Characterization and Aerosolization Performance of HydroxyPropyl-Beta-Cyclodextrin Particles Produced Using Supercritical Assisted Atomization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of HP-β-CD Carrier Particles
2.3. Solid-State Characterization
2.4. In Vitro Aerosol Performance Determined Using an Andersen Cascade Impactor
3. Results and Discussion
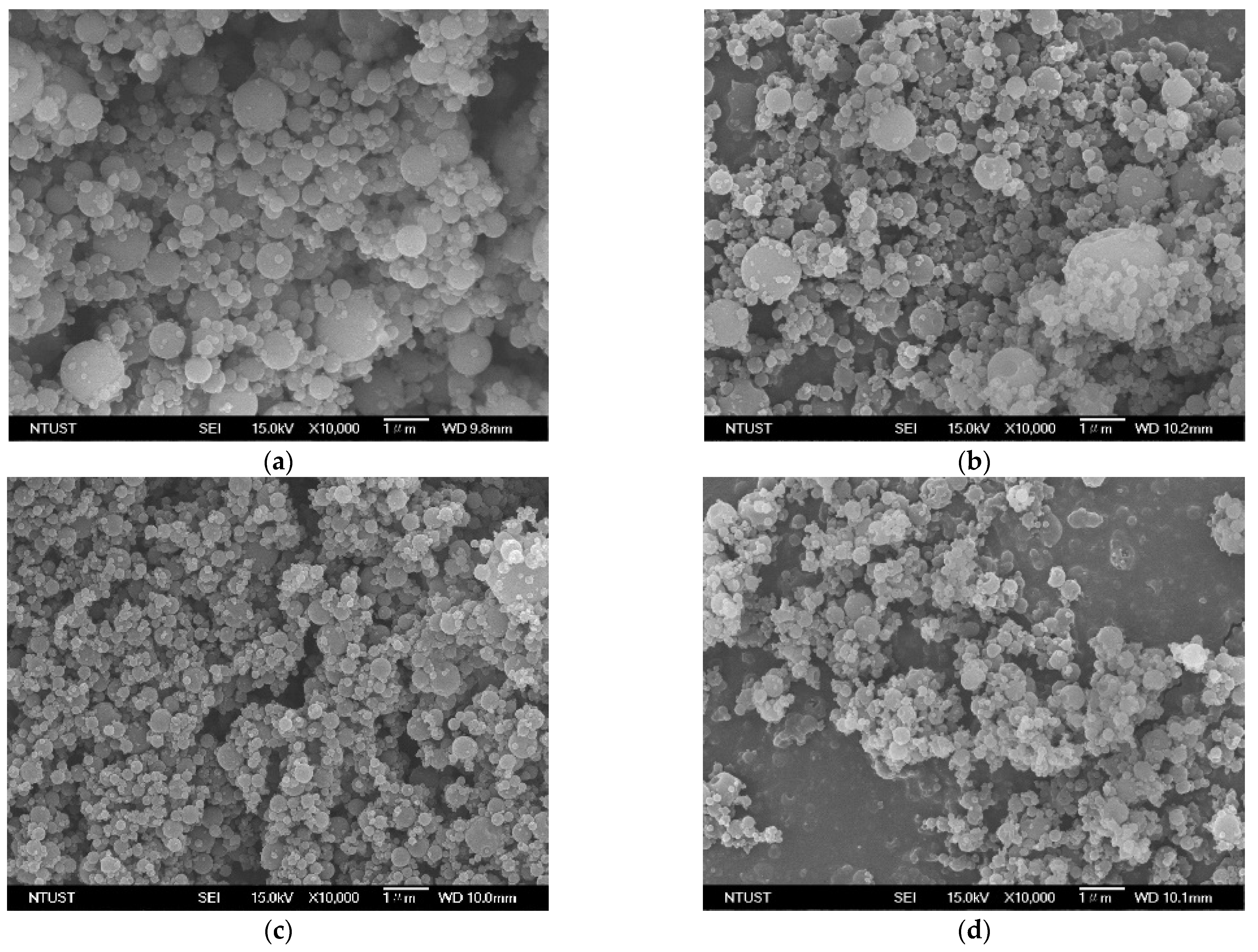
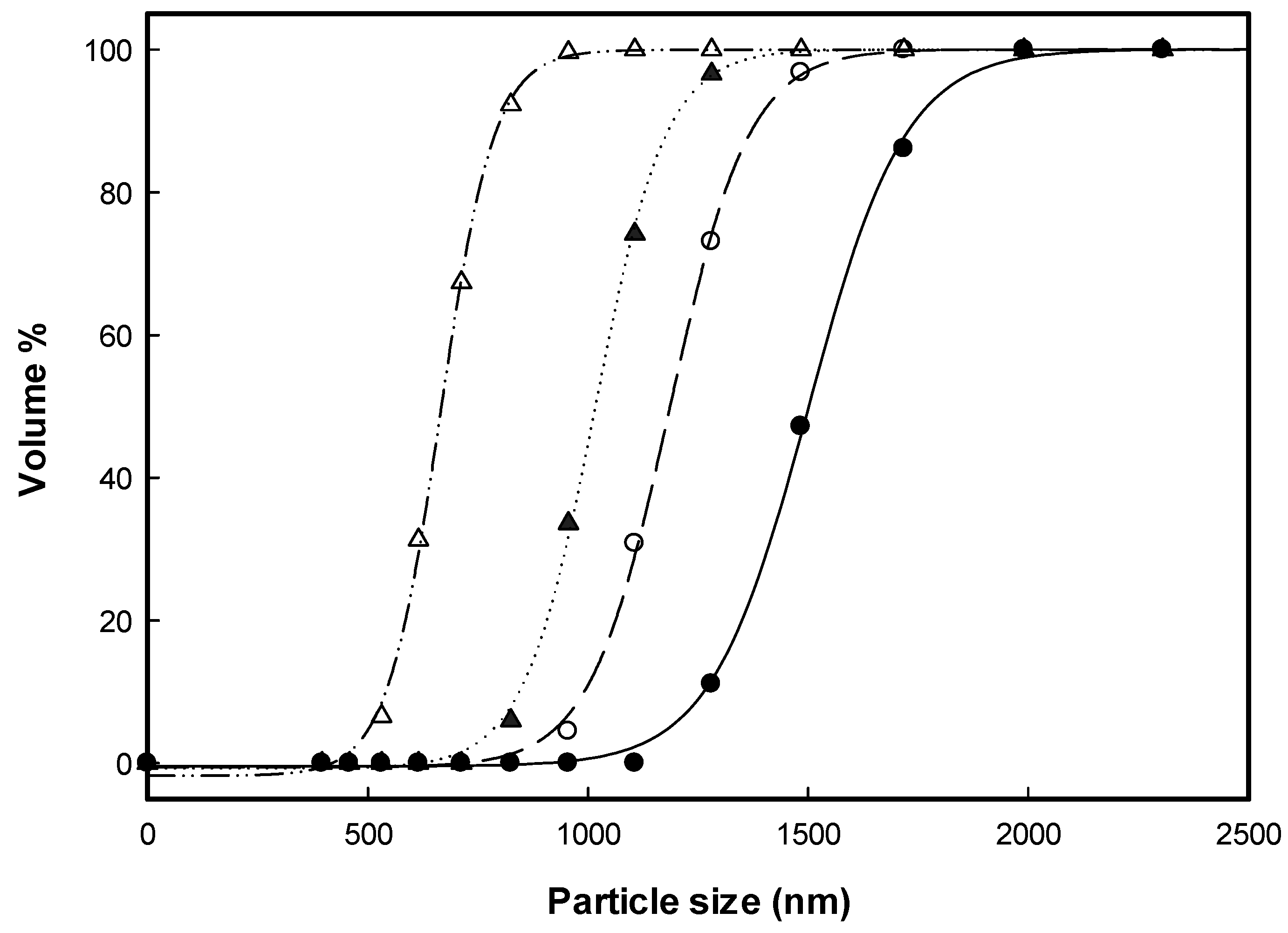
3.1. Solvent Effect on the HP-β-CD Particles
3.2. Effects of the Precipitation Parameters on HP-β-CD Particle Size
3.3. In Vitro Aerosolization Performance of HP-β-CD Carrier Particles with the Addition of L-Leucine
3.4. Solid Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Chelly, J.E.; Lacouture, P.G.; Reyes, C.R.D. Safety of injectable HPbCD-diclofenac in older patients with acute moderate-to-severe postoperative pain: A pooled analysis of three phase III trials. Drugs Aging 2018, 35, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matilainen, L.; Toropainen, T.; Vihola, H.; Hirvonen, J.; Järvinen, T.; Jarho, P.; Järvinen, K. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J. Control. Release 2008, 126, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.I.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Co-spray dried carbohydrate microparticles: Crystallisation delay/inhibition and improved aerosolization characteristics through the incorporation of hydroxypropyl-β-cyclodextrin with amorphous raffinose or trehalose. Pharm. Res. 2015, 32, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, V.; Bimbo, L.M.; Hirvonen, J.; Kauppinen, E.I.; Raula, J. Aerosolization, drug permeation and cellular interaction of dry powder pulmonary formulations of corticosteroids with hydroxypropyl-β-cyclodextrin as a solubilizer. Pharm. Res. 2017, 34, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Dufour, G.; Bigazzi, W.; Wong, N.; Boschini, F.; Tullio, P.; Piel, G.; Cataldo, D.; Evrard, B. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int. J. Pharm. 2015, 495, 869–878. [Google Scholar] [CrossRef]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Suzuki, É.Y.; Amaro, M.I.; de Almeida, G.S.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a new formulation of roflumilast for pulmonary drug delivery to treat inflammatory lung conditions. Int. J. Pharm. 2018, 550, 89–99. [Google Scholar] [CrossRef]
- Tozuka, Y.; Wongmekiat, A.; Sakata, K.; Moribe, K.; Oguchi, T.; Yamamoto, K. Co-grinding with cyclodextrin as a nanoparticle preparation method of a poorly water soluble drug. J. Incl. Phenom. Macrocycl. Chem. 2004, 50, 67–71. [Google Scholar] [CrossRef]
- Kreaz, A.R.M.; Abu-Eida, E.Y.; Erő, I.; Kata, M. Freeze-dried complexes of furosemide with cyclodextrin derivatives. J. Incl. Phenom. Macrocycl. Chem. 1999, 34, 39–48. [Google Scholar] [CrossRef]
- Yurtdaş, G.; Demirel, M.; Genҫ, L. Inclusion complexes of fluconazole with β-cyclodextrin: Physicochemical characterization and in vitro evaluation of its formulation. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 429–435. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G. Supercritical assisted atomization: Performance comparison between laboratory and pilot scale. J. Supercrit. Fluids 2006, 37, 298–306. [Google Scholar] [CrossRef]
- Wu, H.T.; Yang, M.W. Precipitation kinetics of PMMA sub-micrometric particles with a supercritical assisted-atomization process. J. Supercrit. Fluids 2011, 59, 98–107. [Google Scholar] [CrossRef]
- Wu, H.T.; Huang, S.C.; Yang, C.P.; Chien, L.J. Precipitation parameters and the cytotoxicity of chitosan hydrochloride microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2015, 102, 123–132. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Cyclodextrins micrometric powders obtained by supercritical fluid processing. Biotechnol. Bioeng. 2006, 94, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Li, T.-H.; Tsai, H.-M.; Chien, L.-J.; Chuang, Y.-H. Formulation of inhalable beclomethasone dipropionate-mannitol composite particles through low-temperature supercritical assisted atomization. J. Supercrit. Fluids 2021, 168, 105095. [Google Scholar] [CrossRef]
- Day, C.P.F.; Miloserdov, A.; Wildish-Jones, K.; Pearson, E.; Carruthers, A.E. Quantifying the hygroscopic properties of cyclodextrin containing aerosol for drug delivery to the lungs. Phys. Chem. Chem. Phys. 2020, 22, 11327–11336. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.T.; Raabe, O.G. A comparison of cascade impactor data reduction methods. Aerosol Sci. Technol. 2003, 37, 187–200. [Google Scholar] [CrossRef]
- Wu, H.T.; Yang, M.W.; Huang, S.C. Sub-micrometric polymer particles formation by a supercritical assisted-atomization process. J. Taiwan Inst. Chem. Eng. 2014, 45, 1992–2001. [Google Scholar] [CrossRef]
- Wang, X.F.; Lefebvre, A.H. Mean drop sizes from pressure-swirl nozzles. J. Propul. Power 1987, 3, 11–18. [Google Scholar] [CrossRef]
- Kawakami, K.; Sumitani, C.; Yoshihashi, Y.; Yonemochi, E.; Terada, K. Investigation of the dynamic process during spray-drying to improve aerodynamic performance of inhalation particles. Int. J. Pharm. 2010, 390, 250–259. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray: An improved micronization process. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverchon, E. Supercritical-assisted atomization to produce micro- and/or nanoparticles of controlled size and distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Wu, H.T.; Su, Y.C.; Wang, Y.M.; Tsai, H.M. Characterization and aerosolization performance of mannitol particles produced using supercritical assisted atomization. Chem. Eng. Res. Des. 2018, 137, 308–318. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Microparticle formation of sodium cellulose sulfate using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer. Chem. Eng. J. 2010, 159, 220–229. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Chitosan microparticles production by supercritical fluid processing. Ind. Eng. Chem. Res. 2006, 45, 5722–5728. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Reverchon, E.; Spada, A. Erythromycin micro-particles produced by supercritical fluid atomization. Powder Technol. 2004, 141, 100–108. [Google Scholar] [CrossRef]
- Cai, M.-Q.; Guan, Y.-X.; Yao, S.-J.; Zhu, Z.-Q. Supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer (SAA-HCM) for micronization of levofloxacin hydrochloride. J. Supercrit. Fluids 2008, 43, 524–534. [Google Scholar] [CrossRef]
- Wu, H.T.; Yang, M.W. Precipitation kinetics of PMMA-co-BMA sub-micrometric particles with compressed CO2 assisted-atomization process. Powder Technol. 2012, 228, 91–99. [Google Scholar] [CrossRef]
- Wu, H.T.; Lee, H.K.; Chen, H.C.; Chien, L.J. Precipitation kinetics and biological properties of chitosan microparticles produced using supercritical assisted atomization. Chem. Eng. Res. Des. 2015, 104, 615–625. [Google Scholar] [CrossRef]
- Caputo, G.; Liparoti, S.; Adami, R.; Reverchon, E. Use of supercritical CO2 and N2 as dissolved gases for the atomization of ethanol and water. Ind. Eng. Chem. Res. 2012, 51, 11803–11808. [Google Scholar] [CrossRef]
- Mohtar, N.; Taylor, K.M.G.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, B.; Serrano, D.R.; O’Connell, P.; Couet, W.; Marchand, S.; Healy, A.M.; Tewes, F. Use of leucine to improve aerodynamic properties of ciprofloxacin loaded maltose microparticles for inhalation. Eur. J. Pharm. Res. 2019, 1, 2–11. [Google Scholar] [CrossRef]
- Seville, P.C.; Learoyd, T.P.; Li, H.Y.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.H.; Carlberg, R.; Mclntosh, M.P. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Saw, H.Y.; Davies, C.E.; Paterson, A.H.J.; Jones, J.R. Correlation between powder flow properties measured by shear testing and Hausner ratio. Procedia Eng. 2015, 102, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Molina, C.; Kaialy, W.; Nokhodchi, A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of albuterol sulfate. J. Drug Deliv. Sci. Technol. 2019, 49, 97–106. [Google Scholar] [CrossRef]
- Raula, J.; Kuivanen, A.; Lähde, A.; Jiang, H.; Antopolsky, M.; Kansikase, J.; Kauppinen, E.I. Synthesis of L-leucine nanoparticles via physical vapor deposition at varying saturation conditions. J. Aerosol Sci. 2007, 38, 1172–1184. [Google Scholar] [CrossRef]
- Li, L.; Leung, S.S.Y.; Gengenbach, T.; Yu, J.; Gao, G.; Tang, P.; Zhou, Q.; Chan, H.-K. Investigation of L-leucine in reducing the moisture-induced deterioration of spray-dried salbutamol sulfate power for inhalation. Int. J. Pharm. 2017, 530, 30–39. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, X.; Hu, L.; Liu, Y.; Wang, C. Decomposing or subliming? An investigation of thermal behavior of l-leucine. Thermochim. Acta 2006, 447, 147–153. [Google Scholar] [CrossRef]

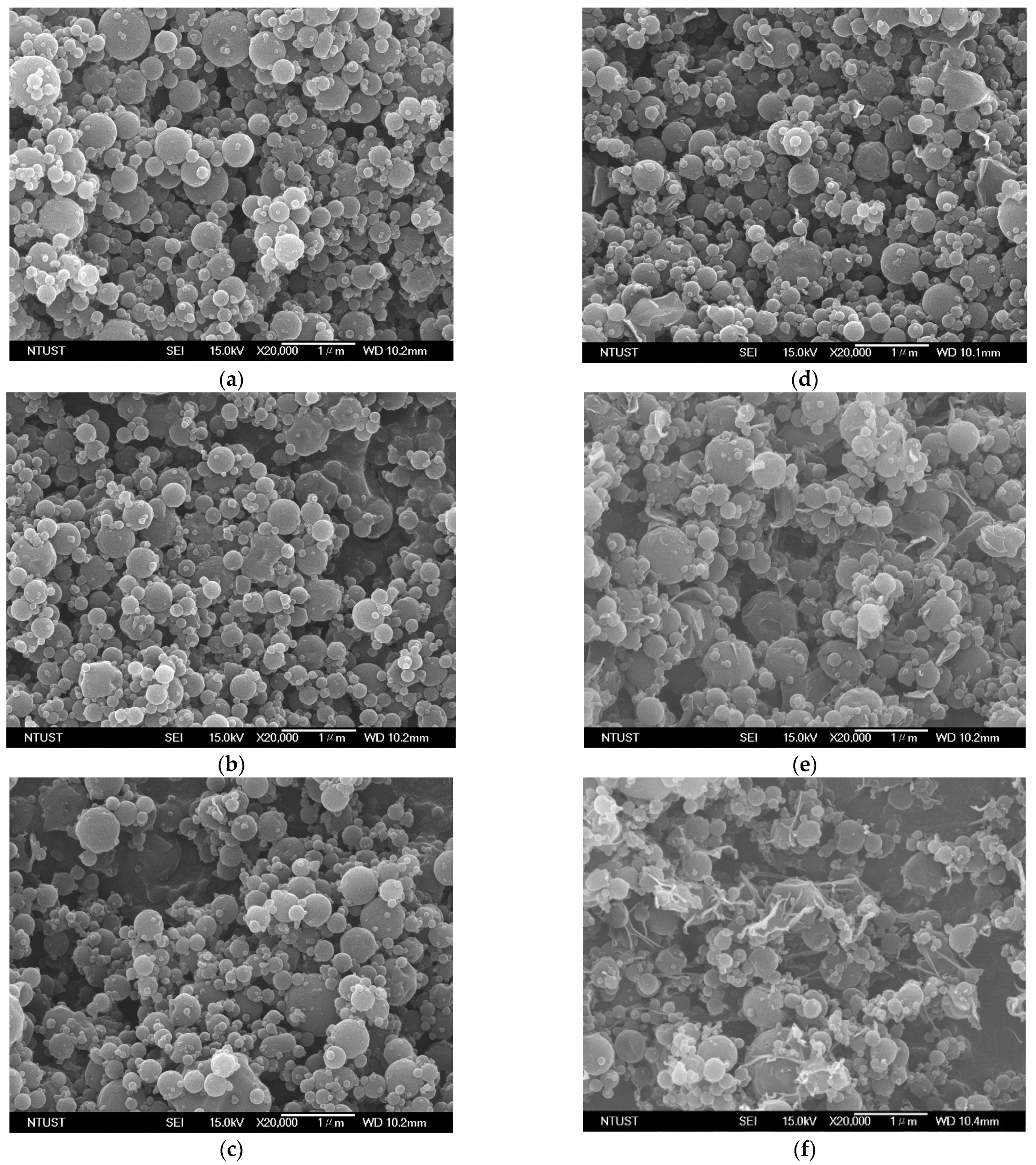

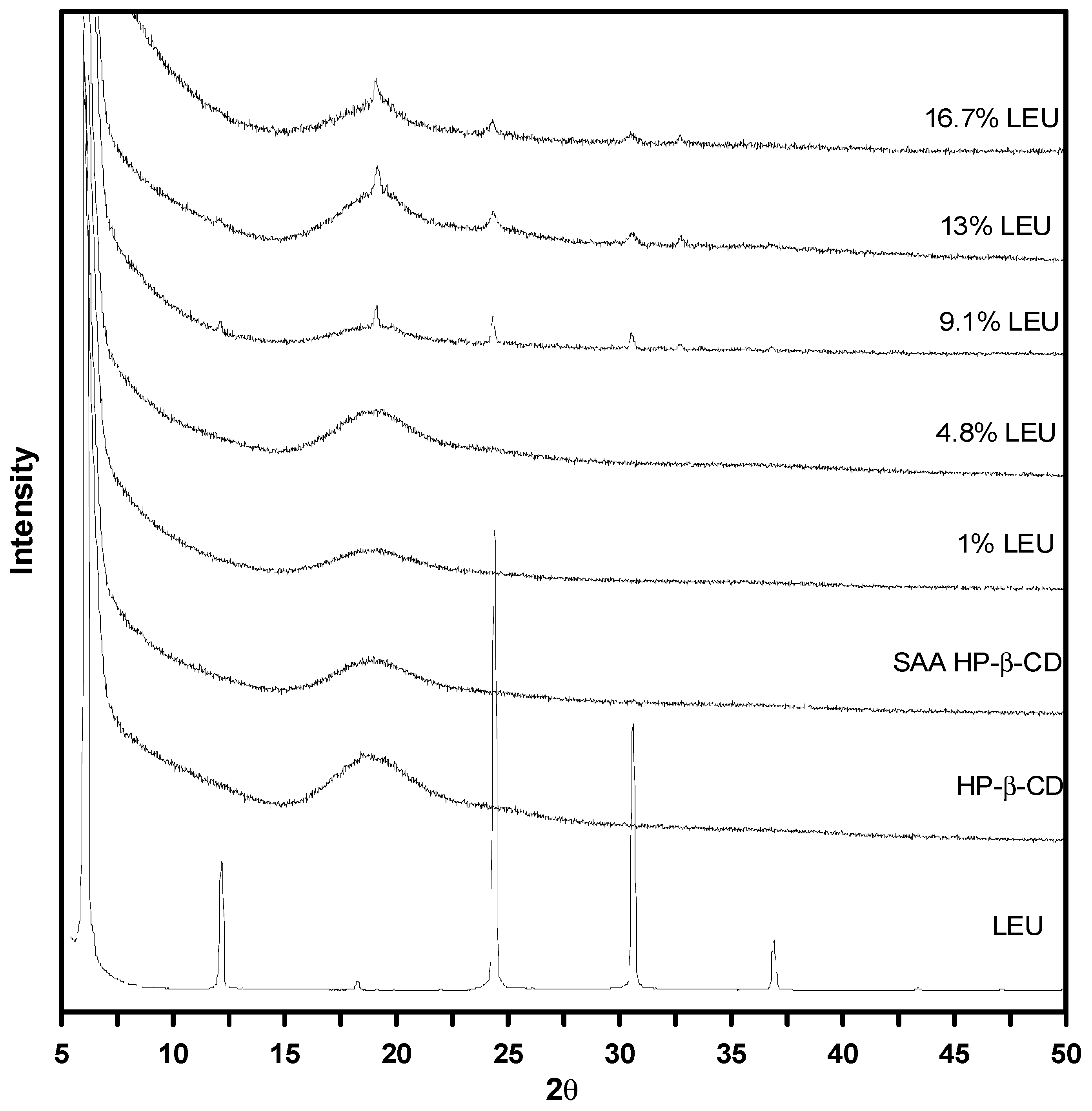
| Run | EtOH% | TP | TS | CHP | R | dno | d4,3 |
|---|---|---|---|---|---|---|---|
| w/w | K | K | mg/mL | FCO2/FL | μm | μm | |
| 1 | 44.1 | 373 | 353 | 10 | 1.8 | 1.28 ± 0.20 | 1.35 ± 010 |
| 2 | 54.2 | 373 | 353 | 10 | 1.8 | 1.10 ± 0.10 | 1.16 ± 0.05 |
| 3 | 70.3 | 373 | 353 | 10 | 1.8 | 0.72 ± 0.15 | 0.78 ± 0.20 |
| 4 | 54.2 | 343 | 353 | 10 | 1.8 | 1.74 ± 0.20 | 1.84 ± 0.20 |
| 5 | 54.2 | 353 | 353 | 10 | 1.8 | 1.64 ± 0.20 | 1.75 ± 0.10 |
| 6 | 54.2 | 363 | 353 | 10 | 1.8 | 1.06 ± 0.10 | 1.10 ± 0.20 |
| 7 | 54.2 | 383 | 353 | 10 | 1.8 | 1.12 ± 0.10 | 1.20 ± 0.20 |
| 8 | 54.2 | 373 | 313 | 10 | 1.8 | 1.13 ± 0.30 | 1.19 ± 0.20 |
| 9 | 54.2 | 373 | 333 | 10 | 1.8 | 1.29 ± 0.10 | 1.36 ± 0.20 |
| 10 | 54.2 | 373 | 363 | 10 | 1.8 | 0.74 ± 0.20 | 0.78 ± 0.10 |
| 11 | 54.2 | 373 | 353 | 3 | 1.8 | 0.85 ± 0.10 | 0.89 ± 0.10 |
| 12 | 54.2 | 373 | 353 | 5 | 1.8 | 0.85 ± 0.05 | 0.89 ± 0.10 |
| 13 | 54.2 | 373 | 353 | 20 | 1.8 | 1.26 ± 0.10 | 1.33 ± 0.10 |
| 14 | 54.2 | 373 | 353 | 30 | 1.8 | 1.34 ± 0.15 | 1.40 ± 0.10 |
| 15 | 54.2 | 373 | 353 | 50 | 1.8 | 1.51 ± 0.10 | 1.57 ± 0.20 |
| 16 | 54.2 | 373 | 353 | 10 | 1.4 | 1.54 ± 0.10 | 1.61 ± 0.20 |
| 17 | 54.2 | 373 | 353 | 10 | 2.4 | 0.98 ± 0.10 | 1.02 ± 0.10 |
| 18 | 54.2 | 373 | 353 | 10 | 2.8 | 0.97 ± 0.15 | 1.00 ± 0.20 |
| Run | CLEU | ED | FPF | MMAD | dno | d4,3 | ρtap | HR |
|---|---|---|---|---|---|---|---|---|
| mg/mL | % | % | µm | µm | µm | g/cm3 | ρtap/ρb | |
| L1 | 0 | 95.4 ± 1.2 | 15.6 ± 1.5 | 9.42 ± 0.60 | 1.10 ± 0.05 | 1.16 ± 0.09 | 0.24 ± 0.03 | 1.40 ± 0.05 |
| L2 | 0.1 | 97.0 ± 1.2 | 16.3 ± 1.6 | 7.06 ± 0.80 | 1.11 ± 0.10 | 1.20 ± 0.08 | 0.22 ± 0.03 | 1.29 ± 0.04 |
| L3 | 0.5 | 96.8 ± 0.1 | 18.3 ± 1.2 | 8.39 ± 0.50 | 1.27 ± 0.12 | 1.32 ± 0.10 | 0.23 ± 0.03 | 1.35 ± 0.04 |
| L4 | 1.0 | 96.2 ± 0.1 | 22.6 ± 0.9 | 5.35 ± 0.80 | 1.32 ± 0.12 | 1.45 ± 0.06 | 0.22 ± 0.04 | 1.32 ± 0.03 |
| L5 | 1.5 | 96.6 ± 0.0 | 27.8 ± 0.4 | 2.32 ± 0.30 | 1.34 ± 0.06 | 1.42 ± 0.05 | 0.23 ± 0.03 | 1.33 ± 0.03 |
| L6 | 2.0 | 82.9 ± 0.2 | 22.8 ± 0.6 | 2.52 ± 0.40 | 1.36 ± 0.14 | 1.43 ± 0.13 | 0.23 ± 0.04 | 1.40 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-T.; Chuang, Y.-H.; Lin, H.-C.; Chien, L.-J. Characterization and Aerosolization Performance of HydroxyPropyl-Beta-Cyclodextrin Particles Produced Using Supercritical Assisted Atomization. Polymers 2021, 13, 2260. https://doi.org/10.3390/polym13142260
Wu H-T, Chuang Y-H, Lin H-C, Chien L-J. Characterization and Aerosolization Performance of HydroxyPropyl-Beta-Cyclodextrin Particles Produced Using Supercritical Assisted Atomization. Polymers. 2021; 13(14):2260. https://doi.org/10.3390/polym13142260
Chicago/Turabian StyleWu, Hsien-Tsung, Yao-Hsiang Chuang, Han-Cyuan Lin, and Liang-Jung Chien. 2021. "Characterization and Aerosolization Performance of HydroxyPropyl-Beta-Cyclodextrin Particles Produced Using Supercritical Assisted Atomization" Polymers 13, no. 14: 2260. https://doi.org/10.3390/polym13142260
APA StyleWu, H.-T., Chuang, Y.-H., Lin, H.-C., & Chien, L.-J. (2021). Characterization and Aerosolization Performance of HydroxyPropyl-Beta-Cyclodextrin Particles Produced Using Supercritical Assisted Atomization. Polymers, 13(14), 2260. https://doi.org/10.3390/polym13142260






