New Star-Shaped Polyether-Pentols (PEPOs) for Fabrication of Crosslinked Polyurethanes—Synthesis and Characterization
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymerization
2.3. Measurements
3. Results and Discussion
3.1. Synthesis of Star-Shaped Polyether-Polyols (PEPOs) by Use of Potassium Salts of 2,2,6,6-Tetrakis(hydroxymethyl)cyclohexanol (THMC) Activated 18C6
3.2. Preparation and Characterization of Crosslinked Polyurethanes
4. Conclusions
- Application of potassium salt of THMC allowed us to synthesize polyether-pentols with the formation of bi- or trimodal polymers with molar masses in the range Mn = 1200–6000.
- The applied initiator system reflects the ability of crown ether to influence the degree of ion-pair separation, and hence the observed multi-fraction composition is prescribed to the formation of ionic aggregates with different reactivities during polymerization.
- MALDI-TOF and FITR spectra confirmed a star-shaped structure and composition of macromolecules with five polyether-arms with OH end groups.
- Polyether-polyols prepared in this work can be used for the synthesis of new polyurethanes.
- Analysis of the carbonyl stretching region in FTIR spectra of crosslinked polyurethanes revealed that in the presence of more bulky isopropyl-substituent, the tendency to phase separation is lower owing to its size.
- Application of aromatic-based PEPOs results in a more profound tendency to phase separation, which reflects the possibility of interaction between neighboring aromatic phenyl rings. Hence in this way range of hard phase dispersion is lower.
- DSC analysis showed that the PUR1 based on benzyl glycidyl ether (PBGE) has a higher glass transition temperature than PUR2 and PUR3 because of the limitation of mobility of the flexible segments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.-J.; Orzesek, H.; Pirkl, H.-G.; Six, C.; et al. Trends in industrial catalysis in the polyurethane industry. Appl. Catal. A Gen. 2001, 221, 303–335. [Google Scholar] [CrossRef]
- Vesterinen, A.; Lipponen, S.; Rich, J.; Seppälä, J. Effect of block composition on thermal properties and melt viscosity of poly [2-(dimethylamino)ethyl methacrylate], poly(ethylene oxide) and poly(propylene oxide) block co-polymers. Express Polym. Lett. 2011, 5, 754–765. [Google Scholar] [CrossRef]
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Jing, L.; Jianchun, J.; Junming, X.; Haihong, X.; Peng, L. Branched polyols based on oleic acid for production of polyurethane foams reinforced with bamboo fiber. Iran. Polym. J. 2016, 25, 811–822. [Google Scholar]
- Brydson, J. Thermoplastic Elastomers: Properties and Applications; Rapra Review Report No. 81; Rapra Technology: Shrewsbury, UK, 1995; Volume 7. [Google Scholar]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [PubMed]
- Brocas, A.-L.; Mantzaridis, C.; Tunc, D.; Carlotti, S. Polyether synthesis: From activated or metal-free anionic ring-opening polymerization of epoxides to functionalization. Prog. Polym. Sci. 2013, 38, 845–873. [Google Scholar] [CrossRef]
- Cendejas, G.; Arreguin, F.; Flores, C.; Villalobos, I.; Flores, E.; Vázquez, F. Novel initiators for the synthesis of propylene oxide oligomers by anionic ring opening polymerization. Catal. Today 2008, 130, 486–491. [Google Scholar] [CrossRef]
- Grobelny, Z.; Matlengiewicz, M.; Golba, S.; Jurek-Suliga, J.; Swinarew, A.; Skrzeczyna, K.; Michalak, M.; Swinarew, B. Application of Dipotassium Glycoxides–Activated 18-Crown-6 for the Synthesis of Poly(propylene oxide) with Increased Molar Mass. Int. J. Polym. Anal. Charact. 2015, 20, 206–222. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Morejko, B.; Stolarzewicz, A.; Grobelny, Z.; Piekarnik, B.; Niedziela, T.; Trzebicka, B. New kind of star-shaped polyethers prepared with cyclic oligo(potassium glycidoxide) as a macroinitiator. React. Funct. Polym. 2007, 67, 669–674. [Google Scholar] [CrossRef]
- Hua, C.; Li, X.-G.; Xu, S.-M. 2,2,6,6-Tetrakis(hydroxymethyl)cyclohexanol. Acta Crystallogr. Sect. E 2007, E63, o547–o548. [Google Scholar] [CrossRef]
- Wittcoff, H. 2,2,6,6-Tetramethylolcyclohexanol. Org. Synth. 2003, 31. [Google Scholar] [CrossRef]
- Karadag, K.; Onaran, G.; Sonmez, H.B. Synthesis and swelling properties of new crosslinked polyorthocarbonates. J. Appl. Polym. Sci. 2011, 121, 3300–3305. [Google Scholar] [CrossRef]
- Bulbul Sonmez, H.; Gonul Kuloglu, F.; Karadag, K.; Wudl, F. Terephthalaldehyde- and isophthalaldehyde-based polyspiroacetals. Polym. J. 2012, 44, 217–223. [Google Scholar] [CrossRef][Green Version]
- Akbulut, G.; Sonmez, H.B.; Wudl, F. Synthesis, characterization and properties of novel polyspiroacetals. J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Luo, Z.; Jiang, X.; Wang, T.; Guo, K. Polyurethane rigid foams formed from different soy-based polyols by the ring opening of epoxidised soybean oil with methanol, phenol, and cyclohexanol. Ind. Crop. Prod. 2015, 74, 76–82. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Szczepkowski, P.; Krzyżowska, M.; Ryszkowska, J. Preparation and characterization of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crop. Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Polyurethane–polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind. Crop. Prod. 2015, 74, 849–857. [Google Scholar] [CrossRef]
- Bak, E. Process for Preparing Polyurethane Compositions Emitting Reduced Amounts of Toxic Fumes on Burning. International Patent Application No. C08K5/098; (IPC1-7): C08G18/16; C08K5/09; European Patent Application No. EP0010931 (A1); Canada Patent Application No. CA1128250 (A). U.S. Patent Application No. US4263411 (A), 15 January 1986. [Google Scholar]
- Smith, S.; Hubin, A.J. The Preparation and Chemistry of Dicationically Active Polymers of Tetrahydrofuran. J. Macromol. Sci. Part A Chem. 1973, 7, 1399–1413. [Google Scholar] [CrossRef]
- Jikei, M.; Jikei, M.; Aikawa, Y.; Matsumoto, K. Synthesis and properties of poly(ether sulfone)–poly(tetrahydrofuran) multiblock copolymers. High Perform. Polym. 2016, 28, 1015–1023. [Google Scholar] [CrossRef]
- Brown, C.A. Saline hydrides and superbases in organic reactions. VII. Potassium hydride, highly active new hydride reagent. Reactivity, applications, and techniques in organic and organometallic reactions. J. Org. Chem. 1974, 39, 3913–3918. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.I. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- Koinuma, H.; Naito, K.; Hirai, H. Anionic polymerization of oxiranes and cyclic siloxanes initiated with potassium salt-crown ether systems. Macromol. Chem. Phys. 1982, 183, 1383–1392. [Google Scholar] [CrossRef]
- Grobelny, Z.; Matlengiewicz, M.; Jurek, J.; Michalak, M.; Kwapulińska, D.; Swinarew, A.; Schab-Balcerzak, E. The influence of macrocyclic ligands and water on propylene oxide polymerization initiated with anhydrous potassium hydroxide in tetrahydrofuran. Eur. Polym. J. 2013, 49, 3277–3288. [Google Scholar] [CrossRef]
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Słomkowski, S. Living ring-opening polymerizations of heterocyclic monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Wang, X.; Ma, P.; Bai, H.; Dong, W.; Xie, Y.; Chen, M. Superior Performance of Polyurethane Based on Natural Melanin Nanoparticles. Biomacromolecules 2016, 17, 3782–3789. [Google Scholar] [CrossRef]
- Hayashi, S. ‘Properties and Applications of Polyurethane’ in series Shape Memory Polymer. In International Progress in Urethanes, 1st ed.; Ashida, K., Frisch, K.C., Eds.; CRC Press: Boca Raton, FL, USA, 1993; Volume 6, pp. 90–115. [Google Scholar]
- Harthcock, M.A. Probing the complex hydrogen bonding structure of urethane block copolymers and various acid containing copolymers using infra-red spectroscopy. Polymer 1989, 30, 1234–1242. [Google Scholar] [CrossRef]
- Tien, T.I.; Wei, K.H. Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. Polymer 2001, 42, 3213–3221. [Google Scholar] [CrossRef]
- Pretsch, T.; Jakob, I.; Müller, W. Hydrolytic degradation and functional stability of a segmented shape memory poly(ester urethane). Polym. Degrad. Stab. 2009, 94, 61–73. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Wang, L.F. Effect of soft segment length on the thermal behaviors of fluorinated polyurethanes. Eur. Polym. J. 2005, 41, 293–301. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galambos, A.F.; Leung, L.M. Compression-molded polyurethane block copolymers. 1. Microdomain morphology and thermomechanical properties. Macromolecules 1992, 25, 6195–6204. [Google Scholar] [CrossRef]
- Wu, Z.-Y. Synthesis and Properties of Moisture-Cured Reactive Polyurethane Containing Castor Oil and Oxime Compounds. Polymers 2020, 12, 1838. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sultan, M.; Imran Sajid, M.; Jubeen, F.; Parveen, S.; Bibi, I.; Safa, Y. Synthesis and characterization of biodegradable and cytocompatible polyurethane-bovine-derived hydroxyapatite biomaterials. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lima, R.D.C.; Cunha, R.H.; Santos, J.F. Evaluation of Polyurethane Elastomers for Encapsulation of Hydroacoustic Transducers. Macromol. Symp. 2020, 394, 2000083. [Google Scholar] [CrossRef]
- Nistor, A.; Toulec, M.; Zubov, A.; Kosek, J. Tomographic Reconstruction and Morphological Analysis of Rigid Polyurethane Foams. Macromol. Symp. 2016, 360, 87–95. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Fuh, J.Y.H.; Lee, H.P. Effect of Porosity on Mechanical Properties of 3D Printed Polymers: Experiments and Micromechanical Modeling Based on X-ray Computed Tomography Analysis. Polymers 2019, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Takase, A.; McNulty, T.; Fitzgibbons, T. Foam Porosity Calculation by X-Ray Computed Tomography and Errors Caused by Insufficient Resolution. Microsc. Microanal. 2018, 24 (Suppl. 2), 546–547. [Google Scholar] [CrossRef][Green Version]
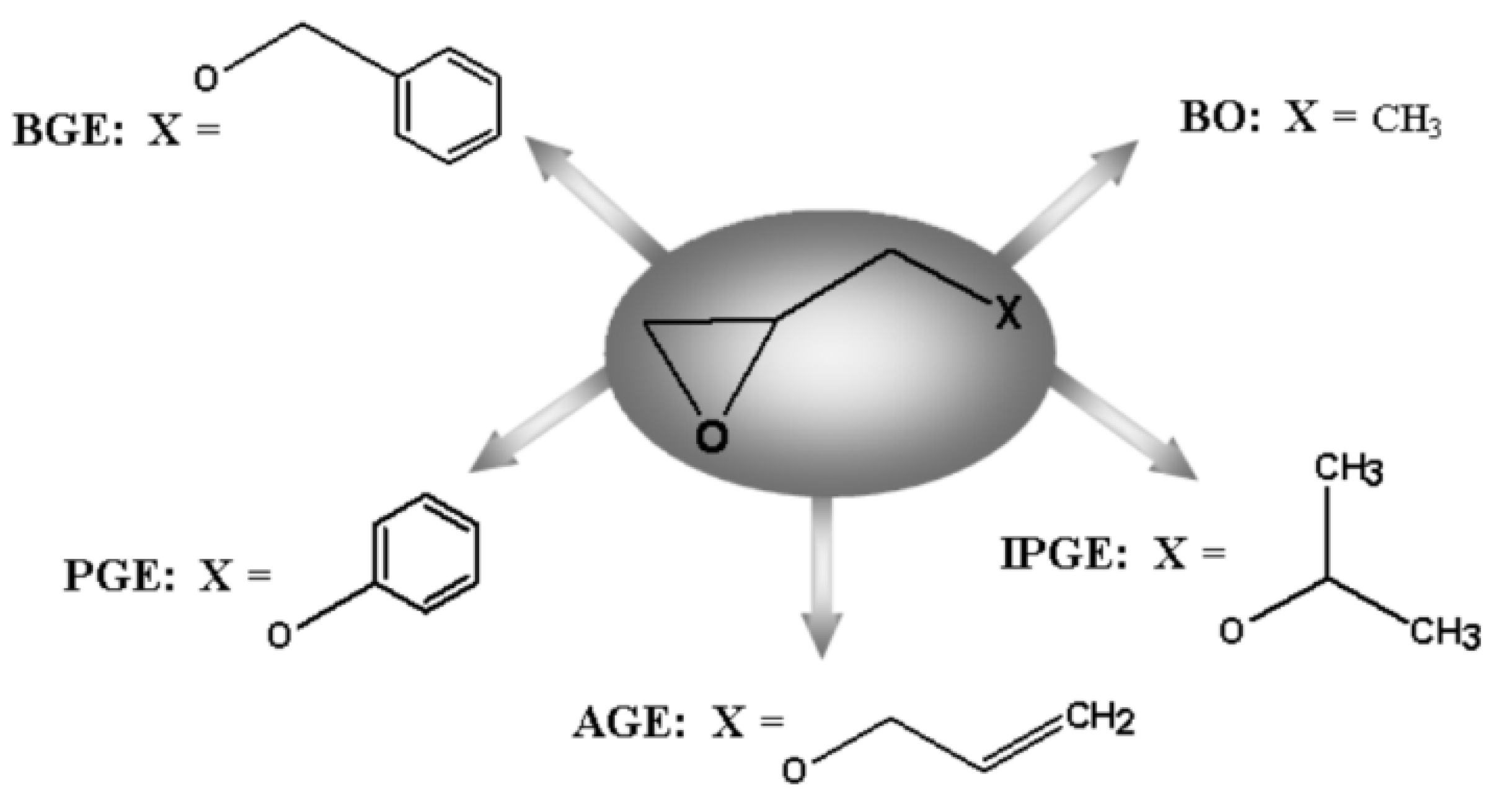

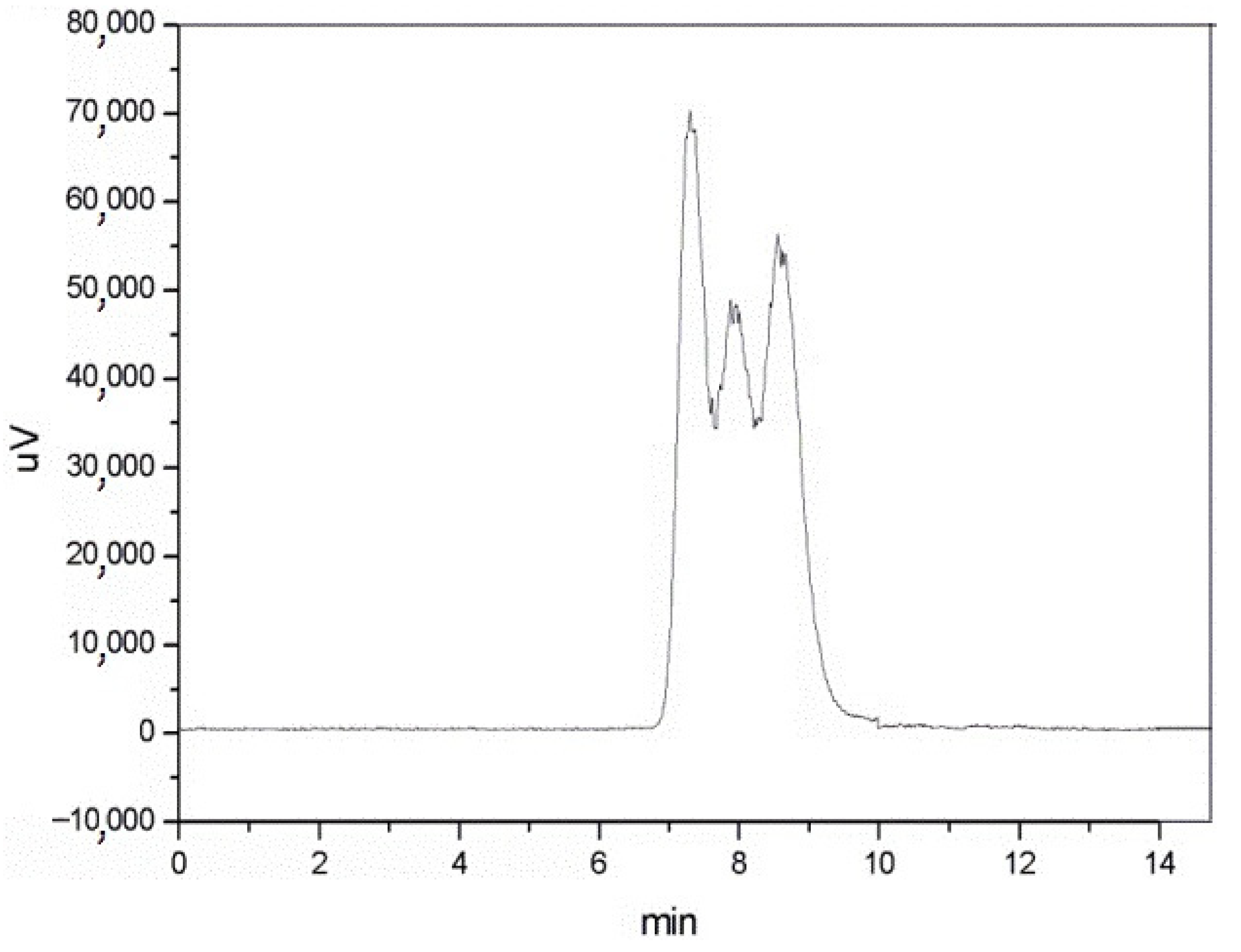
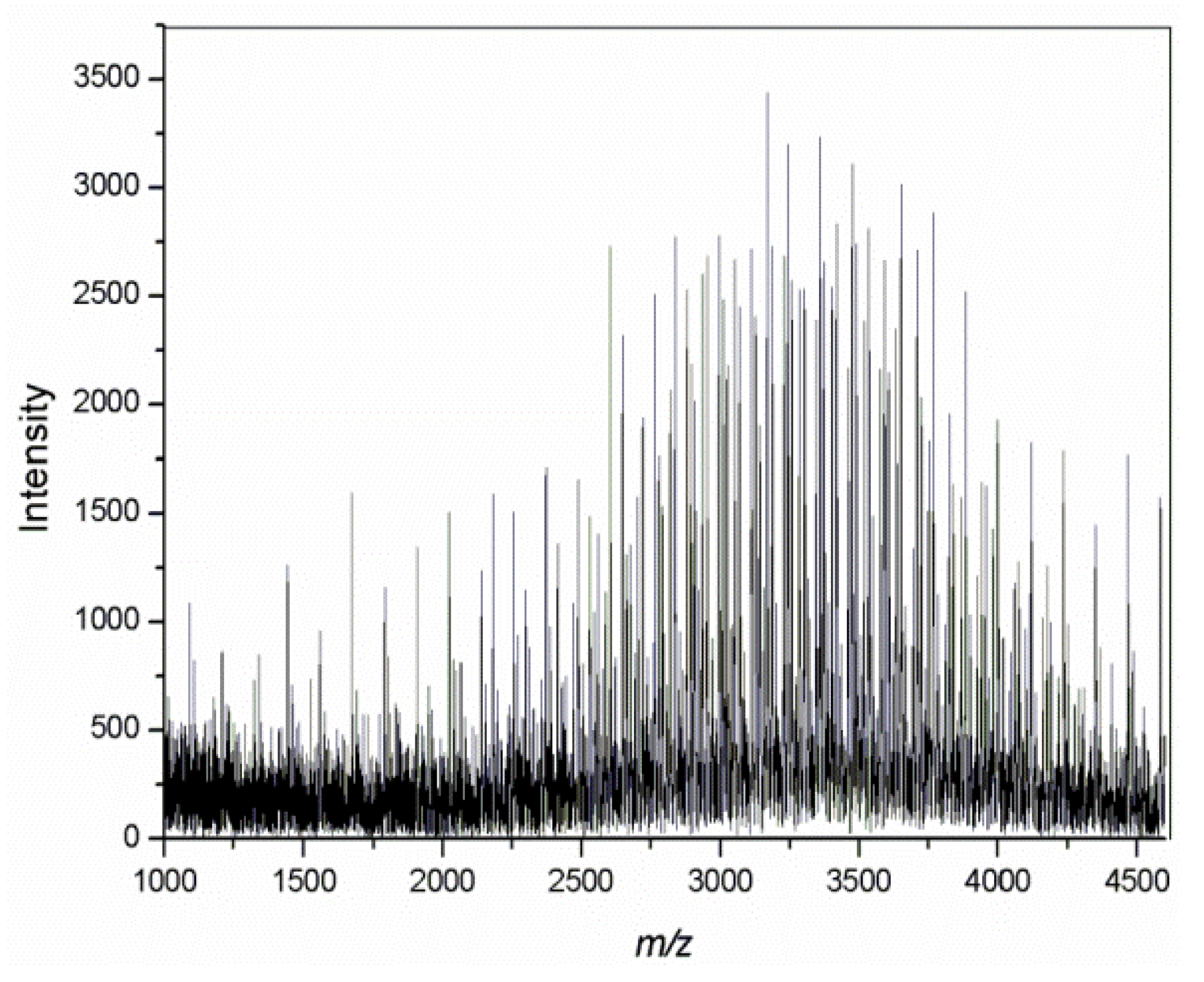

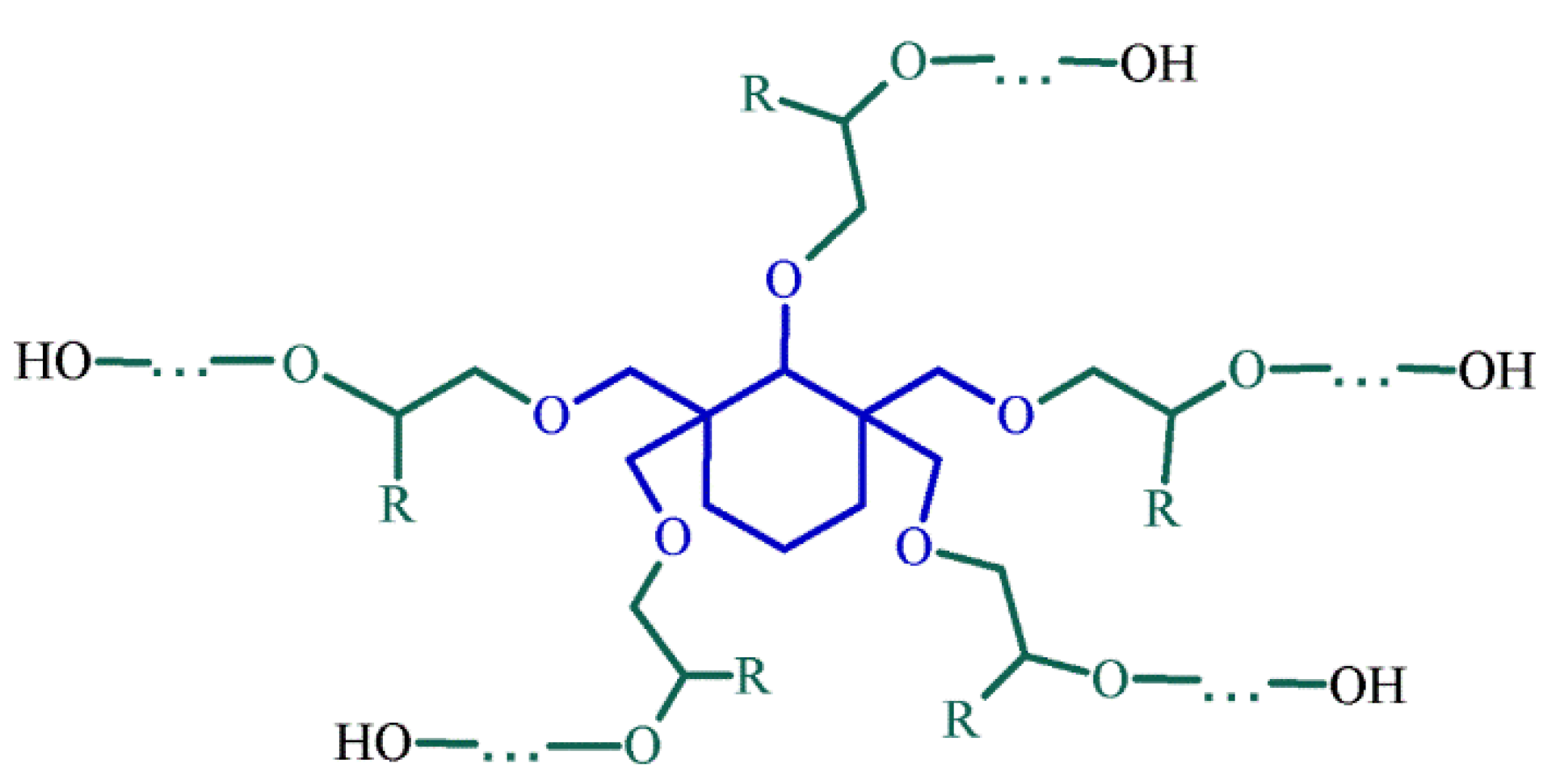
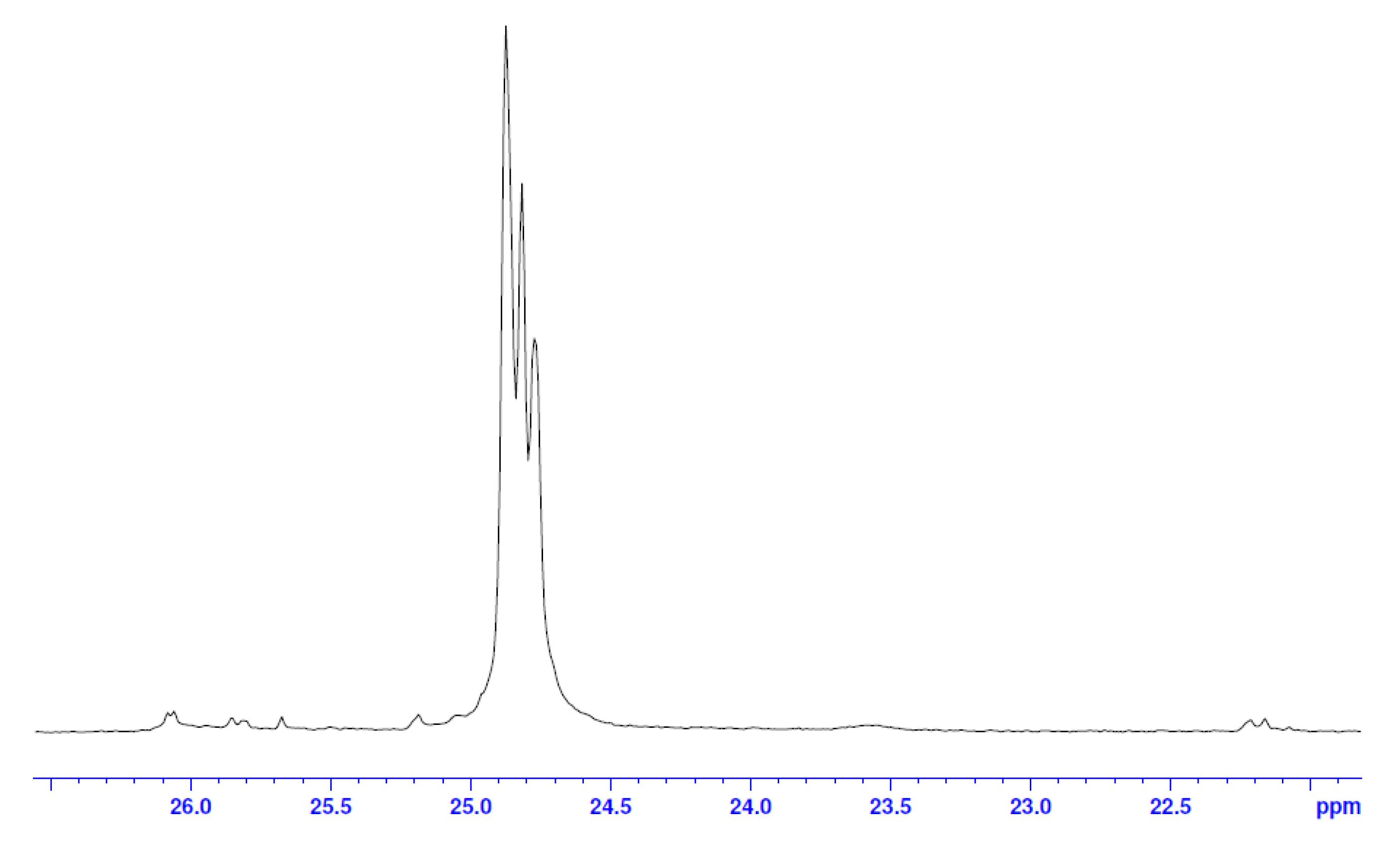




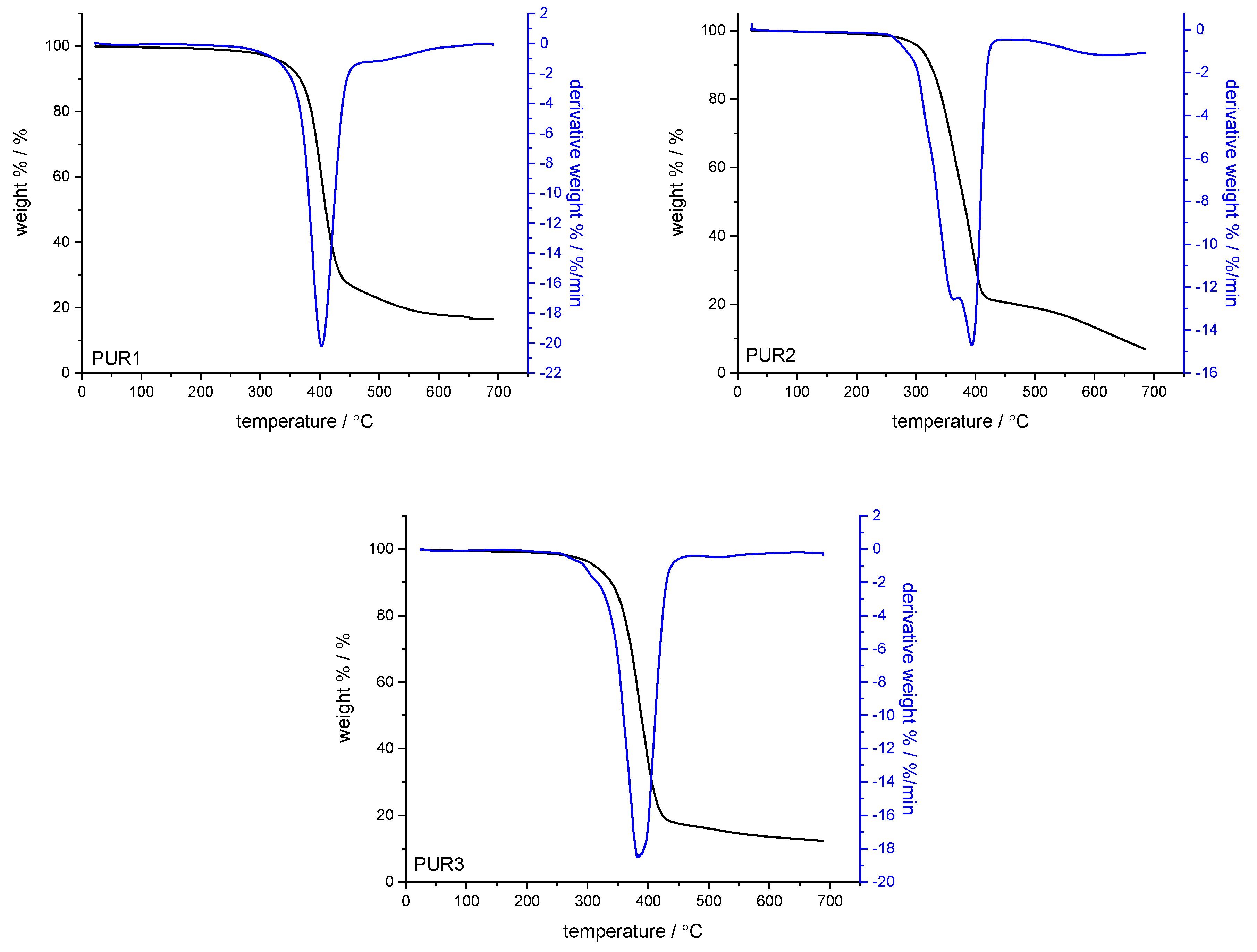
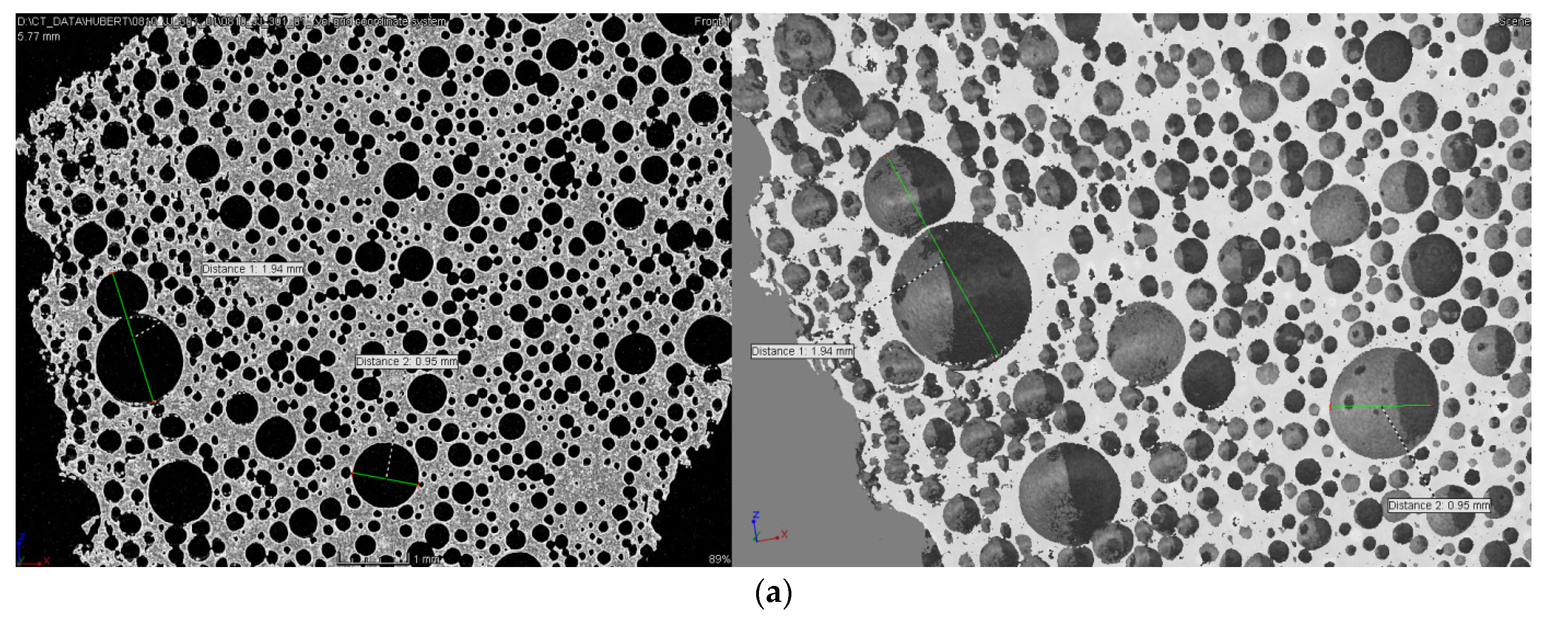
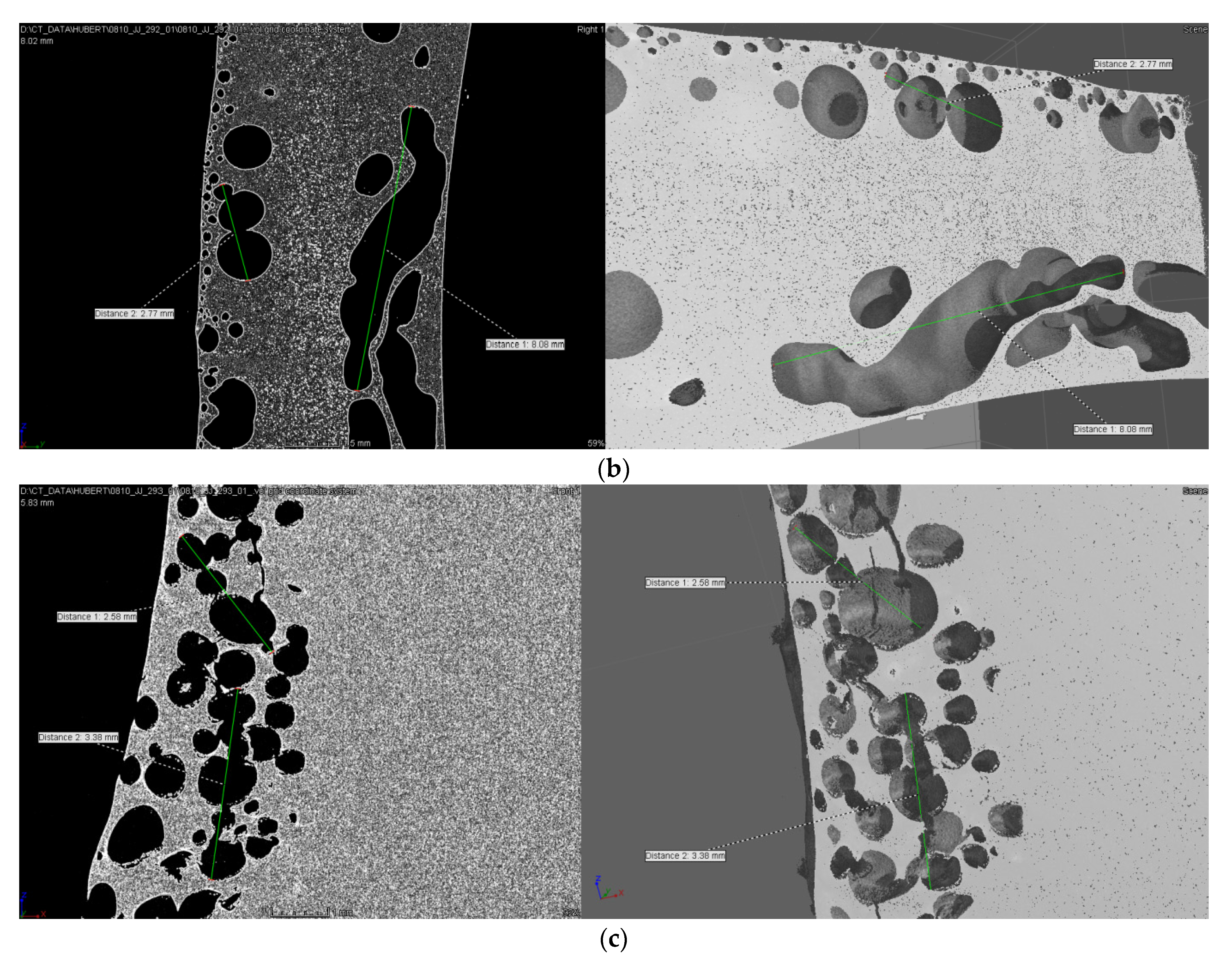
| No. | Monomer | Polymer Fractions (%) | Mn (SEC) | Mw/Mn (SEC) |
|---|---|---|---|---|
| 1 | BO | - | 2600 | 1.08 |
| 2 | IPGE | a(38) | 5800 | 1.03 |
| b(27) | 3200 | 1.04 | ||
| c(35) | 1700 | 1.10 | ||
| 3 | AGE | a(32) | 4800 | 1.05 |
| b(48) | 4000 | 1.08 | ||
| c(20) | 2000 | 1.11 | ||
| 4 | PGE | a(78) | 6000 | 1.08 |
| b(22) | 2100 | 1.10 | ||
| 5 | BGE | a(25) | 4000 | 1.05 |
| b(65) | 2000 | 1.07 | ||
| c(10) | 1200 | 1.09 |
| Name | PEPOs | CO Free ν/cm−1 | CO Bound Non-or ν/cm−1 | CO Bound or ν/cm−1 | R | RPS (1) % | RPD (2) % |
|---|---|---|---|---|---|---|---|
| PUR1 | PBGE | 1730.0 | 1710.1 | 1704.2 | 7.48 | 88 | 12 |
| PUR2 | PBO | 1729.4 | 1715.0 | 1703.9 | 2.66 | 73 | 27 |
| 1747.0 | 1695.2 | ||||||
| 1685.0 | |||||||
| PUR3 | PIPGE | 1744.9 | 1717.8 | 2.37 | 70 | 30 | |
| 1732.1 | 1710.5 |
| Name | T5 wt% | T15 wt% | 1st Decomposition Step | 2nd Decomposition Step | 3rd Decomposition Step | 700 °C Residues | |||
|---|---|---|---|---|---|---|---|---|---|
| [°C] | [°C] | Δ Mass Loss [wt%] | Tmax [°C] | Δ Mass Loss [wt%] | Tmax [°C] | Δ Mass Loss [wt%] | Tmax [°C] | (%) | |
| PUR1 | 338.3 | 378.6 | - | - | 74.57 | 403.0 | - | - | 16.59 |
| PUR2 | 306.4 | 335.5 | 3.41 | 277.9 | 39.22 | 363.5 | 36.10 | 393.9 | 6.95 |
| PUR3 | 311.3 | 352.1 | 6.35 | 309.5 | 75.70 | 381.9 | - | - | 12.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek-Suliga, J.; Grobelny, Z.; Golba, S.; Okła, H.; Bednarczyk, K. New Star-Shaped Polyether-Pentols (PEPOs) for Fabrication of Crosslinked Polyurethanes—Synthesis and Characterization. Polymers 2021, 13, 2150. https://doi.org/10.3390/polym13132150
Jurek-Suliga J, Grobelny Z, Golba S, Okła H, Bednarczyk K. New Star-Shaped Polyether-Pentols (PEPOs) for Fabrication of Crosslinked Polyurethanes—Synthesis and Characterization. Polymers. 2021; 13(13):2150. https://doi.org/10.3390/polym13132150
Chicago/Turabian StyleJurek-Suliga, Justyna, Zbigniew Grobelny, Sylwia Golba, Hubert Okła, and Katarzyna Bednarczyk. 2021. "New Star-Shaped Polyether-Pentols (PEPOs) for Fabrication of Crosslinked Polyurethanes—Synthesis and Characterization" Polymers 13, no. 13: 2150. https://doi.org/10.3390/polym13132150
APA StyleJurek-Suliga, J., Grobelny, Z., Golba, S., Okła, H., & Bednarczyk, K. (2021). New Star-Shaped Polyether-Pentols (PEPOs) for Fabrication of Crosslinked Polyurethanes—Synthesis and Characterization. Polymers, 13(13), 2150. https://doi.org/10.3390/polym13132150







