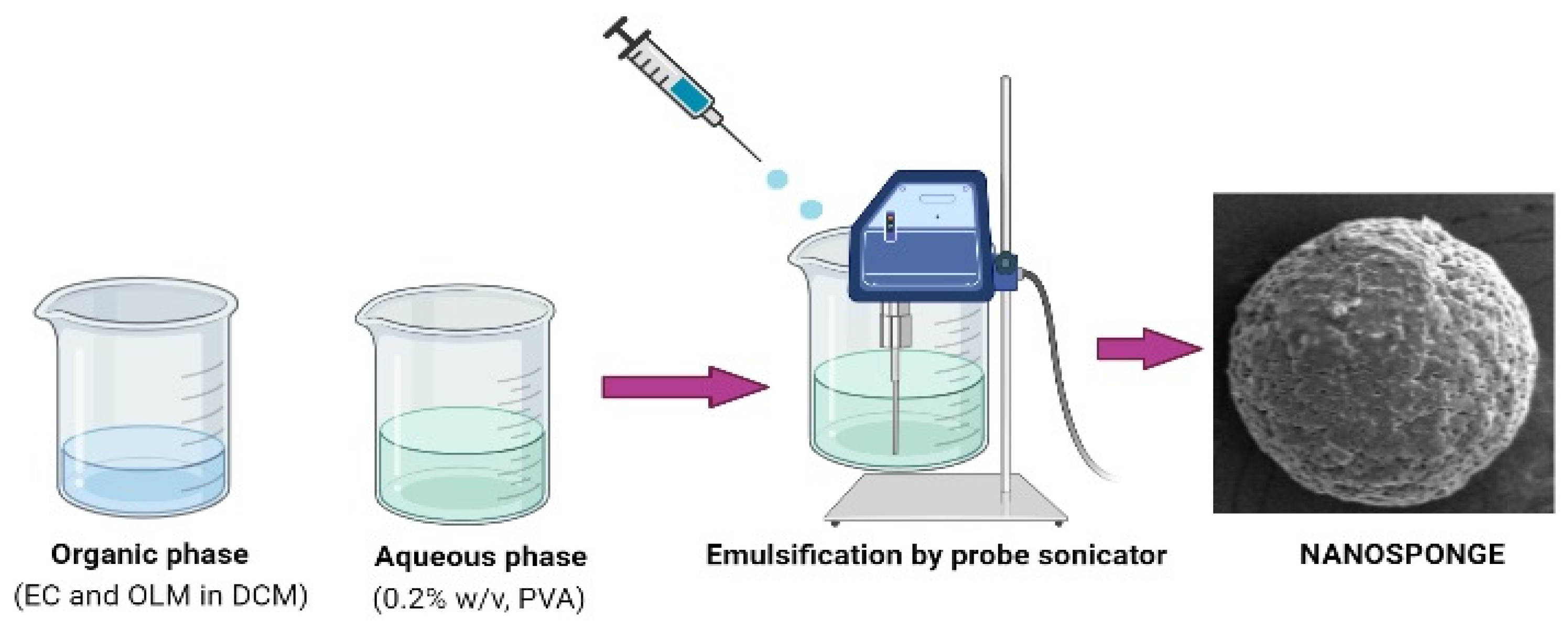
2.2. Preparation of OLM-Loaded Nanosponges
2.3. Measurement of Particle Size, Polydispersity Index (PDI) and Ζeta-Potential (ζP)
2.4. Measurement of Percent Entrapment Efficiency (%EE) and Drug Loading (%DL)
2.5. Fourier Transform Infra-Red (FTIR) Spectroscopy
2.6. Differential Scanning Calorimetry (DSC) Studies
2.7. Powder X-ray Diffraction (PXRD) Studies
2.8. In Vitro Release and Kinetic Studies
2.9. Scanning Electron Microscopy (SEM) Studies
2.10. Nitrogen Adsorption/Desorption Characterization of OLM-Loaded NSs
2.11. Cell Viability MTT Test
2.12. In Vivo Antihypertensive Studies
2.13. Statistical Analysis
3. Results and Discussions
3.1. Measurement of Size, PDI and ζP
3.2. Measurement of %EE and %DL
3.3. FTIR Studies
3.4. DSC Studies
3.5. PXRD Studies
3.6. In Vitro Release and Kinetic Studies
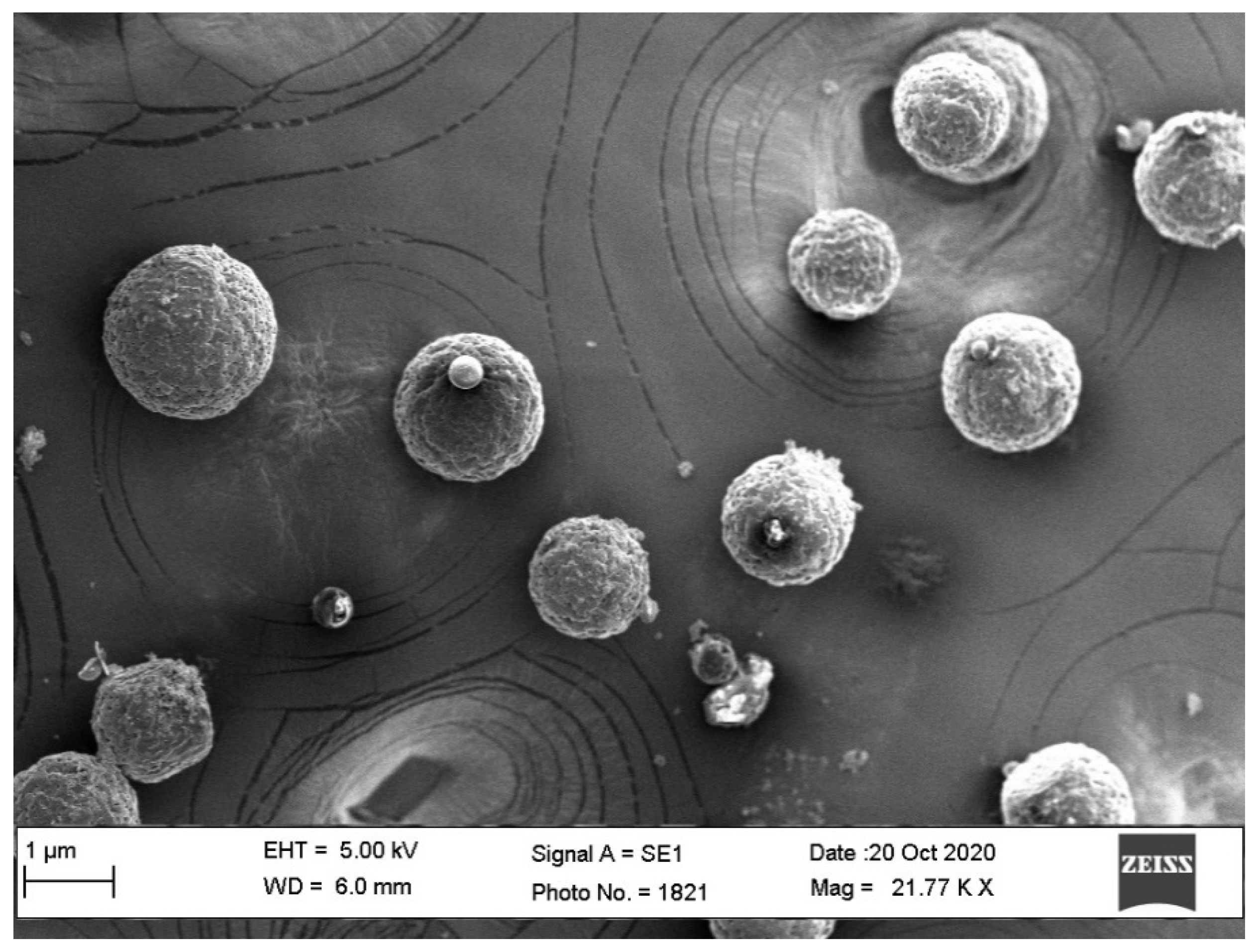
3.7. SEM Studies
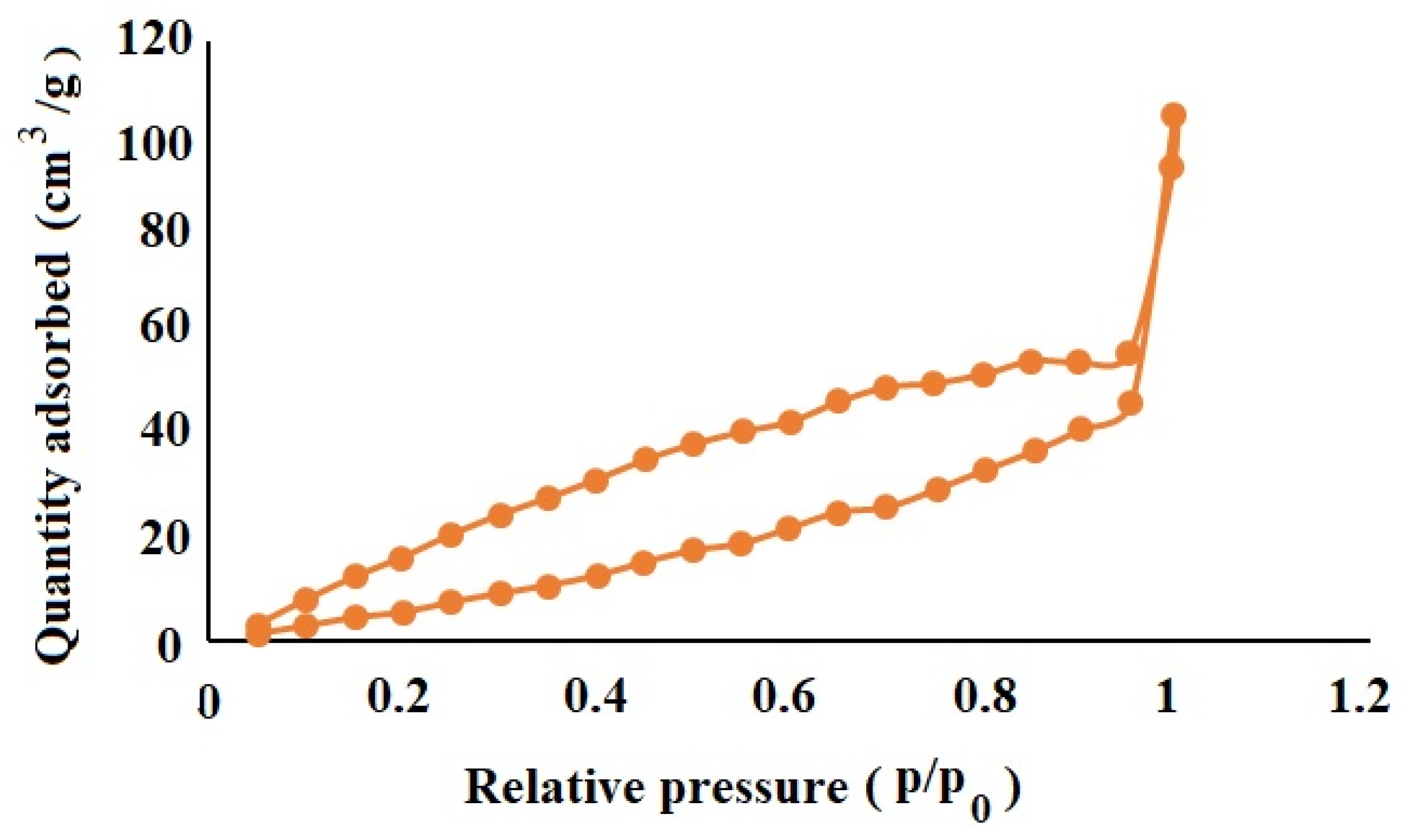
3.8. Nitrogen Adsorption/Desorption Characterization of OLM-Loaded NSs
3.9. Cell Viability MTT Test
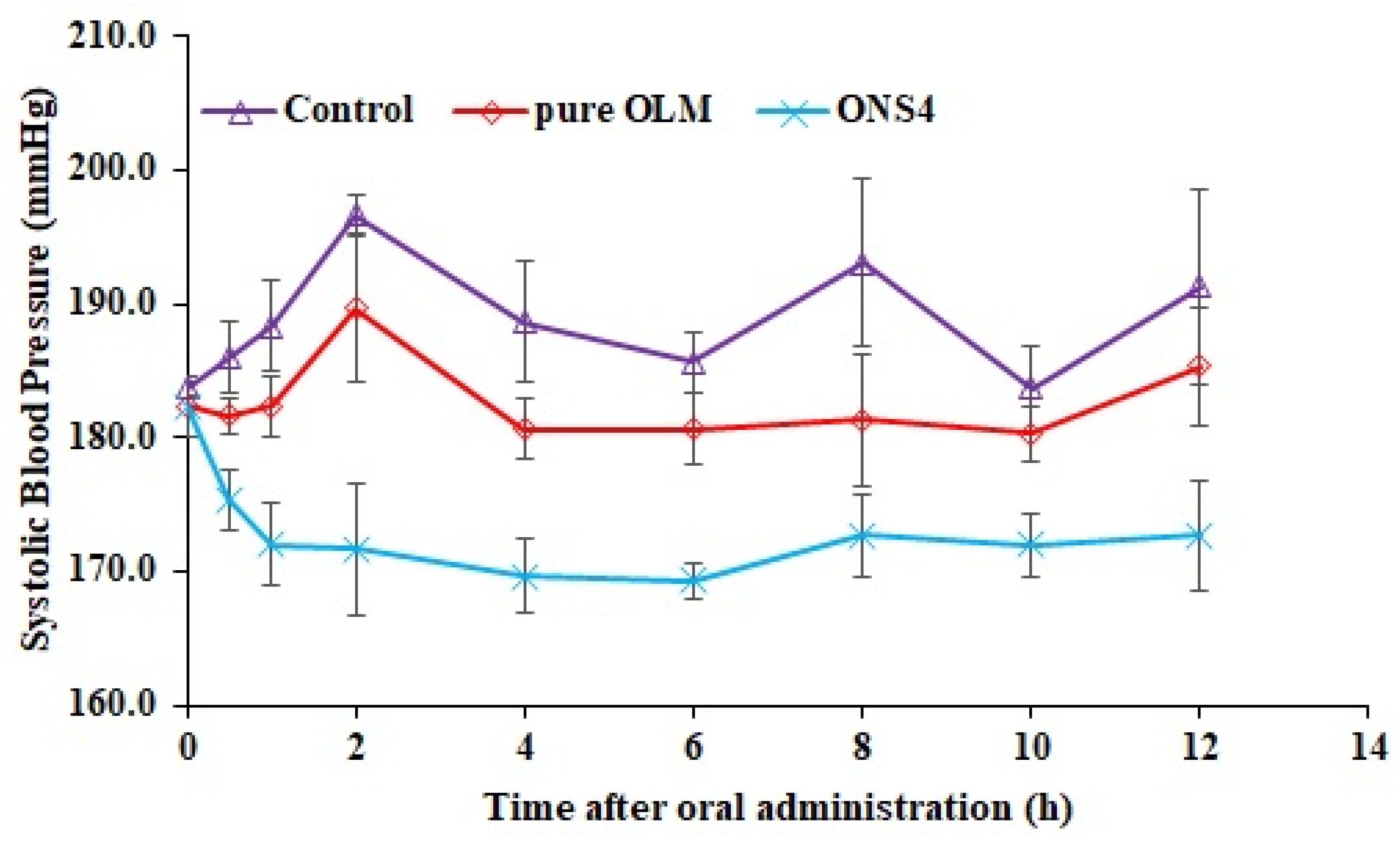
3.10. In Vivo Antihypertensive Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, G.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- De Lima, M.P.B.; Ramos, D.; Freire, A.P.C.F.; Uzeloto, J.S.; de Silva, B.L.; Ramos, E.M.C. Quality of life of smokers and its correlation with smoke load. Fisioter. Pesqui. 2017, 24, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Sabir, F.; Qindeel, M.; Zeeshan, M.; Ul Ain, Q.; Rahdar, A.; Barani, M.; González, E.; Aboudzadeh, M.A. Onco-Receptors Targeting in Lung Cancer via Application of Surface-Modified and Hybrid Nanoparticles: A Cross-Disciplinary Review. Processes 2021, 9, 621. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Saab, S.; Zalzale, H.; Rahal, Z.; Khalifeh, Y.; Sinjab, A.; Kadara, H. Insights into Lung Cancer Immune-Based Biology, Prevention, and Treatment. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of nanotechnology in cancer diagnosis and therapy - A mini-review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Ranjan, M.J.K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; Iqbal, H.M.N.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Iqbal, M.; Ezzeldin, E.; Alalaiwe, A.; Aldawsari, M.F. Development of ethylcellulose based nanosponges of apremilast: In vitro and in vivo pharmacokinetic evaluation. Latin Am. J. Pharm. 2020, 39, 1292–1299. [Google Scholar]
- Bano, N.; Ray, S.K.; Shukla, T.; Upmanyu, N.; Khare, R.; Pandey, S.P.; Jain, P. Multifunctional nanosponges for the treatment of various diseases: A review. Asian J. Pharm. Pharmacol. 2019, 5, 235–248. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and characterization of ethyl cellulose nanosponges for sustained release of brigatinib for the treatment of non-small cell lung cancer. J. Polym. Engn. 2020, 40, 823–832. [Google Scholar] [CrossRef]
- Zhang, Q.; Honko, A.; Zhou, J.; Gong, H.; Downs, S.N.; Vasquez, J.H.; Fang, R.H.; Gao, W.; Griffiths, A.; Zhang, L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020, 20, 5570–5574. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm J. 2021, 29, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Ansari, M.N.; Ezzeldin, E.; Khalil, N.Y.; Ali, R. Improving the Solubilization and Bioavailability of Arbidol Hydrochloride by the Preparation of Binary and Ternary β-Cyclodextrin Complexes with Poloxamer 188. Pharmaceuticals 2021, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Théophile, H.; David, X.R.; Miremont-Salamé, G.; Haramburu, F. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig. Liver Dis. 2014, 46, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Li, H.; Han, X. Sustained release olmesartan medoxomil loaded PLGA nanoparticles with improved oral bioavailability to treat hypertension. J. Drug. Deliv. Sci. Technol. 2020, 55, 101422. [Google Scholar] [CrossRef]
- Anwer, M.K.; Jamil, S.; Ansari, M.J.; Iqbal, M.; Imam, D.; Shakeel, F. Development and evaluation of olmesartan medoxomil loaded PLGA nanoparticles. Mat. Res. Innov. 2016, 20, 193–197. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Kundu, A.; Sarkar, L.; Karmakar, S.; Jaisankar, P.; Pal, T.K. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. Colloids Surf. B 2014, 115, 286–294. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, H.S.; Jeon, H.S.; Park, J.H.; Lee, B.S.; Ahn, B.K.; Moon, K.Y.; Choi, Y.W. In situ intestinal permeability and in vivo absorption characteristics of olmesartan medoxomil in self-microemulsifying drug delivery system. Drug Dev. Ind. Pharm. 2012, 38, 587–596. [Google Scholar] [CrossRef]
- Thakkar, H.P.; Patel, B.V.; Thakkar, S.P. Development and characterization of nanosuspensions of olmesartan medoxomil for bioavailability enhancement. J. Pharm. Bioall. Sci. 2011, 3, 426–434. [Google Scholar] [CrossRef]
- Jain, S.; Patel, K.; Arora, S.; Reddy, V.A.; Dora, C.P. Formulation, optimization, and in vitro-in vivo evaluation of olmesartan medoxomil nanocrystals. Drug Deliv. Transl. Res. 2017, 7, 292–303. [Google Scholar] [CrossRef]
- Prajapati, S.T.; Bulchandani, H.H.; Patel, D.M.; Dumaniya, S.K.; Patel, C.N. Formulation and evaluation of liquisolid compacts for olmesartan medoxomil. J. Drug Deliv. Sci. Technol. 2013, 870579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunda, R.K.; Manchineni, P.R.; Dhachinamoorthi, D. Design, development, and in vitro evaluation of sustained release tablet formulations of olmesartan medoxomil. MOJ Drug Des. Dev. Ther. 2018, 7, 164–169. [Google Scholar] [CrossRef]
- Abd-Alhaseeb, M.M.; Zaitone, S.A.; Abou-El-Ela, S.H.; Moustafa, Y.M. Olmesartan potentiates the anti-angiogenic effect of sorafenib in mice bearing Ehrlich’s ascites carcinoma: Role of angiotensin (1-7). PLoS ONE 2014, 9, e85891. [Google Scholar] [CrossRef] [Green Version]
- Vassiliou, S.; Nkenke, E.; Lefantzis, N.; Ioannidis, A.; Yapijakis, C.; Zoga, M.; Papakosta, V.; Derka, S.; Nikolaou, C.; Vairaktaris, E. Effect of Olmesartan on the Level of Oral Cancer Risk Factor PAI1. Anticancer Res. 2016, 36, 6093–6096. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, E.; Punnagai, K.; Chellathai, D.D. Evaluation of Anticancer Activity of Olmesartan and Ramipril on A549 Cell. Biomed. Pharmacol. J. 2018, 11, 1351–1357. [Google Scholar] [CrossRef]
- Rote, A.R.; Bari, P.D. Spectrophotometric estimation of olmesartan medoxomil and hydrochlorothiazide in tablet. Indian. J. Pharm. Sci. 2010, 72, 111–113. [Google Scholar] [CrossRef] [Green Version]
- Approved Product of OLMETEC® (Olmesartan Medoxomil). 2013. Available online: https://www.tga.gov.au/sites/default/files/auspar-olmesartan-medoxomil-130226-pi.pdf (accessed on 10 July 2021).
- Brunner, H. The new oral angiotensin II antagonist olmesartan medoxomil: A concise overview. J. Hum. Hypertens. 2002, 16, S13–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutz, R. Olmesartan/amlodipine: A review of its use in the management of hypertension. Vasc. Health Risk Manag. 2011, 7, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.R.; Madhur, M.S.; Harrison, D.G.; Dreisbach, A.W.; Riaz, K. What is the global prevalence of hypertension (high blood pressure)? Medscape 2019. Available online: https://www.medscape.com/answers/241381-7614/what-is-the-global-prevalence-of-hypertension-high-blood-pressure (accessed on 10 July 2021).
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, M.K.; Iqbal, M.; Aldawsari, M.F.; Alalaiwe, A.; Ahmed, M.M.; Muharram, M.M.; Ezzeldin, E.; Mahmoud, M.A.; Imam, F.; Ali, R. Improved antimicrobial activity and oral bioavailability of delafloxacin by self-nanoemulsifying drug delivery system (SNEDDS). Drug. Deliv. Sci. Technol. 2021, 64, 102572. [Google Scholar] [CrossRef]
- Anwer, M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L. Surface Area Determination of Porous Materials Using the Brunauer–Emmett–Teller (BET) Method: Limitations and Improvements. J. Phy. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Alshetaili, A.S. Gefitinib loaded PLGA and chitosan coated PLGA nanoparticles with magnified cytotoxicity against A549 lung cancer cell lines. Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Michalowski, C.B.; Arbo, M.D.; Altknecht, L.; Anciuti, A.N.; Abreu, A.; Alencar, L.; Pohlmann, A.R.; Garcia, S.C.; Guterres, S.S. Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules. Pharmaceutics 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interf. Sci. 2009, 14, 3. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Evaluation of particle interactions by in situ visualization of separation behavior. Colloids Surf. A 2014, 440, 122–130. [Google Scholar] [CrossRef]
- Rahdar, A.; Sargazi, S.; Barani, M.; Shahraki, S.; Sabir, F.; Aboudzadeh, M.A. Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments. Polymers 2021, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Taboada, P.; Hajinezhad, M.R.; Barani, M.; Beyzaei, H. Effect of tocopherol on the properties of Pluronic F127 microemulsions: Physico-chemical characterization and in vivo toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Aloorkar, N.H.; Kulkarni, A.S.; Ingale, D.J.; Patil, R.A. Microsponges as Innovative Drug Delivery Systems. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1597–1606. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian. J. Pharm Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Pandav, S.; Naik, J. Preparation and In Vitro Evaluation of Ethylcellulose and Polymethacrylate Resins Loaded Microparticles Containing Hydrophilic Drug. J. Pharm. 2014, 904036. [Google Scholar] [CrossRef] [Green Version]
- Maji, R.; Ray, S.; Das, B.; Nayak, A. Ethyl Cellulose Microparticles Containing Metformin HCl by Emulsification-Solvent Evaporation Technique: Effect of Formulation Variables. Int. Schol. Res. Not. 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Perrin, J.H. Sustained and Controlled Release Drug Delivery Systems; Robinson, J. Dekker: New York, NY, USA, 1978; Volume 6, p. 773. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose–A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]
- Trofimiuk, M.; Wasilewska, K.; Winnicka, K. How to modify drug release in paediatric dosage forms? Novel technologies and modern approaches with regard to children’s population. Int. J. Mol. Sci. 2019, 20, 3200. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.T.; Devi, S.G.; KV, S. Formulation and evaluation of ketorolac tromethamine-loaded albumin microspheres for potential intramuscular administration. AAPS PharmSciTech. 2007, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Walker, R.B.; Pathak, K. Evaluation of the Kinetics and Mechanism of Drug Release from Econazole nitrate Nanosponge Loaded Carbapol Hydrogel. Indian J. Pharm. Edu. Res. 2011, 45, 25–31. [Google Scholar]
- Sadjadi, S.; Heravi, M.M.; Daraie, M. Cyclodextrin nanosponges: A potential catalyst and catalyst support for synthesis of xanthenes. Res. Chem. Intermed. 2017, 43, 843–857. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Hosseini, A.; Boroushaki, M.B.; Mousavi, S.H. Synergistic, cytotoxic and apoptotic activities of olmesartan with NF-κB inhibitor against HeLa human cell line. Toxicol. Mech. Methods 2015, 25, 614–621. [Google Scholar] [CrossRef]



| Nanosponges | OLM (mg) | EC (mg) | PVA (w/v%) |
|---|---|---|---|
| ONS1 | 40 | 50 | 0.2 |
| ONS2 | 40 | 100 | 0.2 |
| ONS3 | 40 | 150 | 0.2 |
| ONS4 | 40 | 200 | 0.2 |
| Nanosponges | Physicochemical Properties * | ||||
|---|---|---|---|---|---|
| PS ± SD (nm) | PDI | ζP ± SD (mV) | %EE ± SD | %DL ± SD | |
| ONS1 | 381 ± 8.2 * | 0.312 ± 0.02 * | −15.7 ± 1.6 * | 87.1 ± 4.1 | 1.89 ± 0.15 |
| ONS2 | 387 ± 9.5 * | 0.446 ± 0.02 * | −19.0 ± 2.1 * | 87.9 ± 5.2 | 1.61 ± 0.28 |
| ONS3 | 404 ± 12.4 * | 0.355 ± 0.05 * | −18.2 ± 1.2 * | 88.6 ± 4.6 | 1.52 ± 0.16 |
| ONS4 | 487 ± 12.8 * | 0.386 ± 0.03 * | −18.1 ± 1.8 * | 91.2 ± 1.9 | 0.88 ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. https://doi.org/10.3390/polym13142272
Almutairy BK, Alshetaili A, Alali AS, Ahmed MM, Anwer MK, Aboudzadeh MA. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers. 2021; 13(14):2272. https://doi.org/10.3390/polym13142272
Chicago/Turabian StyleAlmutairy, Bjad K., Abdullah Alshetaili, Amer S. Alali, Mohammed Muqtader Ahmed, Md. Khalid Anwer, and M. Ali Aboudzadeh. 2021. "Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments" Polymers 13, no. 14: 2272. https://doi.org/10.3390/polym13142272
APA StyleAlmutairy, B. K., Alshetaili, A., Alali, A. S., Ahmed, M. M., Anwer, M. K., & Aboudzadeh, M. A. (2021). Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers, 13(14), 2272. https://doi.org/10.3390/polym13142272









