The Proposal and Necessity of the Numerical Description of Nano- and Microplastics’ Surfaces (Plastisphere)
Abstract
:1. Introduction
1.1. Microplastics, Nanoplastics and the Plastisphere
1.2. SEM Pictures as a Base for Image Analysis
1.3. The Picture Analyses in Various Research Areas and Different Numerical Approaches
2. Materials and Methods
2.1. Microplastics and Nanoplastics Classes and Collection of Samples
- the size and distribution of fibres in a standard glass fibre filter (GFF);
- debris mapping on the filter and their grain distribution;
- the degradation of fibres (ghost nets);
- the different patterns of degradation for polymer spheres;
- number of pores per surface;
- description and visualisation of a texture;
- classification of different morphologies present on a surface;
- comprehensive characterization of weathered polymers found inside animals (e.g., fishes).
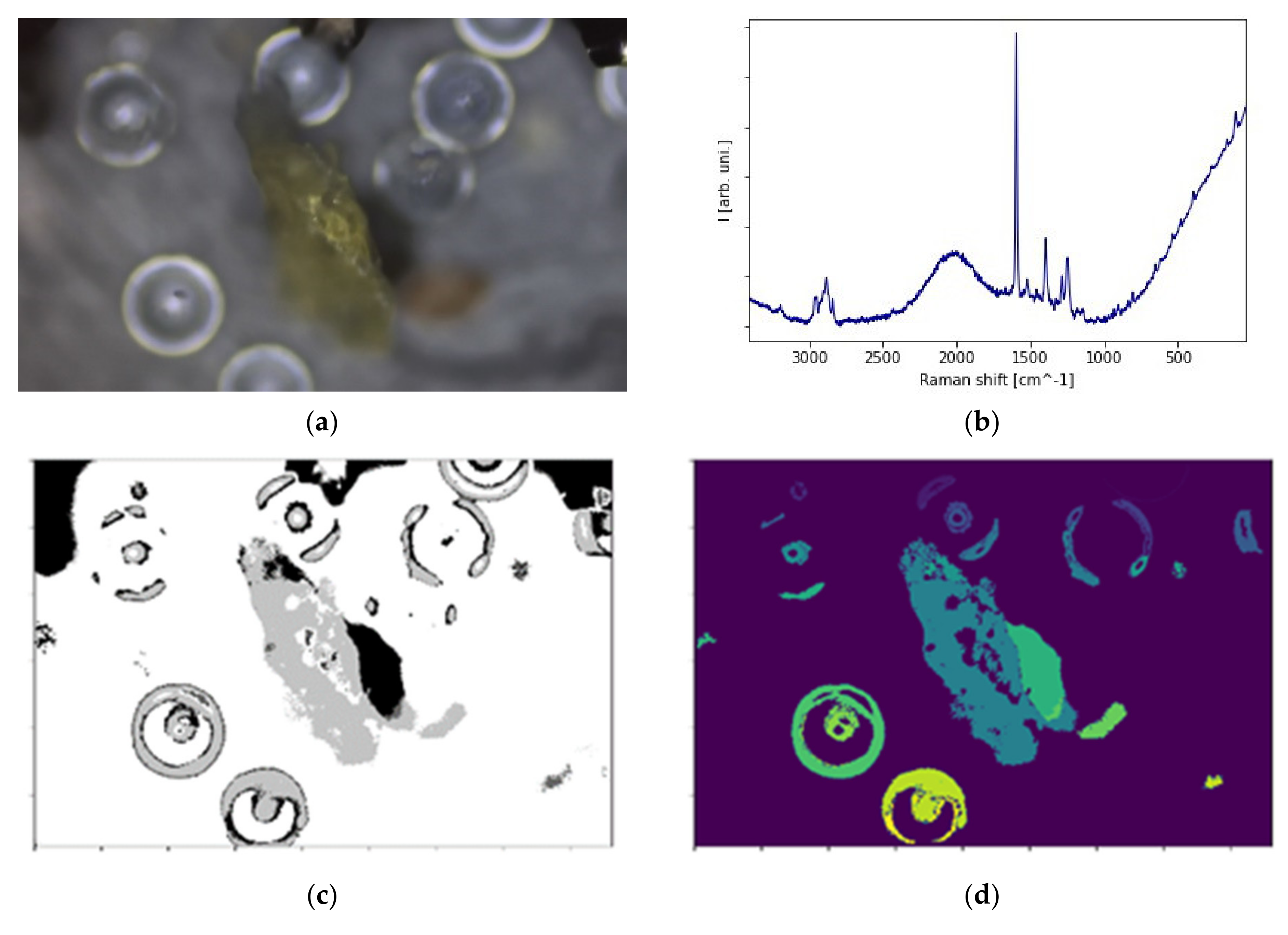
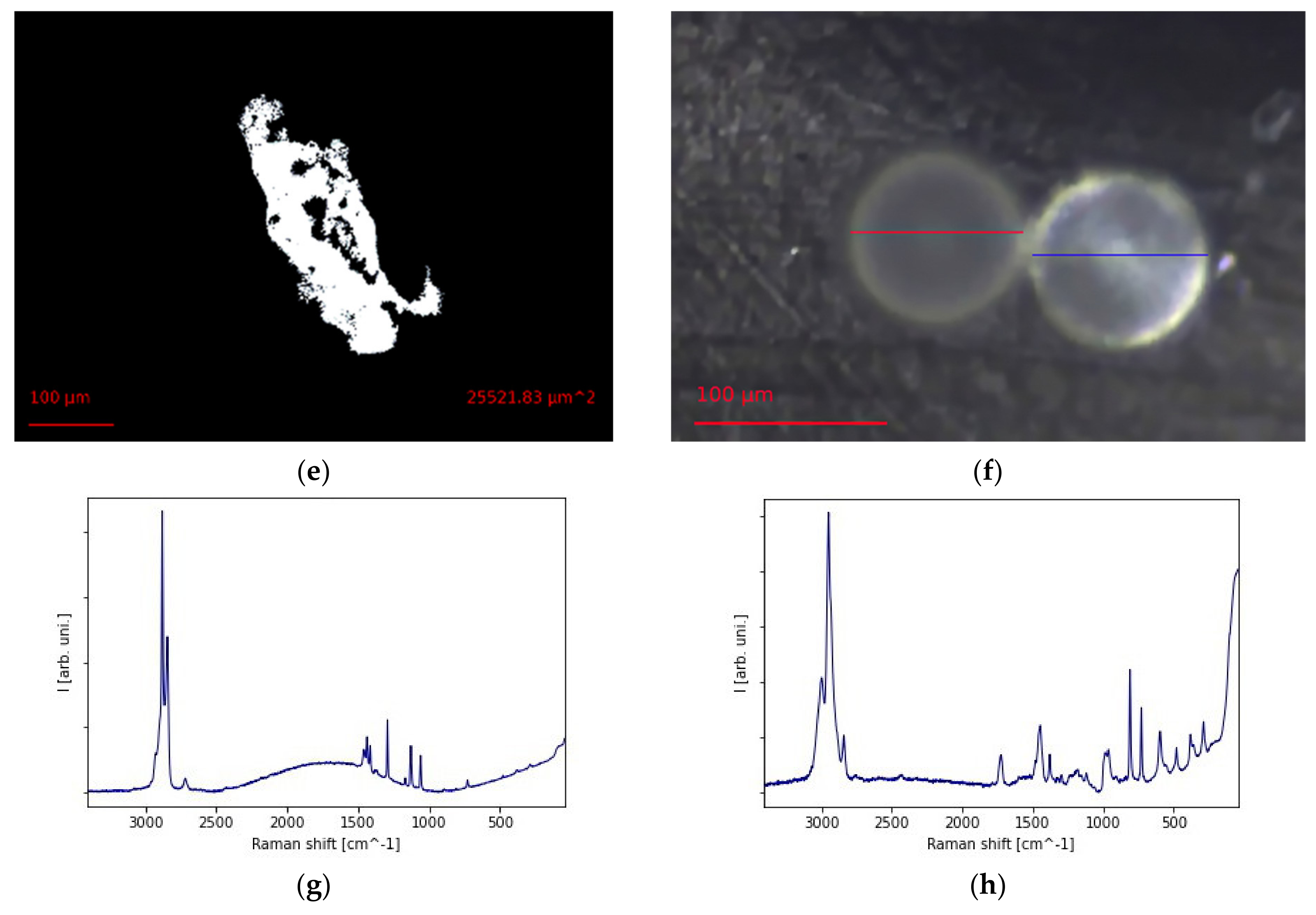
2.2. The Physical and Chemical Qualitative Characterization
2.3. The Picture Analysis and the Quantitative Description of Surface
- the user-friendly and fast-developing programming language;
- interactive and standard mode;
- several distributions available (e.g., Anaconda);
- a powerful programming language with clear and elegant syntax making it easy to read and write;
- highly portable, easy to extend;
- an open-source software, with a large community of users.
3. Results and Discussion
3.1. The Characterization of Glass Fibre Filters (GFF)
3.2. The Detection of Particles and Their Grain Sizes
3.3. Ghost Netting
3.4. Primary Sources and Nurdles
3.5. Natural Weathering of Polymers on a Seacoast
3.6. Natural Textures and Patterns
3.7. The Variety of Substrates within One Debris
3.8. Microplastics Detected Inside the Comestible Fishes
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, X.; Chen, F.; Zhao, F.; Zhao, Y.; Ruan, L.; Wang, Y.; Yang, Y. Fate and transport of nanoplastics in complex natural aquifer media: Effect of particle size and surface functionalization. Sci. Total Environ. 2019, 669, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Huvet, A.; Paul-Pont, I. Surface functionalization determines behavior of nanoplastic solutions in model aquatic environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, F.F.; Wang, S.C.; Huang, T.Y.; Li, M.R.; Zhu, Z.L.; Liu, G.Z. Sorption of fluoroquinolones to nanoplastics as affected by surface functionalization and solution chemistry. Environ. Pollut. 2020, 262, 114347. [Google Scholar] [CrossRef] [PubMed]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J.; Klaassen, M.A.; Gallager, S.M. Biofouling impacts on polyethylene density and sinking in coastal waters: A macro/micro tipping point? Water Res. 2021, 201, 117289. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A. A roadmap for a plastisphere. Mar. Pollut. Bull. 2021, 167. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Int. 2019, 123, 79–86. [Google Scholar] [CrossRef]
- Laganà, P.; Caruso, G.; Corsi, I.; Bergami, E.; Venuti, V.; Majolino, D.; La Ferla, R.; Azzaro, M.; Cappello, S. Do plastics serve as a possible vector for the spread of antibiotic resistance? First insights from bacteria associated to a polystyrene piece from King George Island (Antarctica). Int. J. Hyg. Environ. Health 2019, 222, 89–100. [Google Scholar] [CrossRef]
- Vos, M. The evolution of bacterial pathogens in the Anthropocene. Infect. Genet. Evol. 2020, 86, 104611. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dussud, C.; Meistertzheim, A.L.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.L.; et al. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. Pollut. 2018, 236, 807–816. [Google Scholar] [CrossRef]
- Gong, J.; Kong, T.; Li, Y.; Li, Q.; Li, Z.; Zhang, J. Biodegradation of microplastic derived from poly(ethylene terephthalate) with bacterial whole-cell biocatalysts. Polymers (Basel) 2018, 10, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H. Insight into Chain Scission and Release Profiles from Photodegradation of Polycarbonate Microplastics Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef] [PubMed]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Jiang, J.Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Morley, S.A.; Bell, J.; Brewin, P.; Brigden, K.; Collins, M.; Glass, T.; Goodall-Copestake, W.P.; Henry, L.; Laptikhovsky, V.; et al. Marine plastics threaten giant Atlantic Marine Protected Areas. Curr. Biol. 2018, 28, R1137–R1138. [Google Scholar] [CrossRef] [Green Version]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Li, X.; Liu, H.; Lu, G.; Dang, Z.; Yin, H. Bacterial communities on soil microplastic at Guiyu, an E-Waste dismantling zone of China. Ecotoxicol. Environ. Saf. 2020, 195, 110521. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.M.; Di Cesare, A.; Kettner, M.T.; Arias-Andres, M.; Fontaneto, D.; Grossart, H.P.; Corno, G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018, 234, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Iruthayam, A.; Shukor, M.Y.A.; Alias, S.A.; Smykla, J.; Yasid, N.A. Biodeterioration of untreated polypropylene microplastic particles by antarctic bacteria. Polymers (Basel) 2020, 12, 2616. [Google Scholar] [CrossRef]
- Cowger, W.; Gray, A.; Christiansen, S.H.; DeFrond, H.; Deshpande, A.D.; Hemabessiere, L.; Lee, E.; Mill, L.; Munno, K.; Ossmann, B.E.; et al. Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research. Appl. Spectrosc. 2020, 74, 989–1010. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130. [Google Scholar] [CrossRef]
- Serranti, S.; Palmieri, R.; Bonifazi, G.; Cózar, A. Characterization of microplastic litter from oceans by an innovative approach based on hyperspectral imaging. Waste Manag. 2020, 76, 117–125. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-bonaca, M. Ten inconvenient questions about plastics in the sea. Environ. Sci. Policy 2018, 85, 146–154. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Cristina, M.; Corsolini, S.; Sea, R. Chemosphere Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, M.; Pietroletti, M.; Monakhova, Y.B. FTIR spectroscopy supported by statistical techniques for the structural characterization of plastic debris in the marine environment : Application to monitoring studies. MPB 2016, 106, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, P.; Ni, F.; Cizdziel, J.; Wu, D.; Zhao, Q.; Zhou, Y. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Mar. Pollut. Bull. 2019, 145, 153–160. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Ghosal, S.; Chen, M.; Wagner, J.; Wang, Z.M.; Wall, S. Molecular identification of polymers and anthropogenic particles extracted from oceanic water and fish stomach—A Raman micro-spectroscopy study. Environ. Pollut. 2018, 233, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Corami, F.; Rosso, B.; Bravo, B.; Gambaro, A.; Barbante, C. A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere 2020, 238, 124564. [Google Scholar] [CrossRef]
- Renner, G.; Nellessen, A.; Schwiers, A.; Wenzel, M.; Schmidt, T.C.; Schram, J. Data preprocessing & evaluation used in the microplastics identification process: A critical review & practical guide. TrAC Trends Anal. Chem. 2019, 111, 229–238. [Google Scholar] [CrossRef]
- Frère, L.; Maignien, L.; Chalopin, M.; Huvet, A.; Rinnert, E.; Morrison, H.; Kerninon, S.; Cassone, A.L.; Lambert, C.; Reveillaud, J.; et al. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Burrows, S.D.; Frustaci, S.; Thomas, K.V.; Galloway, T. Expanding exploration of dynamic microplastic surface characteristics and interactions. TrAC Trends Anal. Chem. 2020, 130, 115993. [Google Scholar] [CrossRef]
- Wang, Z.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. Science of the Total Environment SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fi sh guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef]
- Gniadek, M.; Dąbrowska, A. The marine nano- and microplastics characterisation by SEM-EDX: The potential of the method in comparison with various physical and chemical approaches. Mar. Pollut. Bull. 2019, 148, 210–216. [Google Scholar] [CrossRef]
- Gontard, L.C.; López-Castro, J.D.; González-Rovira, L.; Vázquez-Martínez, J.M.; Varela-Feria, F.M.; Marcos, M.; Calvino, J.J. Assessment of engineered surfaces roughness by high-resolution 3D SEM photogrammetry. Ultramicroscopy 2017. [Google Scholar] [CrossRef] [PubMed]
- Sartipi, Y.; Ross, A.; Zhang, W.; Norris, S.; El-Sherif, H.; Anand, C.K.; Bassim, N.D. Scanning Electron Microscope 3D Surface Reconstruction via Optimization. Microsc. Microanal. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dunn, R.M.; Schrlau, M.G. Numerical processing of CNT arrays using 3D image processing of SEM images. Robot. Comput. Integr. Manuf. 2018, 53, 21–27. [Google Scholar] [CrossRef]
- Hameed, N.A.; Ali, I.M.; Hassun, H.K. Calculating surface roughness for a large scale sem images by mean of image processing. Energy Procedia 2019, 157, 84–89. [Google Scholar] [CrossRef]
- Gerke, K.M.; Korostilev, E.V.; Romanenko, K.A.; Karsanina, M.V. Going submicron in the precise analysis of soil structure: A FIB-SEM imaging study at nanoscale. Geoderma 2021, 383, 114739. [Google Scholar] [CrossRef]
- Kleinendorst, S.M.; Fleerakkers, R.; Cattarinuzzi, E.; Vena, P.; Gastaldi, D.; van Maris, M.P.F.H.L.; Hoefnagels, J.P.M. Micron-scale experimental-numerical characterization of metal-polymer interface delamination in stretchable electronics interconnects. Int. J. Solids Struct. 2020, 204–205, 52–64. [Google Scholar] [CrossRef]
- Qiu, H.; Chauhan, K.; Lei, C. A numerical study of drag reduction performance of simplified shell surface microstructures. Ocean Eng. 2020, 217, 107916. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, W.; Wang, D.; Sun, H.; Chen, K.; Yang, B. The role of surface roughness in the wettability and floatability of quartz particles. Appl. Surf. Sci. 2020, 527, 146799. [Google Scholar] [CrossRef]
- Muhammad, W.; Ali, U.; Brahme, A.P.; Kang, J.; Mishra, R.K.; Inal, K. Experimental analyses and numerical modeling of texture evolution and the development of surface roughness during bending of an extruded aluminum alloy using a multiscale modeling framework. Int. J. Plast. 2017, 117, 93–121. [Google Scholar] [CrossRef]
- Barreau, M.; Méthivier, C.; Sturel, T.; Allely, C.; Drillet, P.; Cremel, S.; Grigorieva, R.; Nabi, B.; Podor, R.; Lautru, J.; et al. In situ surface imaging: High temperature environmental SEM study of the surface changes during heat treatment of an Al[sbnd]Si coated boron steel. Mater. Charact. 2020, 163, 110266. [Google Scholar] [CrossRef]
- Song, H.; Liu, C.; Zhang, H.; Du, J.; Yang, X.; Leen, S.B. In-situ SEM study of fatigue micro-crack initiation and propagation behavior in pre-corroded AA7075-T7651. Int. J. Fatigue 2020, 137, 105655. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Development of an SEM image analysis method to characterize intumescent fire retardant char layer. Prog. Org. Coatings 2020, 139, 105461. [Google Scholar] [CrossRef]
- Gesho, M.; Chaisoontornyotin, W.; Elkhatib, O.; Goual, L. Auto-segmentation technique for SEM images using machine learning: Asphaltene deposition case study. Ultramicroscopy 2020, 217, 113074. [Google Scholar] [CrossRef] [PubMed]
- Sitko, M.; Mojzeszko, M.; Rychlowski, L.; Cios, G.; Bala, P.; Muszka, K.; Madej, L. Numerical procedure of three-dimensional reconstruction of ferrite-pearlite microstructure data from SEM/EBSD serial sectioning. Procedia Manuf. 2020, 47, 1217–1222. [Google Scholar] [CrossRef]
- Kohler, R.; Sowards, K.; Medina, H. Numerical model for acid-etching of titanium: Engineering surface roughness for dental implants. J. Manuf. Process. 2020, 59, 113–121. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Pan, Z.; Tong, W. Nanoscale pore structure and mechanical property analysis of coal : An insight combining AFM and SEM images. Fuel 2020, 260, 116352. [Google Scholar] [CrossRef]
- Ng, K.; Dai, Q. Numerical investigation of internal frost damage of digital cement paste samples with cohesive zone modeling and SEM microstructure characterization. Constr. Build. Mater. 2014, 50, 266–275. [Google Scholar] [CrossRef]
- Nafar Dastgerdi, J.; Sheibanian, F.; Remes, H.; Lehto, P.; Hosseini Toudeshky, H. Numerical modelling approach for considering effects of surface integrity on micro-crack formation. J. Constr. Steel Res. 2020, 175, 106387. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, W.; Wang, G. Simulation of the meso-macro-scale fracture network development law of coal water injection based on a SEM reconstruction fracture COHESIVE model. Fuel 2020, 119475. [Google Scholar] [CrossRef]
- Bargmann, S.; Klusemann, B.; Markmann, J.; Eike, J.; Schneider, K.; Soyarslan, C.; Wilmers, J. Progress in Materials Science Generation of 3D representative volume elements for heterogeneous materials : A review. Prog. Mater. Sci. 2018, 96, 322–384. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Stelfox, M.; Hudgins, J.; Sweet, M. A review of ghost gear entanglement amongst marine mammals, reptiles and elasmobranchs. Mar. Pollut. Bull. 2016, 111, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, E.M.; de Bruyn, P.J.N. Pinniped entanglement in oceanic plastic pollution: A global review. Mar. Pollut. Bull. 2019, 145, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.P.; Hoellein, T.J.; Sapp, M.; Tagg, A.S.; Ju-Nam, Y.; Ojeda, J.J. Microplastic-Associated Biofilms: A Comparison of Freshwater and Marine Environments; Springer: Berlin, Germany, 2018; Volume 58, ISBN 9783319616148. [Google Scholar]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef]
- Godoy, V.; Martín-Lara, M.A.; Calero, M.; Blázquez, G. Physical-chemical characterization of microplastics present in some exfoliating products from Spain. Mar. Pollut. Bull. 2019, 139, 91–99. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Urban-malinga, B.; Wodzinowski, T.; Witalis, B.; Zalewski, M.; Radtke, K.; Grygiel, W. Marine litter on the sea fl oor of the southern Baltic. Mar. Pollut. Bull. 2018, 127, 612–617. [Google Scholar] [CrossRef]
- Wagner, J.; Wang, Z.M.; Ghosal, S.; Rochman, C.; Gassel, M.; Wall, S. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal. Methods 2017, 9, 1479–1490. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Ballerini, T.; Zettler, E.R.; Asbun, A.A.; Adame, A.; Casotti, R.; Dumontet, B.; Donnarumma, V.; Engelmann, J.C.; Frère, L.; et al. Diversity and predicted inter- and intra-domain interactions in the Mediterranean Plastisphere. Environ. Pollut. 2021, 286, 117439. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wu, N.; Li, W.; Song, X.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on plastic debris: The localization of immigrant bacterial communities. Water Res. 2021, 193, 116883. [Google Scholar] [CrossRef]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment—Application of the source-pathway-receptor model (review). Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef]
- Lorusso, G.F.; Rutigliani, V.; Van Roey, F.; Mack, C.A. Unbiased roughness measurements: Subtracting out SEM effects. Microelectron. Eng. 2018, 190, 33–37. [Google Scholar] [CrossRef]
- Ranjan, S.; Mishra, J.; Palai, G. Analysing roughness of surface through fractal dimension: A review ☆. Image Vis. Comput. 2019, 89, 21–34. [Google Scholar] [CrossRef]
- Giannatou, E.; Papavieros, G.; Constantoudis, V.; Papageorgiou, H.; Gogolides, E. Deep learning denoising of SEM images towards noise-reduced LER measurements. Microelectron. Eng. 2019, 216, 111051. [Google Scholar] [CrossRef]
- Kedzierski, M.; Falcou-Préfol, M.; Kerros, M.E.; Henry, M.; Pedrotti, M.L.; Bruzaud, S. A machine learning algorithm for high throughput identification of FTIR spectra: Application on microplastics collected in the Mediterranean Sea. Chemosphere 2019, 234, 242–251. [Google Scholar] [CrossRef]
- Tarafdar, A.; Lee, J.U.; Jeong, J.E.; Lee, H.; Jung, Y.; Oh, H.B.; Woo, H.Y.; Kwon, J.H. Biofilm development of Bacillus siamensis ATKU1 on pristine short chain low-density polyethylene: A case study on microbe-microplastics interaction. J. Hazard. Mater. 2021, 409, 124516. [Google Scholar] [CrossRef]
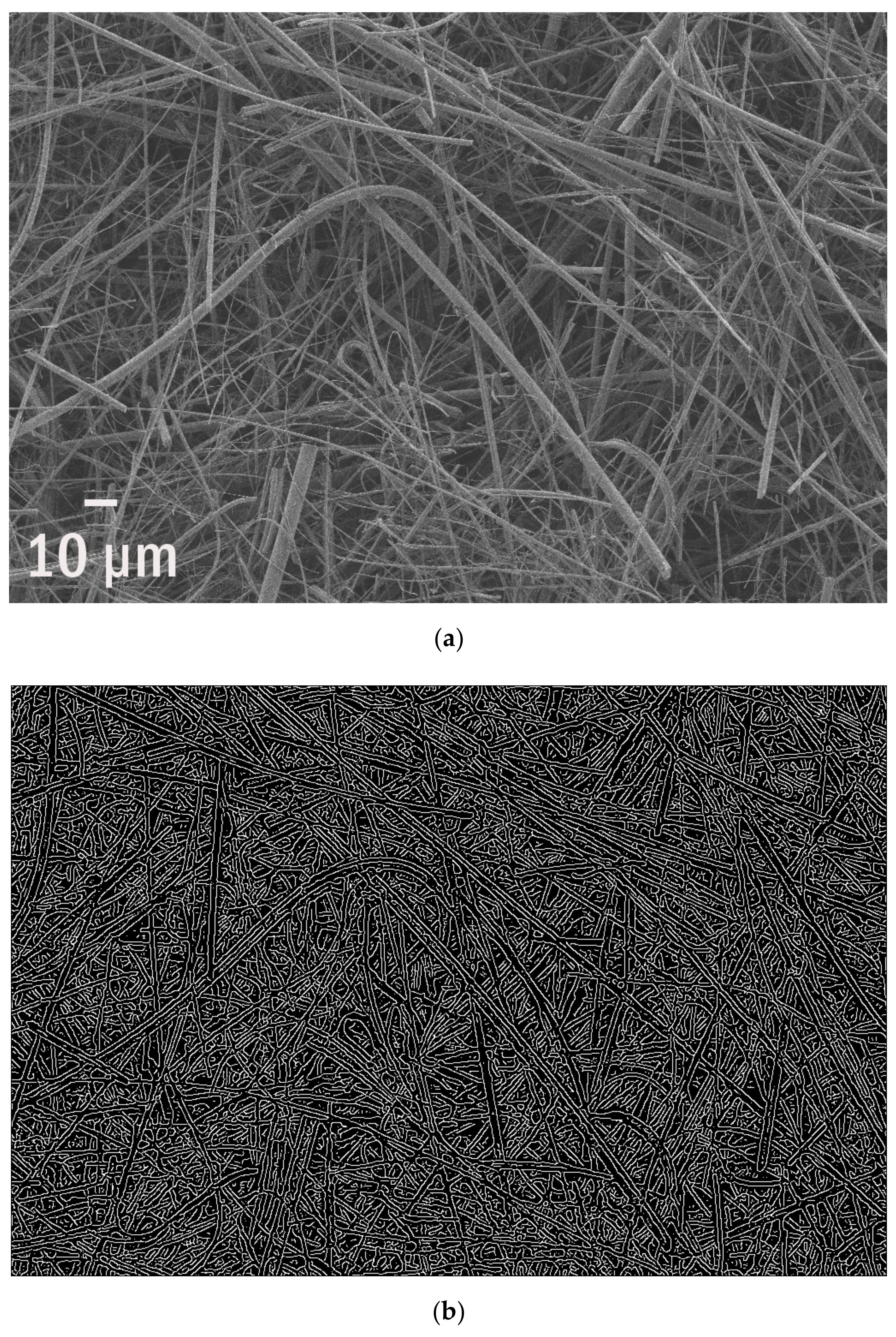
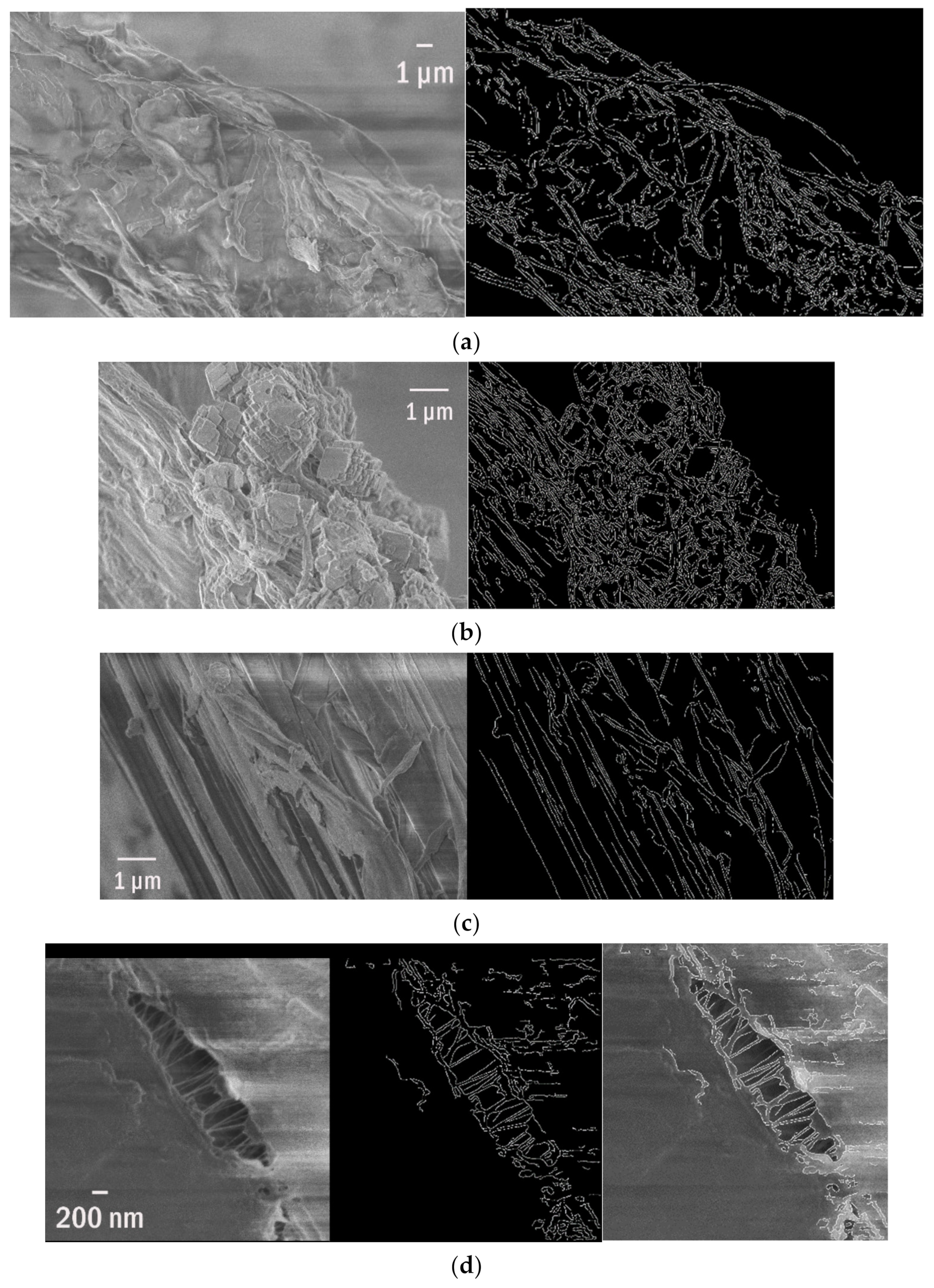


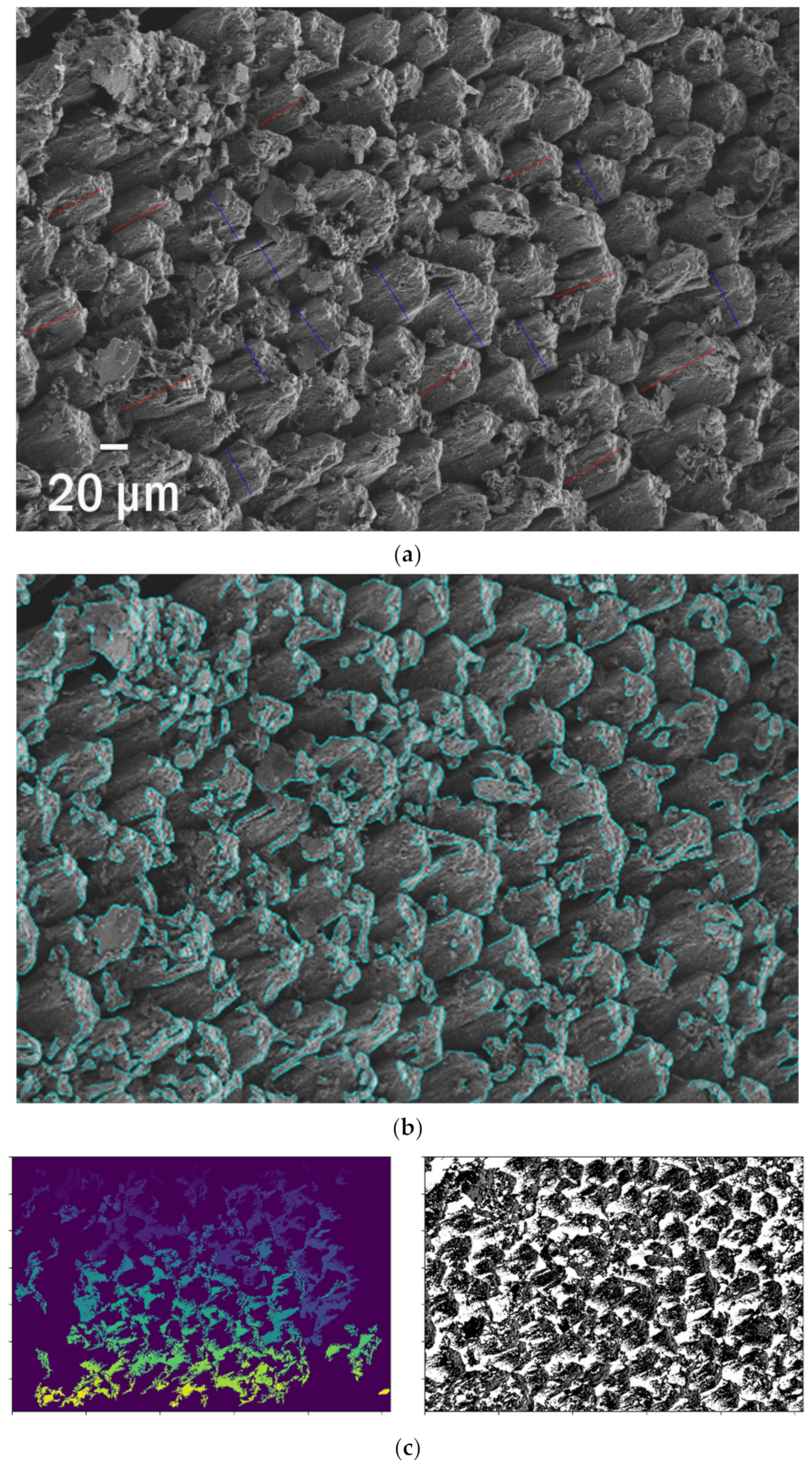
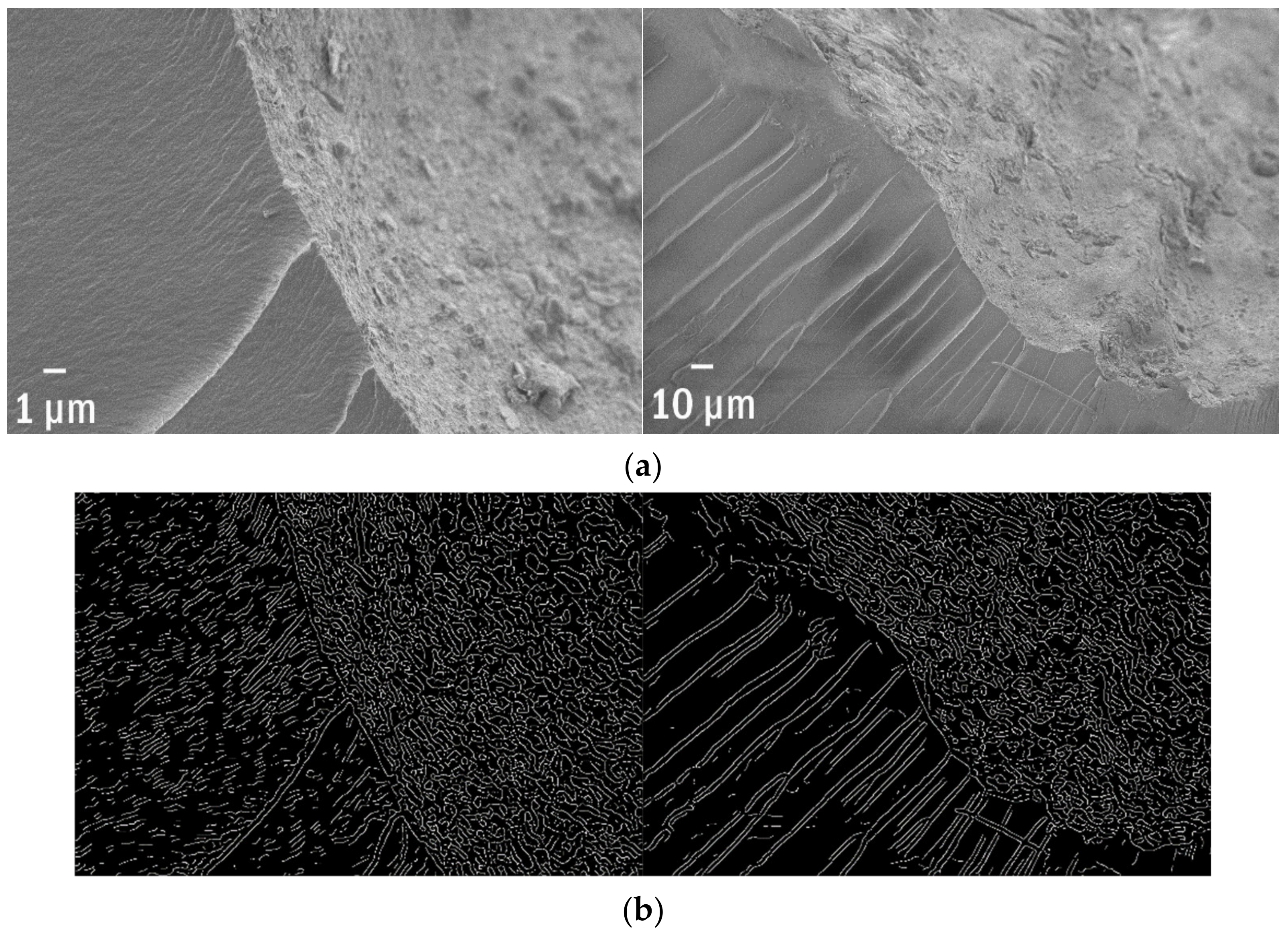
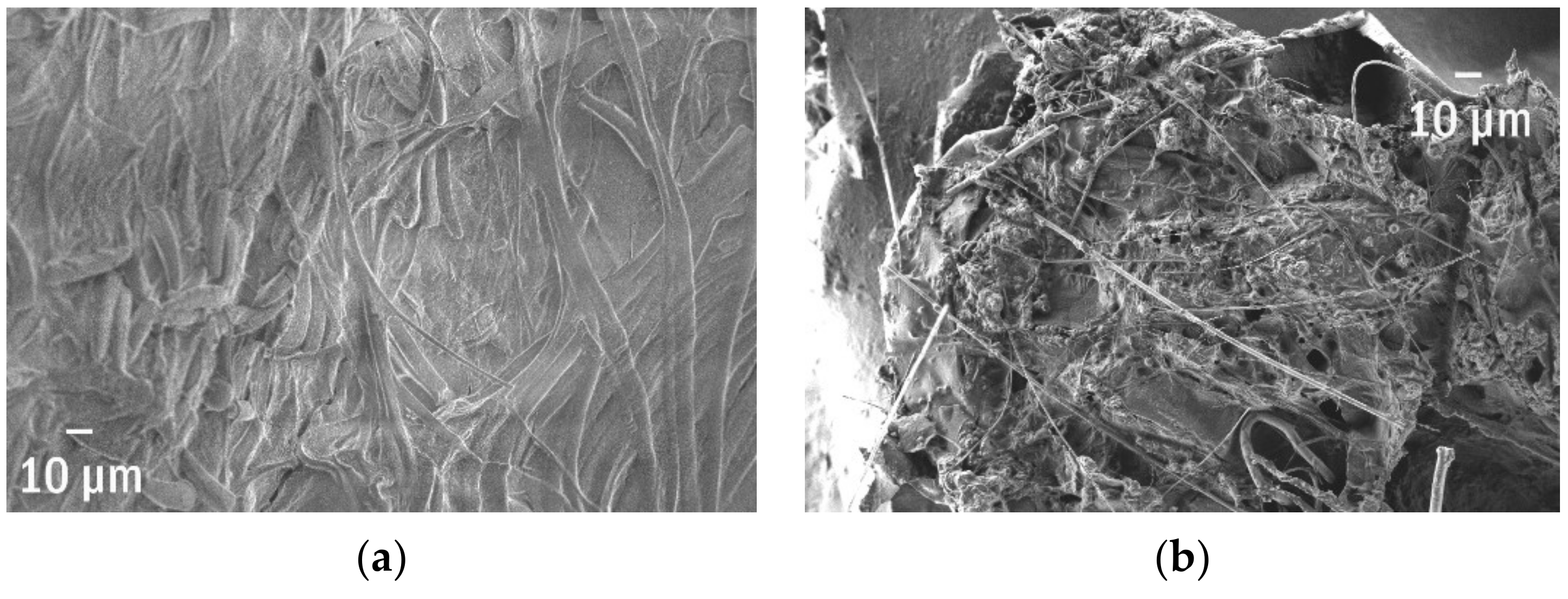


| SEM of a Plastisphere | % of Edges | Edge Per 1 µm2 | Edge/Perimeter |
|---|---|---|---|
 | 6.65 % | 2.394 µm | 6.80 |
 | 10.96 % | 3.944 µm | 11.20 |
 | 10.84 % | 204.25 nm | 5.80 |
 | 5.67 % | 390.41 nm | 11.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, A.; Gniadek, M.; Machowski, P. The Proposal and Necessity of the Numerical Description of Nano- and Microplastics’ Surfaces (Plastisphere). Polymers 2021, 13, 2255. https://doi.org/10.3390/polym13142255
Dąbrowska A, Gniadek M, Machowski P. The Proposal and Necessity of the Numerical Description of Nano- and Microplastics’ Surfaces (Plastisphere). Polymers. 2021; 13(14):2255. https://doi.org/10.3390/polym13142255
Chicago/Turabian StyleDąbrowska, Agnieszka, Marianna Gniadek, and Piotr Machowski. 2021. "The Proposal and Necessity of the Numerical Description of Nano- and Microplastics’ Surfaces (Plastisphere)" Polymers 13, no. 14: 2255. https://doi.org/10.3390/polym13142255
APA StyleDąbrowska, A., Gniadek, M., & Machowski, P. (2021). The Proposal and Necessity of the Numerical Description of Nano- and Microplastics’ Surfaces (Plastisphere). Polymers, 13(14), 2255. https://doi.org/10.3390/polym13142255







