Synthesis and Biological Evaluation of a Novel Glycidyl Metharcylate/Phaytic Acid-Based on Bagasse Xylan Composite Derivative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Bagasse Xylan
2.3. Preparation of 1-Ally-3-methylimidazole Chloride
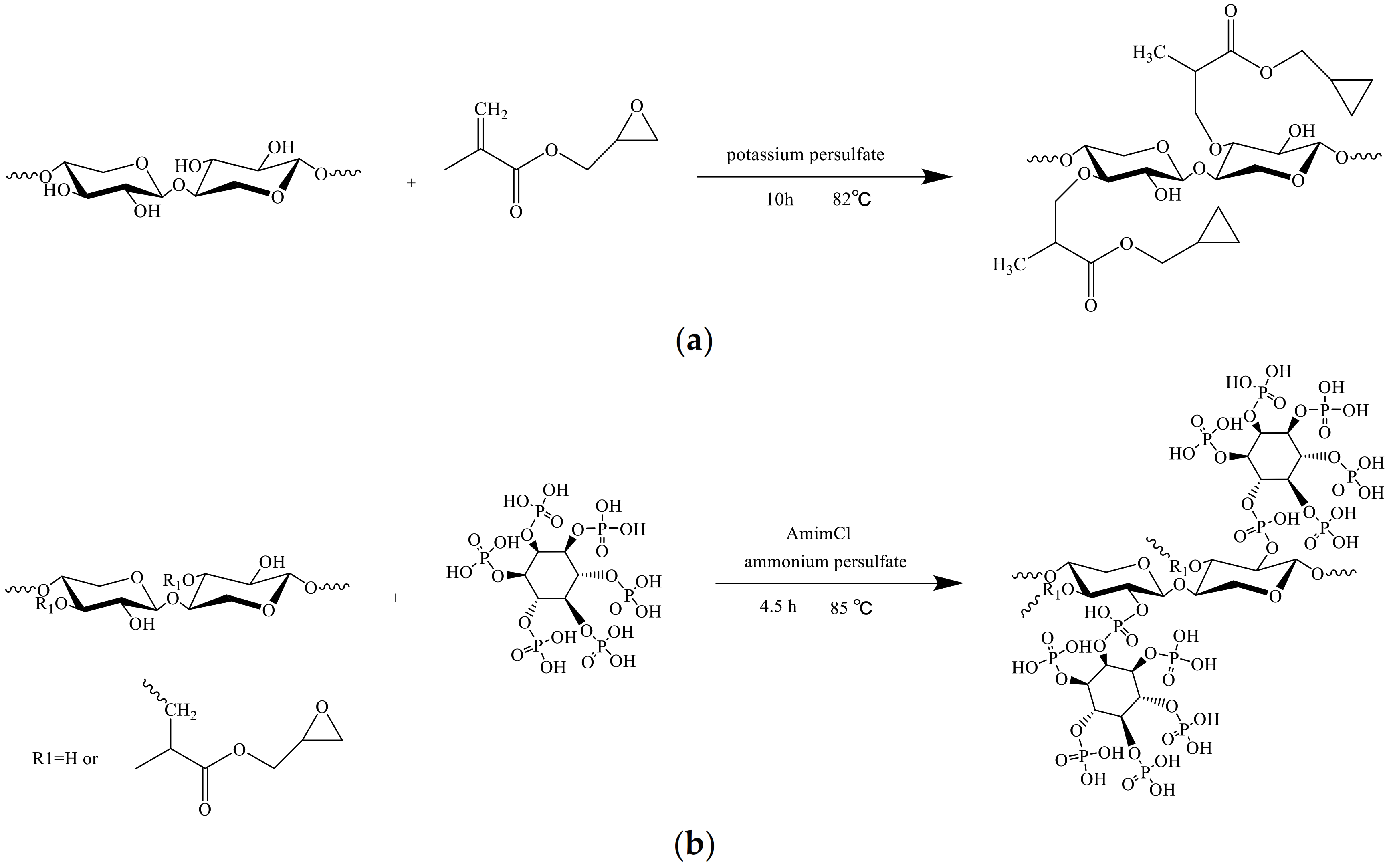
2.4. Graft Copolymerization Modification of Bagasse Xylan with Glycidyl Methacrylate (GMABX)
2.5. Bagasse Xlyan Graft Copolymer Functionalization with Phytic Acid (GMABX-PA)
2.6. Determination of Grafting Rate and Monomer Conversion Rate
2.7. Determination of Degree of Substitution
2.8. Characterization
2.8.1. Fourier Transform Infrared (FTIR)
2.8.2. Thermogravimetric Analysis (TG-DTG)
2.8.3. X-ray Diffraction (XRD)
2.8.4. Scanning Electron Microscope (SEM)
2.8.5. Hydrogen Nuclear Magnetic Resonance (1H NMR)
2.9. Molecular Docking
2.10. Tumor Cell Proliferation Inhibitory Assay
3. Results
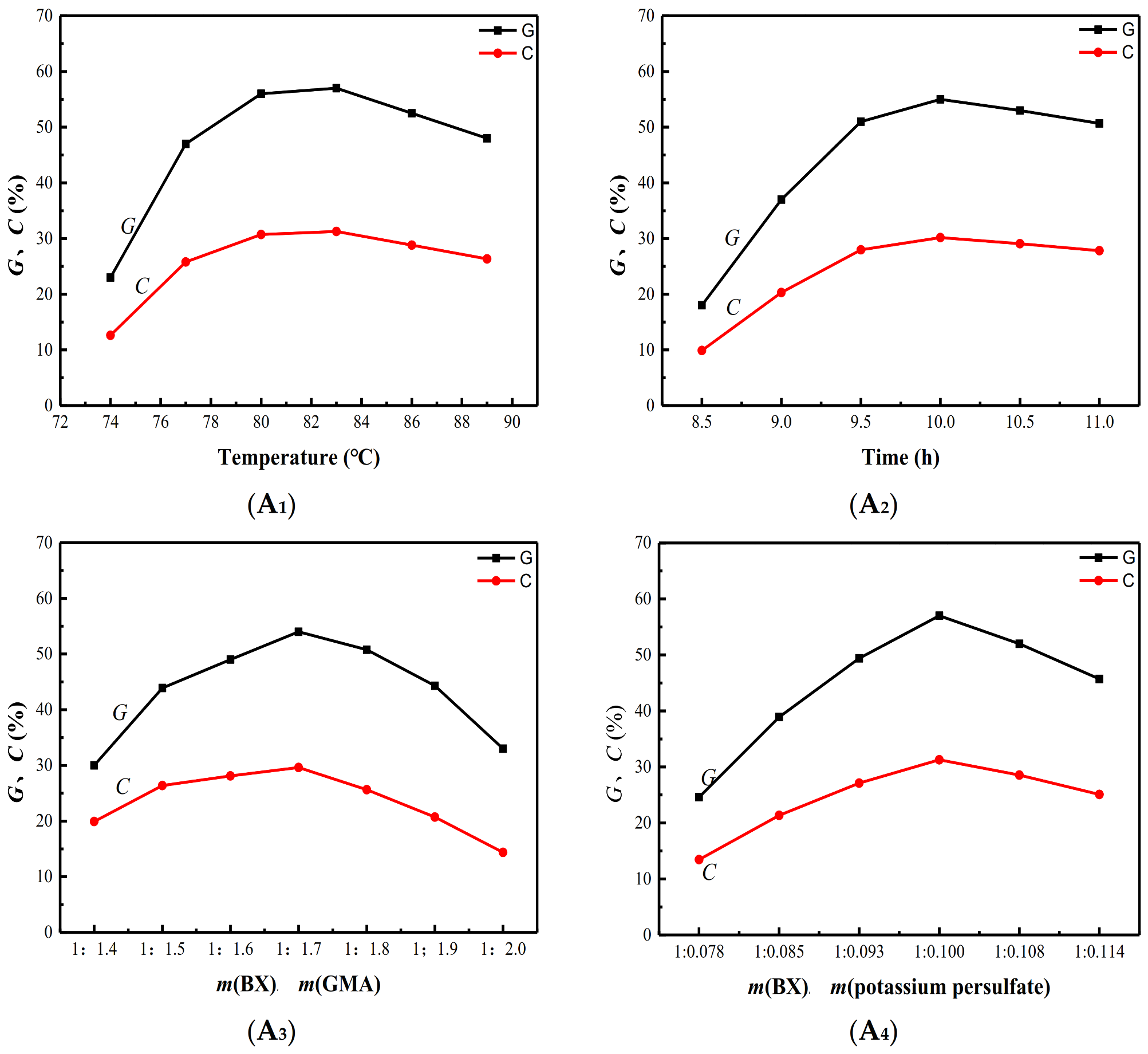
3.1. Single Factor Analysis of Graft Copolymerization Reaction
3.2. Single Factor Analysis of Esterification Reaction
3.3. Structure Analysis
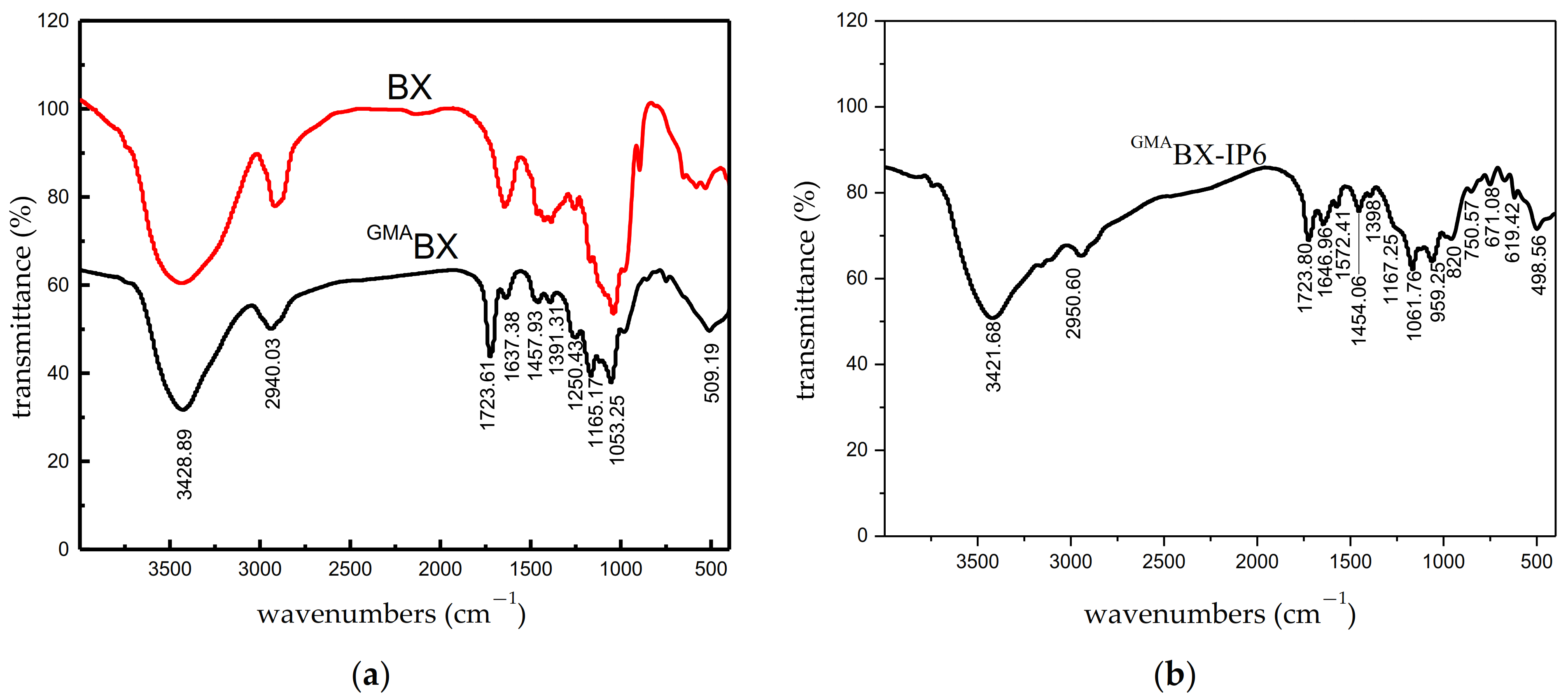
3.3.1. FTIR Analysis of GMABX-PA
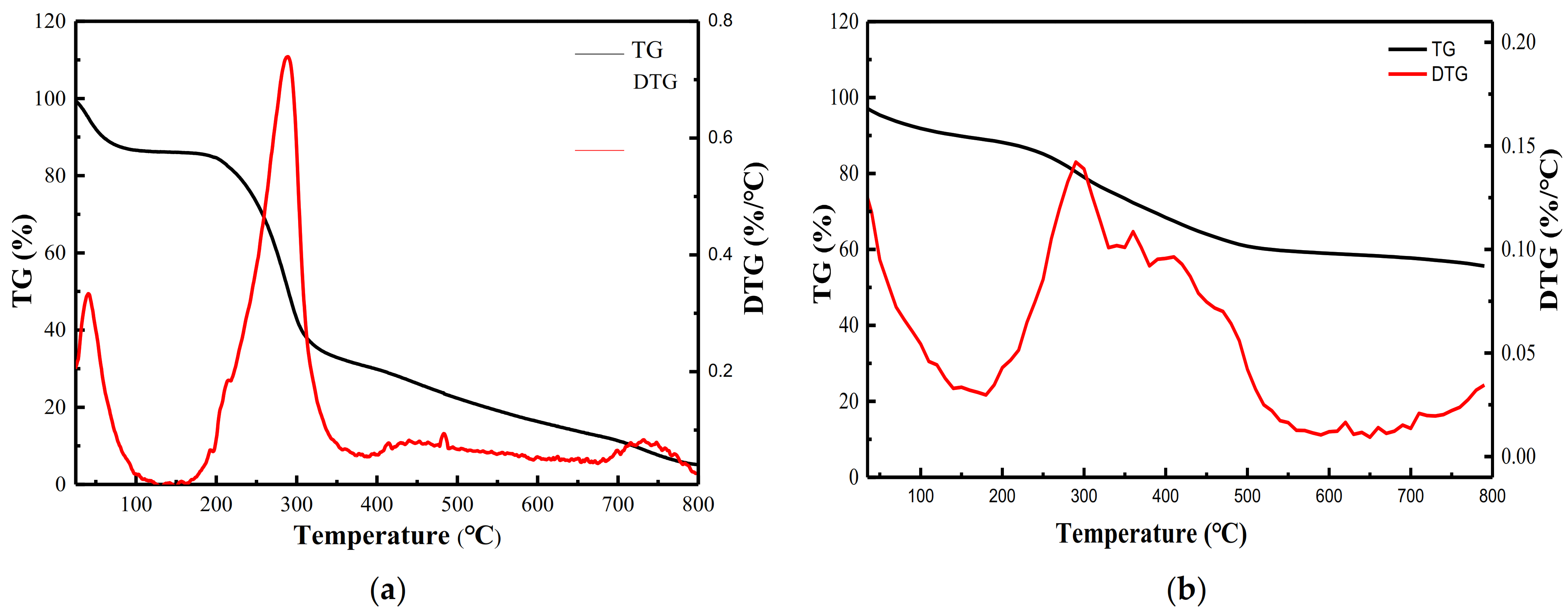
3.3.2. TG-DTG Analysis of GMABX-PA
3.3.3. XRD Analysis of GMABX-PA
3.3.4. SEM Analysis of GMABX-PA
3.3.5. 1H NMR Analysis of GMABX-PA
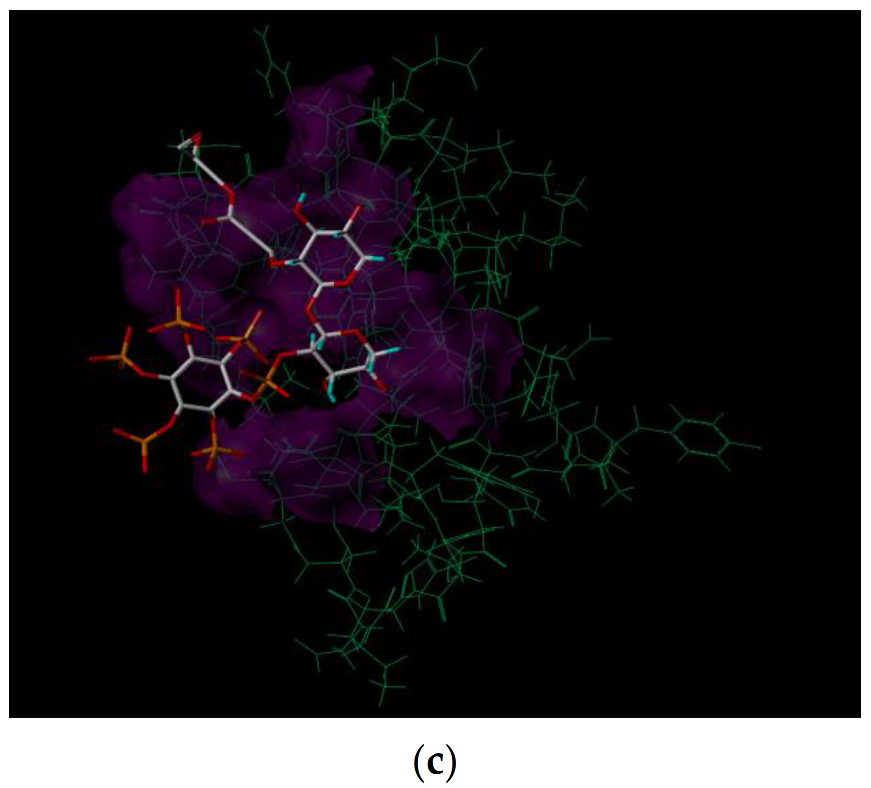
3.4. Molecular Docking Study
3.5. Inhibition Analysis of Tumor Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constantin, M.; Bucatariu, S.; Sacarescu, L.; Daraba, O.M.; Anghelache, M.; Fundueanu, G. Pullulan derivative with cationic and hydrophobic moieties as an appropriate macromolecule in the synthesis of nanoparticles for drug delivery. Biol. Macromol. 2020, 164, 4487–4498. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Mitra, K.; Arumugam, S.K.; Doble, M. Preparation of Curdlan sulphate—Chitosan nanoparticles as a drug carrier to target Mycobacterium smegmatis infected macrophages. Carbohydr. Polym. 2021, 258, 117686. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y. Synthesis of porous starch microgels for the encapsulation, delivery and stabilization of anthocyanins. Food Eng. 2021, 302, 110552. [Google Scholar] [CrossRef]
- Kumamoto, K.; Maeda, T.; Hayakawa, S.; Mustapha, N.; Wang, M.-J.; Shirosaki, Y. Antibacterial Chitosan Nanofiber Thin Films with Bacitracin Zinc Salt. Polymers 2021, 13, 1104. [Google Scholar] [CrossRef]
- Vilas, D.C.; Pal, K.; Sarkar, P. Synthesis, characterization, and antimicrobial efficacy of composite films from guar gum/sago starch/whey protein isolate loaded with carvacrol, citral and carvacrol-citral mixture. Mater. Sci. 2019, 30, 117. [Google Scholar]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef]
- Xu, G.; Luo, Y.; Song, T.; He, B.; Chang, M.; Ren, J. Preparation and Application of a Xylan-based Antibacterial Papermaking Additive to Protect against Escherichia coli Bacteria. Bioresources 2020, 15, 4781–4801. [Google Scholar] [CrossRef]
- Wang, X.H.; Dai, Q.Q.; Zhong, H.Q.; Liu, X.X.; Ren, J.L. Fast-responsive temperature-sensitive hydrogels. Bioresources 2019, 14, 8543–8558. [Google Scholar] [CrossRef]
- Fu, C.; Dong, X.; Wang, S.; Kong, F. Synthesis of nanocomposites using xylan and graphite oxide for remediation of cationic dyes in aqueous solutions. Int. J. Biol. Macromol. 2019, 137, 886–894. [Google Scholar] [CrossRef]
- Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Xylans from Scinaia hatei: Structural featuRes, sulfation and anti-HSV activity. Biol. Macromol. 2010, 46, 173–178. [Google Scholar] [CrossRef]
- Drozd, N.N.; Kuznetsova, S.A.; Malyar, Y.N.; Vasilyeva, N.Y.; Kuznetsov, B.N. Study of the Blood Compatibility of Sulfated Organosolv Lignins Derived from Abies sibirica and Larix sibirica Wood Pulp [J]. Bull. Exp. Biol. Med. 2020, 169, 815–820. [Google Scholar] [CrossRef]
- Fu, G.Q.; Su, L.Y.; Yue, P.P.; Huang, Y.H.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. Syntheses of xylan stearate nanoparticles with loading function from by-products of viscose fiber mills. Cellulose 2019, 26, 7195–7206. [Google Scholar] [CrossRef]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X.; et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Biol. Macromol. 2019, 156, 1217–1233. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, Z.; Hui, L.; Ma, L.; Wang, X.; Zhang, B. Utilization of Xylan-rich Steam Explosion Liquid from Processing of Poplar for Hydrogel Synthesis. Bioresources 2020, 15, 2525–2539. [Google Scholar]
- Li, N.; Hu, Y.J.; Bian, J.; Li, M.-F.; Hao, X.; Peng, F.; Sun, R.-C. Enhanced mechanical performance of xylan-based composite hydrogel via chain extension and semi-interpenetrating networks. Cellulose 2020, 27, 4407–4416. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Dong, C.; An, Y.; Liu, J.; Li, J.; Li, X.; Si, C.; Zhang, M. Synthesis and characterization of temperature/pH dual sensitive hemicellulose-based hydrogels from eucalyptus APMP waste liquor. Bioresources 2020, 15, 2525–2539. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Y.X.; Sun, B.; Zhang, R.F. Improving the wet strength of hemicelluloses based composite films by citric acid crosslinking. Wood Chem. Technol. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Qian, J.X.; Li, H.P.; Zuo, K.; Feng, X.; Hu, Y.; Zhang, S. Methacrylic acid/butyl acrylate onto feruloylated bagasse xylan: Graft copolymerization and biological activity. Mater. Sci. Eng. 2019, 98, 594–601. [Google Scholar] [CrossRef]
- Kong, W.; Gao, C.; Hu, S.; Ren, J.-L.; Zhao, L.-H.; Sun, R.-C. Xylan-modified-based hydrogels with temperature/pH dual sensitivity and controllable drug delivery behavior. Materials 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Simkovic, I.; Kelnar, I.; Uhliariková, I.; Mendichi, R.; Mandalika, A.; Elder, T. Cabroxymethylated-hydroxypropylsulfonated and quaternized xylan derivative films. Cabrohydrate Polym. 2014, 110, 464–471. [Google Scholar] [CrossRef]
- Pczkowski, P.; Gawdzik, B. Studies on preparation, characterization and application of porous functionalized glycidyl methacrylate-based microspheres. Materials 2021, 14, 1438. [Google Scholar] [CrossRef]
- Gad, Y.H.; Elbarbary, A.M. Radiation synthesis of Fe3O4/SiO2/glycidyl methacrylate/acrylonitrile nanocomposite for adsorption of basic violet 7 dye: Kinetic, isotherm, and thermodynamic study. Appl. Orgamomet. Chem. 2021, 4, e6258. [Google Scholar]
- Roshanali, M.; Nodehi, A.; Atai, M. Synthesis and characterization of core-shell nanoparticles and their application in dental resins. Mech. Behav. Biomed. Mater. 2020, 110, 103926. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Li, J.; Li, Y.; Li, N.; Niu, S. A scalable ultrasonic-assisted and foaming combination method preparation polyvinyl alcohol/phytic acid polymer sponge with thermal stability and conductive capability. Ultarasonics Sonochemistry 2018, 42, 18–25. [Google Scholar] [CrossRef]
- Sheikhi, P.; Petrody, S.R.D. Comparative study of xylan extracted by sodium and potassium hydroxides (NaOH and KOH) from bagasse pulp: Characterization and morphological properties. Polym. Environ. 2018, 26, 3710–3717. [Google Scholar] [CrossRef]
- Gomes, T.M.; Sousa, A.R.; Belenkiy, Y.L.; Evtuguin, D.V. Xylan accessibility of bleached eucalypt pulp in alkaline solutions. Holzforschung 2020, 74, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.J.; Shi, Q.S. Transforming Sugarcane bagasse into bioplastics via homogeneous modification with phthalic anhydride in Ionic Liquid. ACS Sustain. Chem. Eng. 2015, 3, 2510–2515. [Google Scholar] [CrossRef]
- Jin, W.U.; Zhang, H.; Zhang, J.; He, J.S. Homogeneous Acetylation and Regioselectivity of Cellulose in a New Ionic Liquid. Chem. J. Chin. Univ.-Chin. 2006, 27, 592–594. [Google Scholar]
- Vismara, E.; Bertolini, G.; Bongio, C.; Massironi, N.; Zarattini, M.; Nanni, D.; Cosentino, C.; Torri, G. Nanocellulose from cotton waste and its glycidyl methacrylate grafting and allylation: Synthesis, characterization and adsorption properties. Nanomaterials 2021, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Pella, M.C.; Simao, A.R.; Lima-Tenorio, M.K.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C.; Rubira, A.F. Magnetic chitosan microgels: Synthesis, characterization, and evaluation of magnetic field effect over the drug release behavior. Carbohydr. Polym. 2020, 250, 116879. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Guan, J.P.; Kiekens, P.; Yang, X.-H.; Tang, R.-C. Preparation and evaluation of an eco-friendly, reactive, and phytic acid-based flame retardant for wool. React. Funct. Polym. 2019, 134, 58–66. [Google Scholar] [CrossRef]
- Zuo, K.; Qian, J.X.; Gong, J.; Zhang, J.; Li, H.; Zhou, G. Synthesis, Characterization, Molecular Docking and Cytotoxicity Studies of Bagasse Xylem Ferulate-Acrylamide/Methyl Methacrylate Composite. Iran. J. Chem. Chem. Eng. 2019, 38, 107–116. [Google Scholar]
- Na, J.H.; Geong, G.A.; Park, H.J.; Lee, C.J. Impact of esterification with malic acid on the structural characteristics and in vitro digestibilities of different starches. Int. J. Biol. Macromol. 2021, 174, 540–548. [Google Scholar] [CrossRef]
- Yuriev, E.; Holien, J.; Ramsland, P.A. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. Mol. Recognit. 2015, 28, 581–604. [Google Scholar] [CrossRef]
- Zaid, A.N.; Shraim, N.; Radwan, A.; Jaradat, N.; Hirzallah, S.; Issa, I.; Khraim, A. Does gastroplus support similarity and dissimilarity factors of in vitro-in vivo prediction in biowaiver studies? A lower strength amlodipine as a model drug. Drug Res. 2018, 68, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.H.; Shao, Y.J.; Yuan, Y. Pinocembrin flavanone inhibits cell viability in PC-3 human prostate cancer by inducing cellular apoptosis, ROS production and cell cycle arrest. Acta Pharm. 2021, 71, 669–678. [Google Scholar] [CrossRef]
- Sun, X.M.; Hou, L.T.; Qiu, C.P.; Kong, B.H. MiR-501 promotes tumor proliferation and metastasis by targeting HOXD10 in endometrial cancer. Cell. Mol. Biol. Lett. 2021, 26, 1. [Google Scholar] [CrossRef]

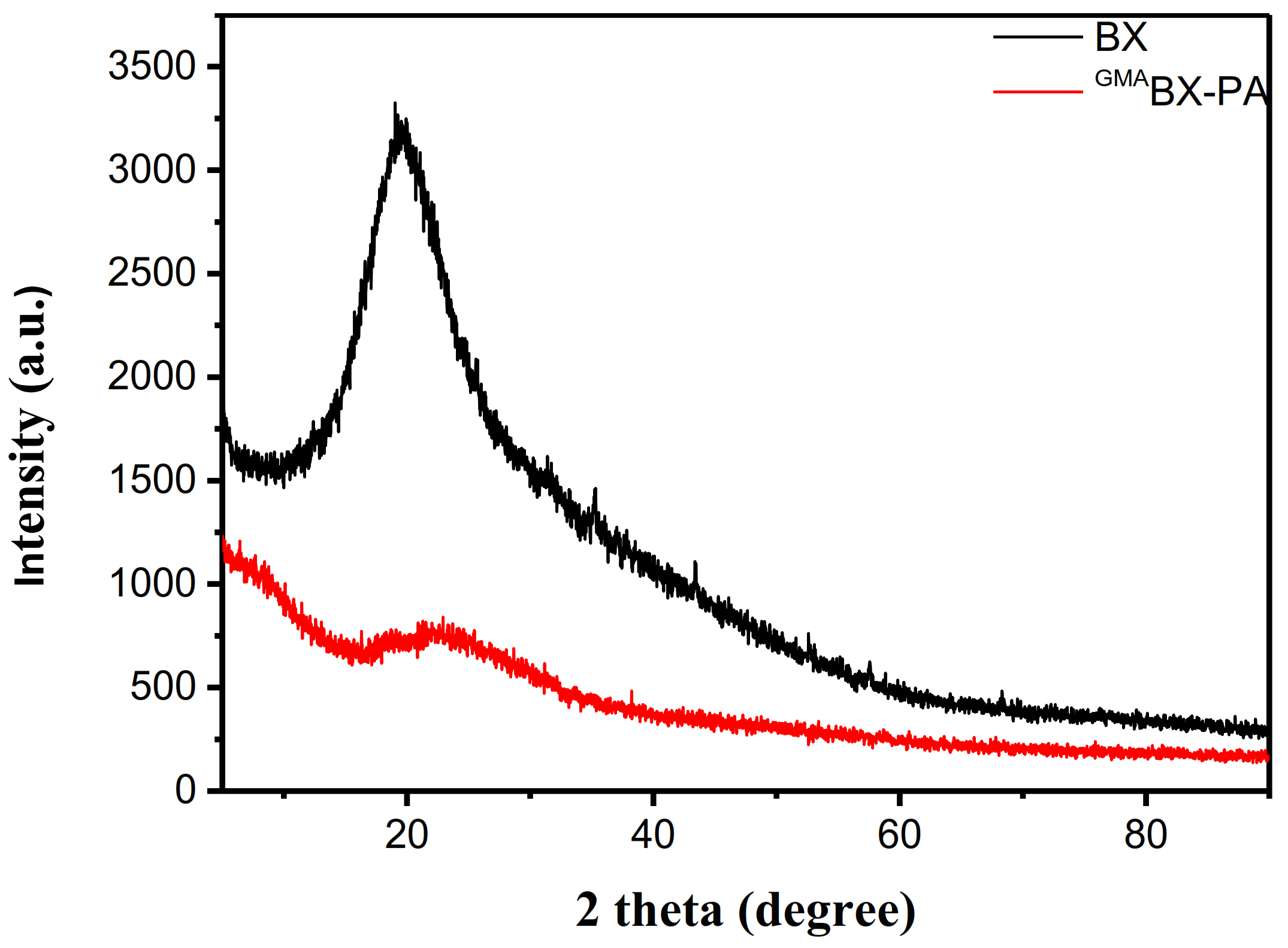


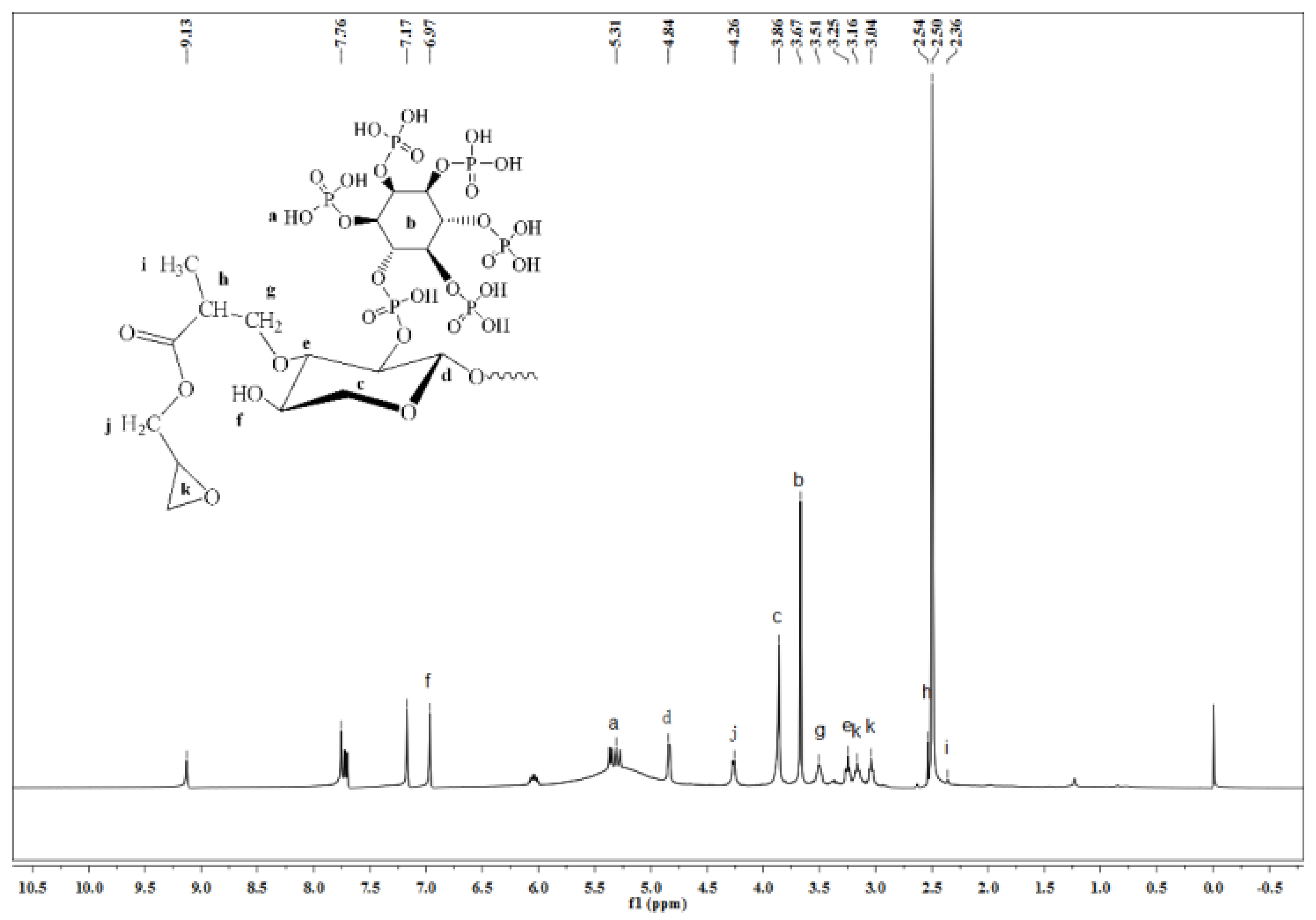
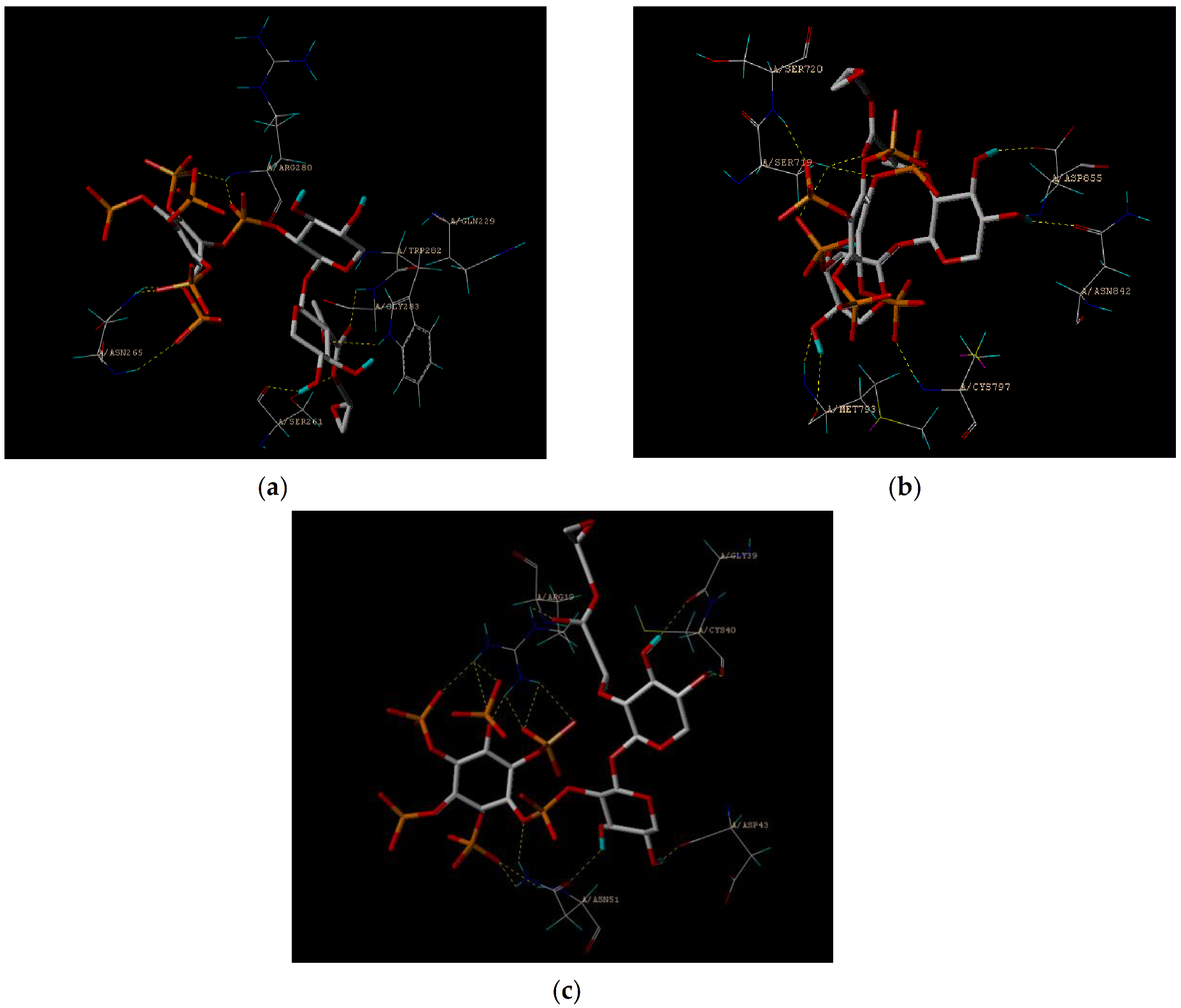
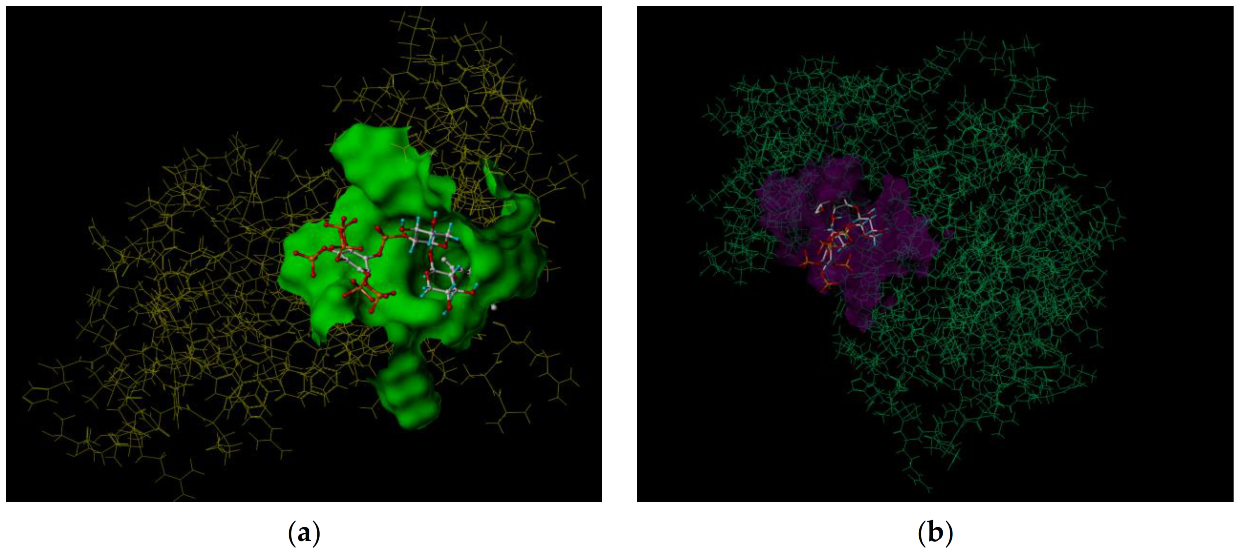
| No. | Pos. [°2Th.] | FWHM [°2Th.] | Area [cts*°2Th.] | d-Spacing | Height [cts] | Rel.Int. [%] |
|---|---|---|---|---|---|---|
| 1 | 10.2107 | 0.1692 | 360.3 | 7.89281 | 2129.5 | 47.9 |
| 2 | 16.4689 | 0.1033 | 138.1 | 7.09903 | 2005.05 | 45.11 |
| 3 | 20.4251 | 0.7768 | 2302.04 | 4.56975 | 4445.27 | 100 |
| 4 | 23.4479 | 0.0199 | 30.69 | 3.50022 | 2309.12 | 51.95 |
| 5 | 31.9634 | 0.5492 | 608.36 | 2.80004 | 1661.48 | 37.38 |
| No. | Pos. [°2Th.] | FWHM [°2Th.] | Area [cts*°2Th.] | d-Spacing | Height [cts] | Rel.Int. [%] |
|---|---|---|---|---|---|---|
| 1 | 6.4121 | 0.992 | 891.65 | 13.78465 | 1348.2 | 100 |
| 2 | 7.5506 | 0.9055 | 745.31 | 11.70863 | 1234.66 | 91.58 |
| 3 | 10.0881 | 0.304 | 233.69 | 8.76842 | 1153.2 | 85.54 |
| 4 | 16.4347 | 0.4085 | 190.36 | 5.39385 | 698.94 | 51.84 |
| 5 | 20.9515 | 0.4507 | 265.83 | 4.24013 | 884.81 | 65.63 |
| 6 | 23.1301 | 1.3628 | 1115.96 | 3.84544 | 818.87 | 60.74 |
| 7 | 28.658 | 0.5834 | 269.56 | 3.11503 | 693.04 | 51.41 |
| 8 | 31.1086 | 0.1764 | 64.96 | 2.875 | 552.27 | 40.96 |
| 9 | 38.1321 | 0.09 | 13.98 | 2.36007 | 233 | 17.28 |
| 10 | 47.2061 | 0.09 | 14.85 | 1.92543 | 165 | 12.24 |
| 11 | 51.8601 | 0.09 | 12.96 | 1.76305 | 144 | 10.68 |
| 12 | 68.0321 | 0.09 | 6.03 | 1.37809 | 67 | 4.97 |
| 13 | 75.9101 | 0.09 | 6.03 | 1.25347 | 67 | 4.97 |
| 14 | 76.2481 | 0.09 | 3.15 | 1.24875 | 35 | 2.6 |
| Total Score | D-Score | PMF-Score | G-Score | CHEM-Score | CScore | Global-Score | ||
|---|---|---|---|---|---|---|---|---|
| 5XNV | BX | 5.18 | −182.58 | −43.83 | −235.78 | −11.02 | 4 | 4 |
| GMABX-PA | 5.16 | −685.39 | −119.04 | −357.38 | 14.14 | 5 | 5 | |
| 2EB2 | BX | 8.60 | −96.13 | −108.38 | −141.77 | −17.66 | 4 | 4 |
| GMABX-PA | 8.89 | −456.02 | −179.09 | −450.19 | 9.44 | 5 | 5 | |
| 2M6N | BX | 5.44 | −105.00 | −22.04 | −136.06 | −15.61 | 5 | 5 |
| GMABX-PA | 5.83 | −453.63 | −72.50 | −268.03 | −9.05 | 5 | 5 |
| Sample | Mass Concentration/(μg/mL) | Inhibition Ratio/% | |||
|---|---|---|---|---|---|
| BEAS-2B | NCI-H460 | MGC80-3 | MDA-MB-231 | ||
| BX | 100 | 1.93 ± 0.48 | 4.62 ± 2.79 | 2.02 ± 0.57 | 3.16 ± 0.94 |
| 50 | 1.72 ± 0.76 | 0.71 ± 0.22 | 0.24 ± 0.08 | 2.35 ± 0.72 | |
| 20 | −0.26 ± 0.57 | −0.24 ± 0.19 | −0.15 ± 0.13 | 1.62 ± 0.47 | |
| 10 | −2.94 ± 0.35 | −2.97 ± 1.43 | −2.99 ± 1.11 | 0.98 ± 0.33 | |
| 1 | −5.61 ± 0.23 | −4.33 ± 2.03 | −3.27 ± 1.61 | 0.17 ± 0.12 | |
| GMABX-PA | 100 | 1.72 ± 0.59 | 29.68 ± 4.45 | 11.37 ± 3.29 | 20.65 ± 3.82 |
| 50 | 0.96 ± 0.82 | 25.21 ± 4.56 | 7.39 ± 2.03 | 16.02 ± 3.71 | |
| 20 | 0.89 ± 0.77 | 17.02 ± 3.29 | 4.18 ± 1.37 | 13.29 ± 1.86 | |
| 10 | −1.32 ± 0.39 | 9.77 ± 1.62 | 2.38 ± 0.24 | 10.41 ± 2.65 | |
| 1 | −2.54 ± 0.43 | 2.95 ± 1.13 | 0.97 ± 0.28 | 7.16 ± 1.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, H.; Liu, H.; Zou, Z.; Xie, C. Synthesis and Biological Evaluation of a Novel Glycidyl Metharcylate/Phaytic Acid-Based on Bagasse Xylan Composite Derivative. Polymers 2021, 13, 2084. https://doi.org/10.3390/polym13132084
Li M, Li H, Liu H, Zou Z, Xie C. Synthesis and Biological Evaluation of a Novel Glycidyl Metharcylate/Phaytic Acid-Based on Bagasse Xylan Composite Derivative. Polymers. 2021; 13(13):2084. https://doi.org/10.3390/polym13132084
Chicago/Turabian StyleLi, Mingkun, Heping Li, Hongli Liu, Zhiming Zou, and Chaoyu Xie. 2021. "Synthesis and Biological Evaluation of a Novel Glycidyl Metharcylate/Phaytic Acid-Based on Bagasse Xylan Composite Derivative" Polymers 13, no. 13: 2084. https://doi.org/10.3390/polym13132084
APA StyleLi, M., Li, H., Liu, H., Zou, Z., & Xie, C. (2021). Synthesis and Biological Evaluation of a Novel Glycidyl Metharcylate/Phaytic Acid-Based on Bagasse Xylan Composite Derivative. Polymers, 13(13), 2084. https://doi.org/10.3390/polym13132084





