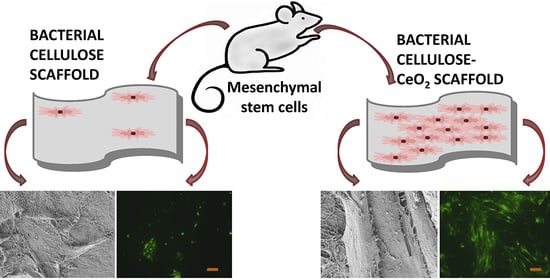
Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Cellulose
2.3. CeO2 Nanoparticles
2.4. Thin Film Fabrication
2.5. Film Characterisation Techniques
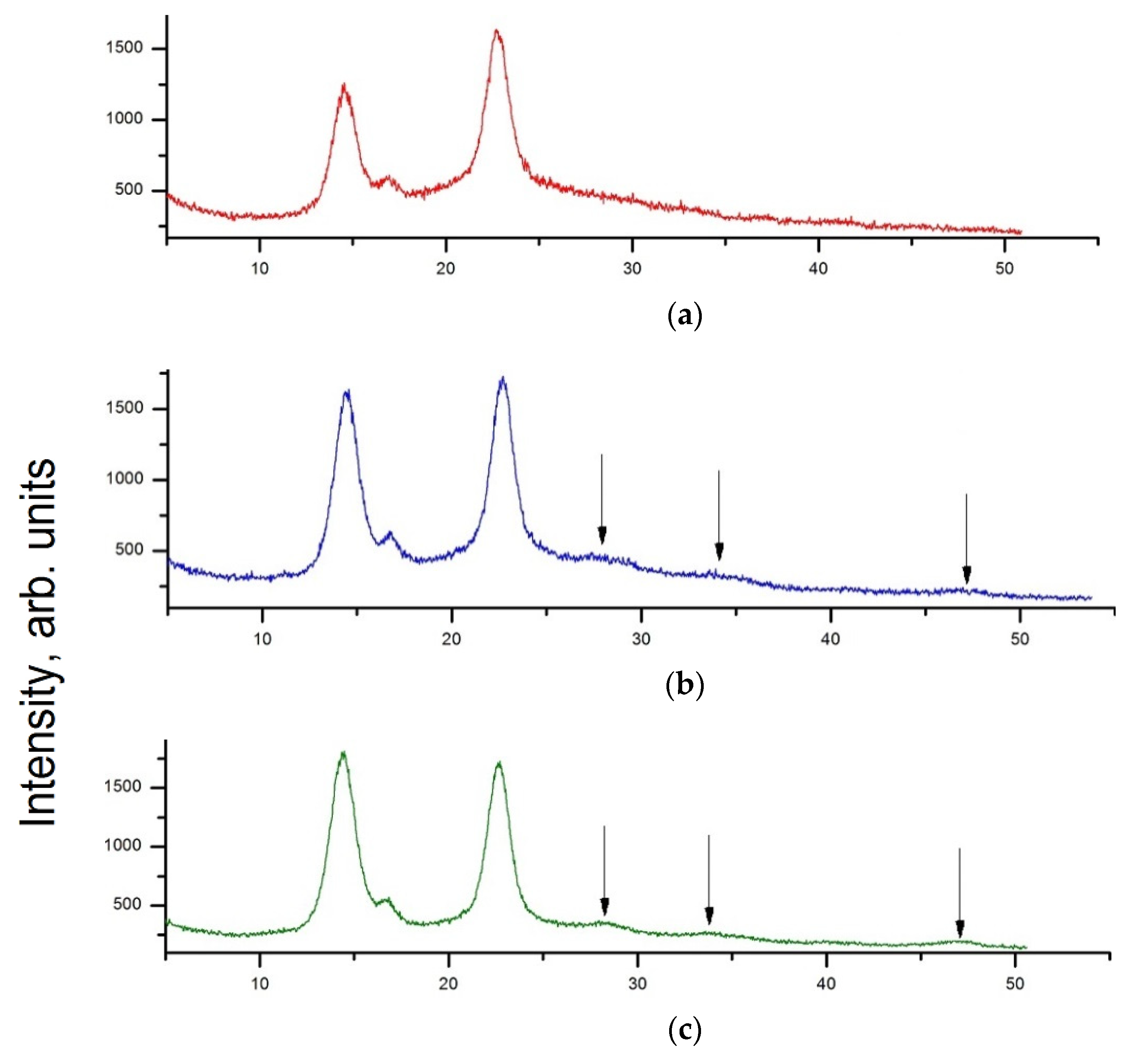
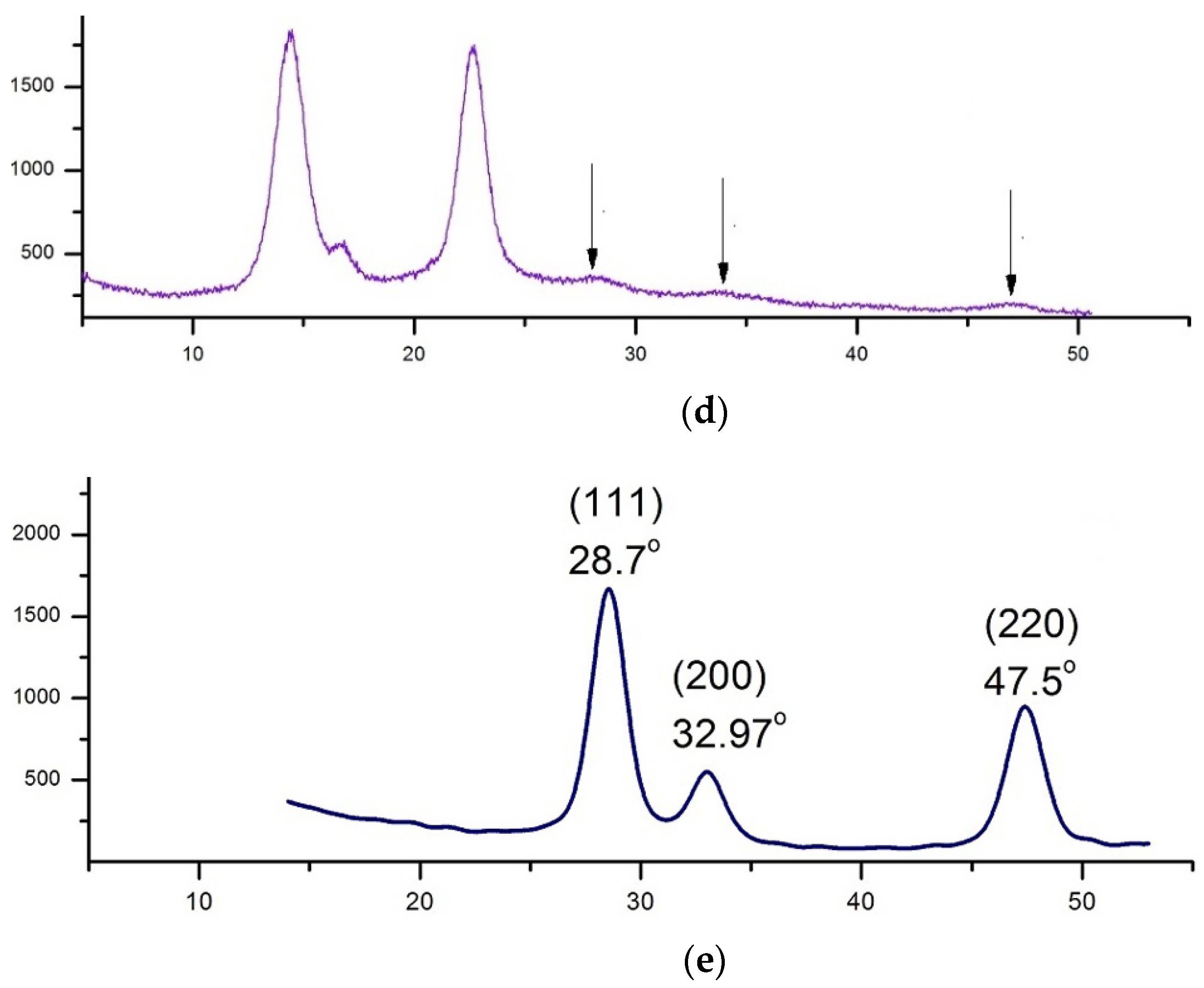
XRD Analysis
2.6. Scanning Electron Microscopy
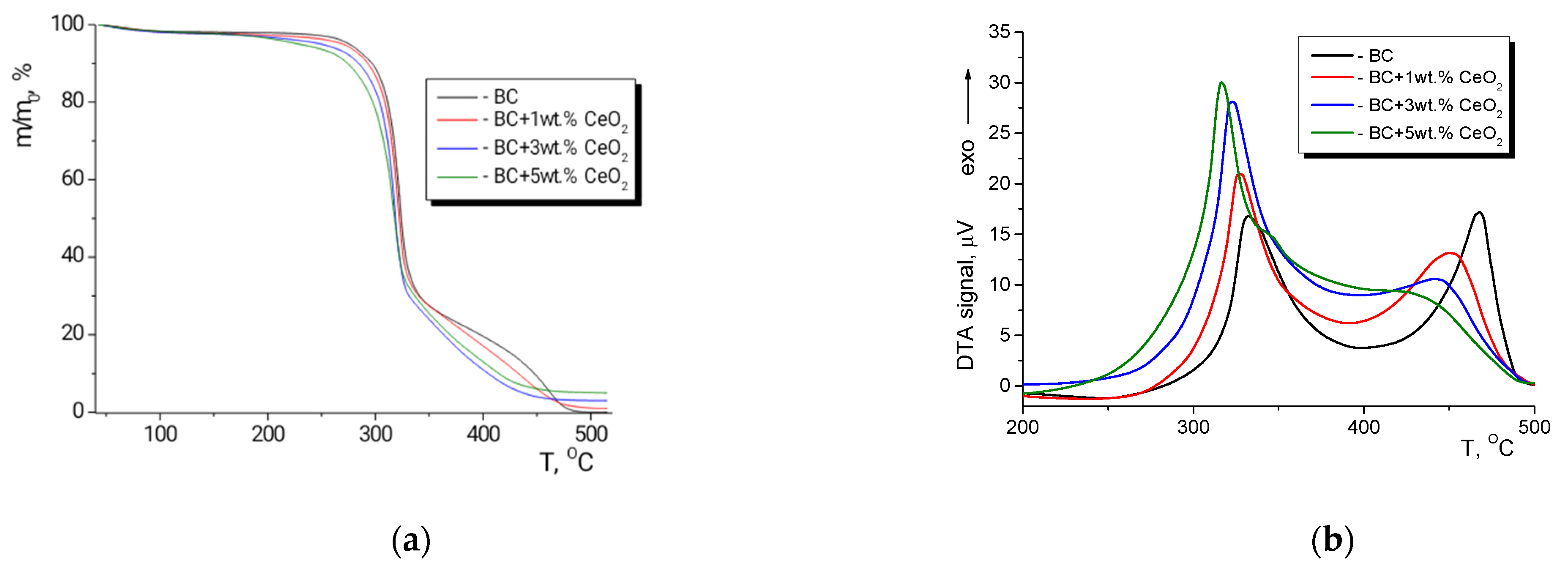
2.7. Thermal Analysis
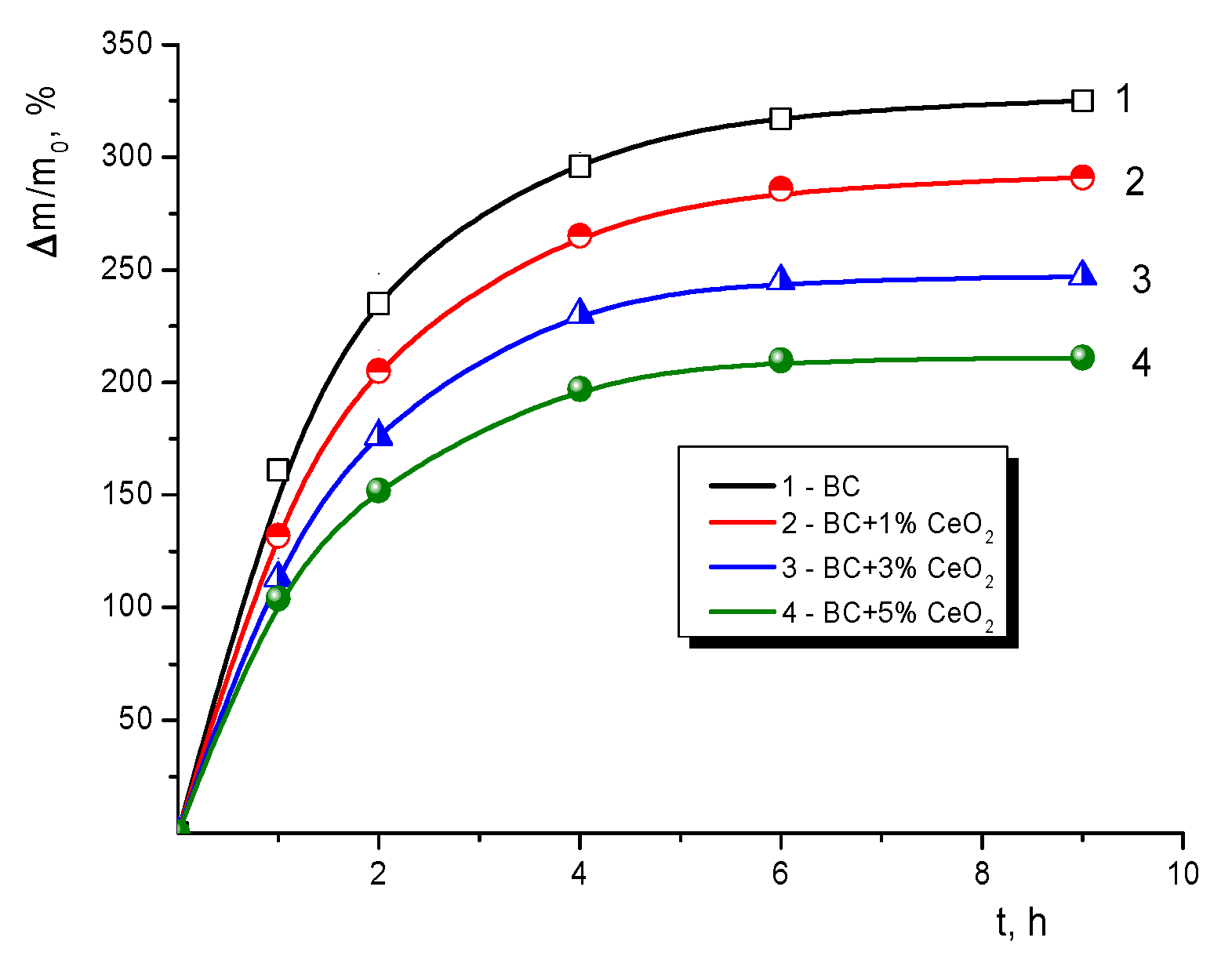
2.8. Swelling Properties
2.9. Mechanical Properties
2.10. Cell Proliferation Control Techniques
Cell Culture
2.11. Fluorescent Microscopy
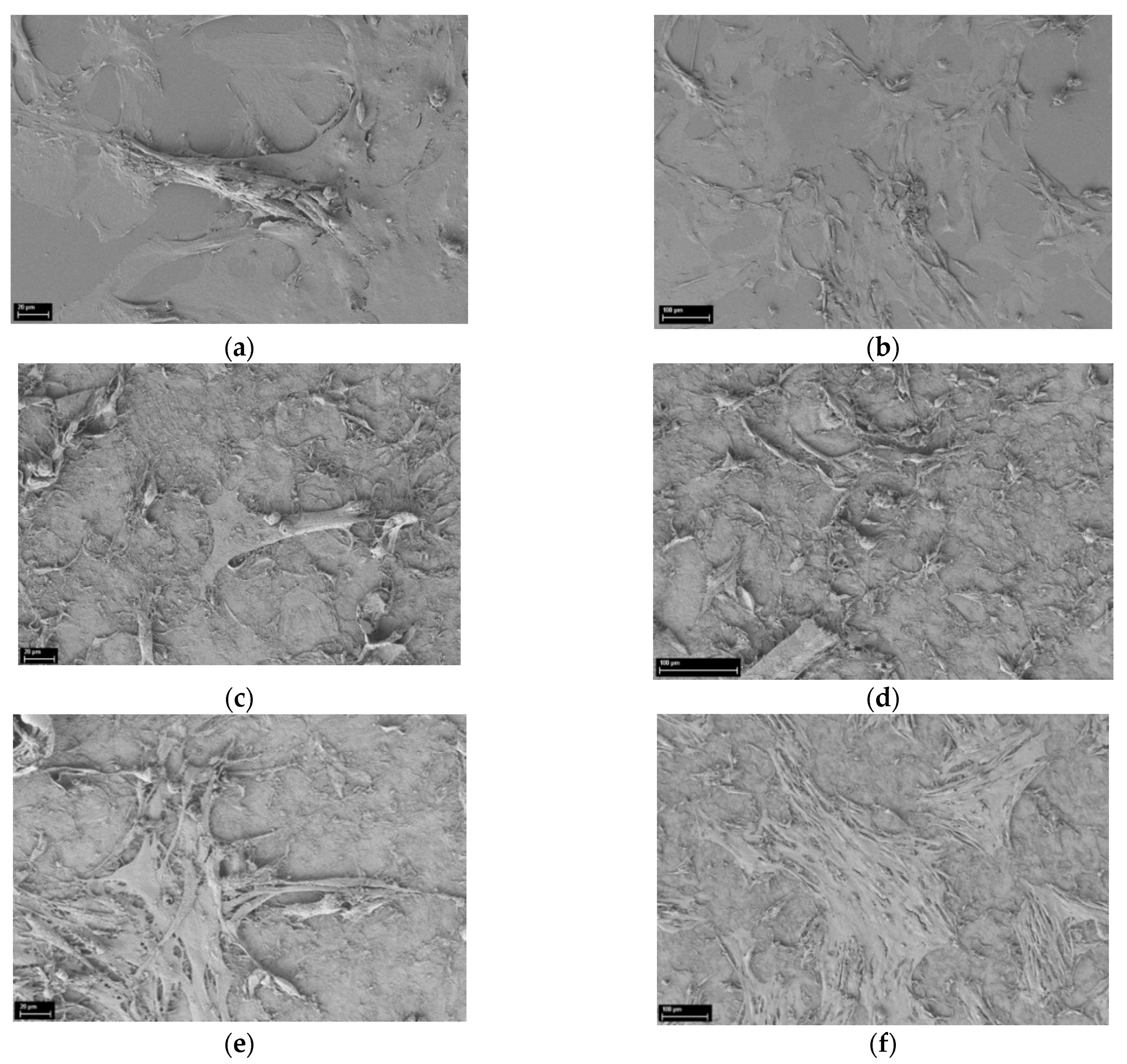
2.12. Scanning Electron Microscopy
2.13. Reverse Transcription Polymerase Chain Reaction (PCR-RT)
3. Results
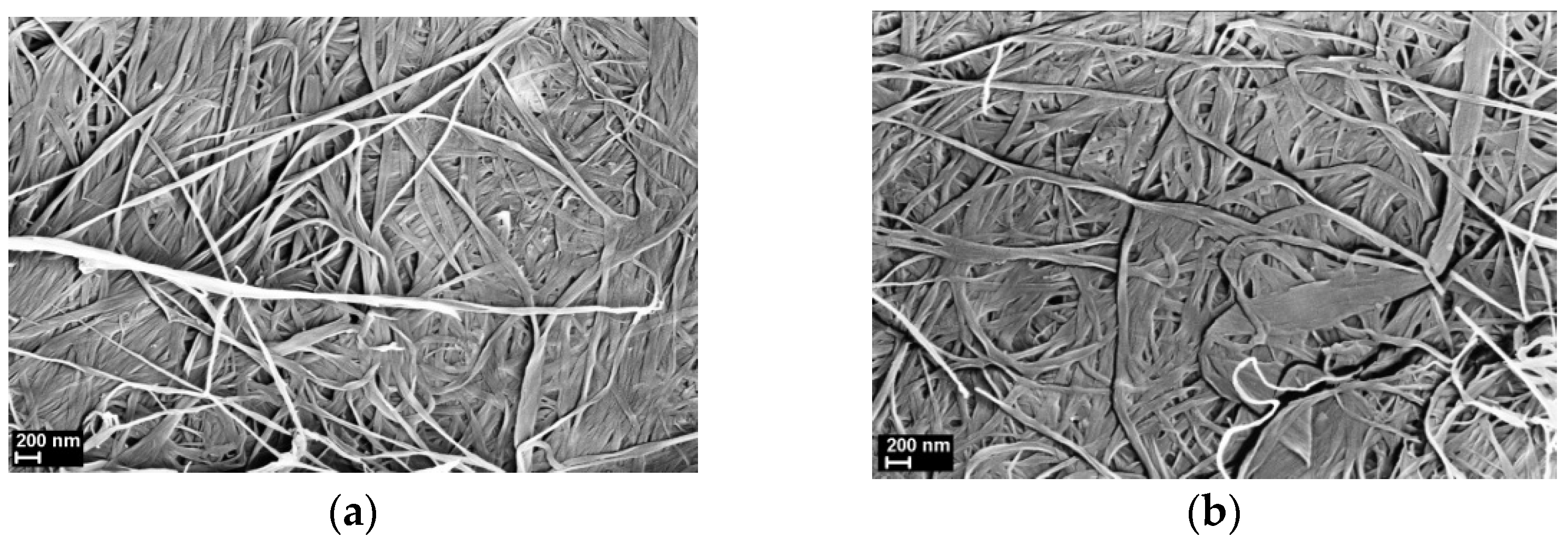
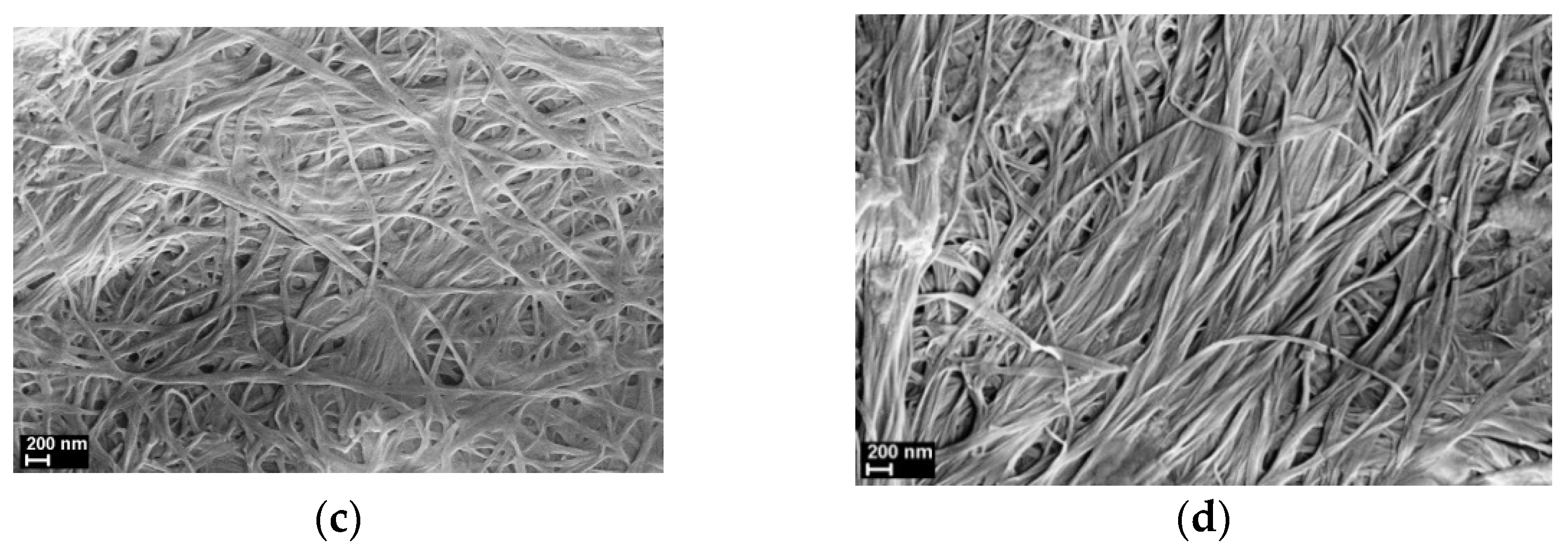
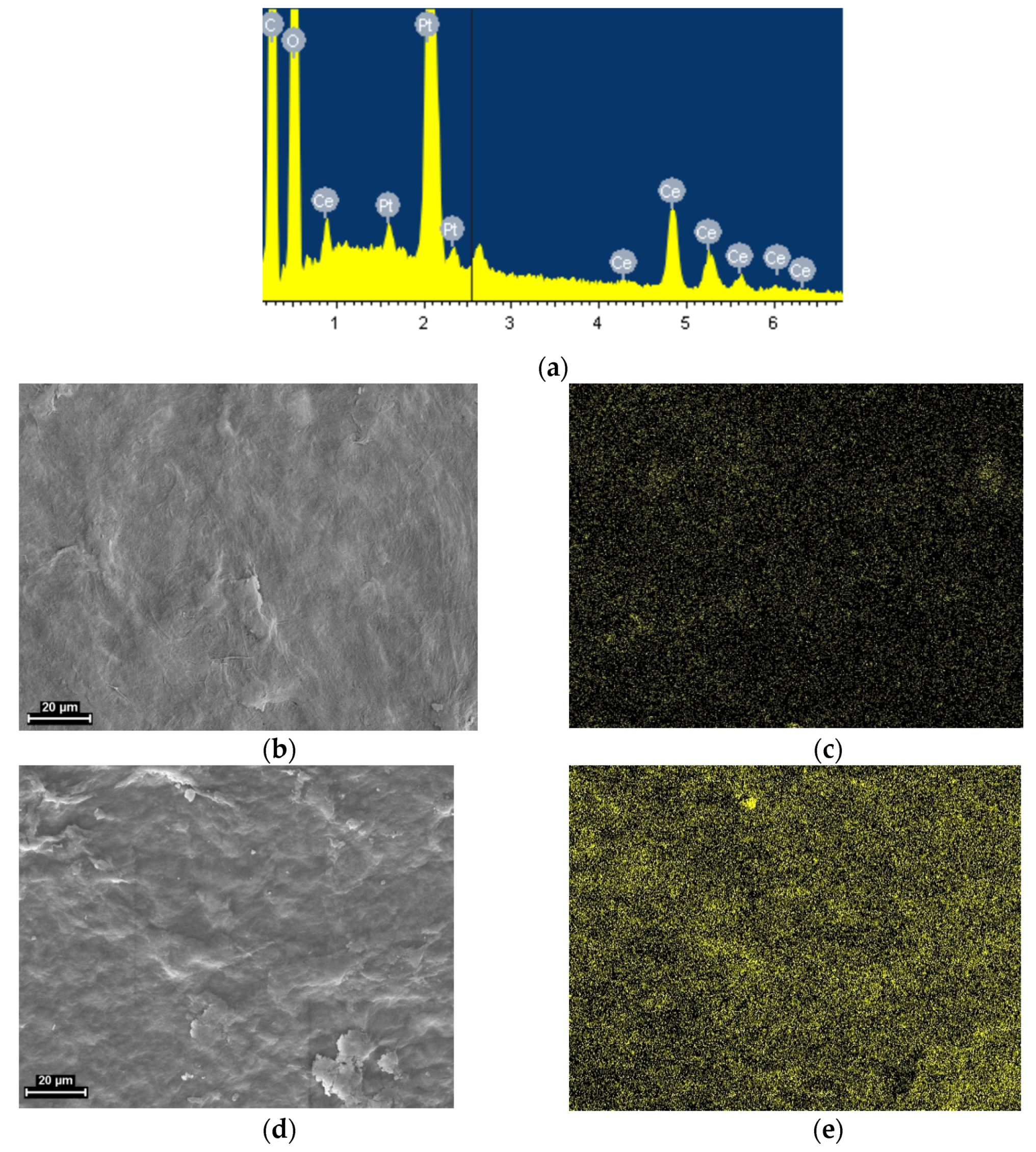
3.1. SEM
3.2. XRD Analysis
3.3. EDX Analysis
3.4. Thermal Analysis
3.5. Mechanical Properties
3.6. Ability to Swell in Water
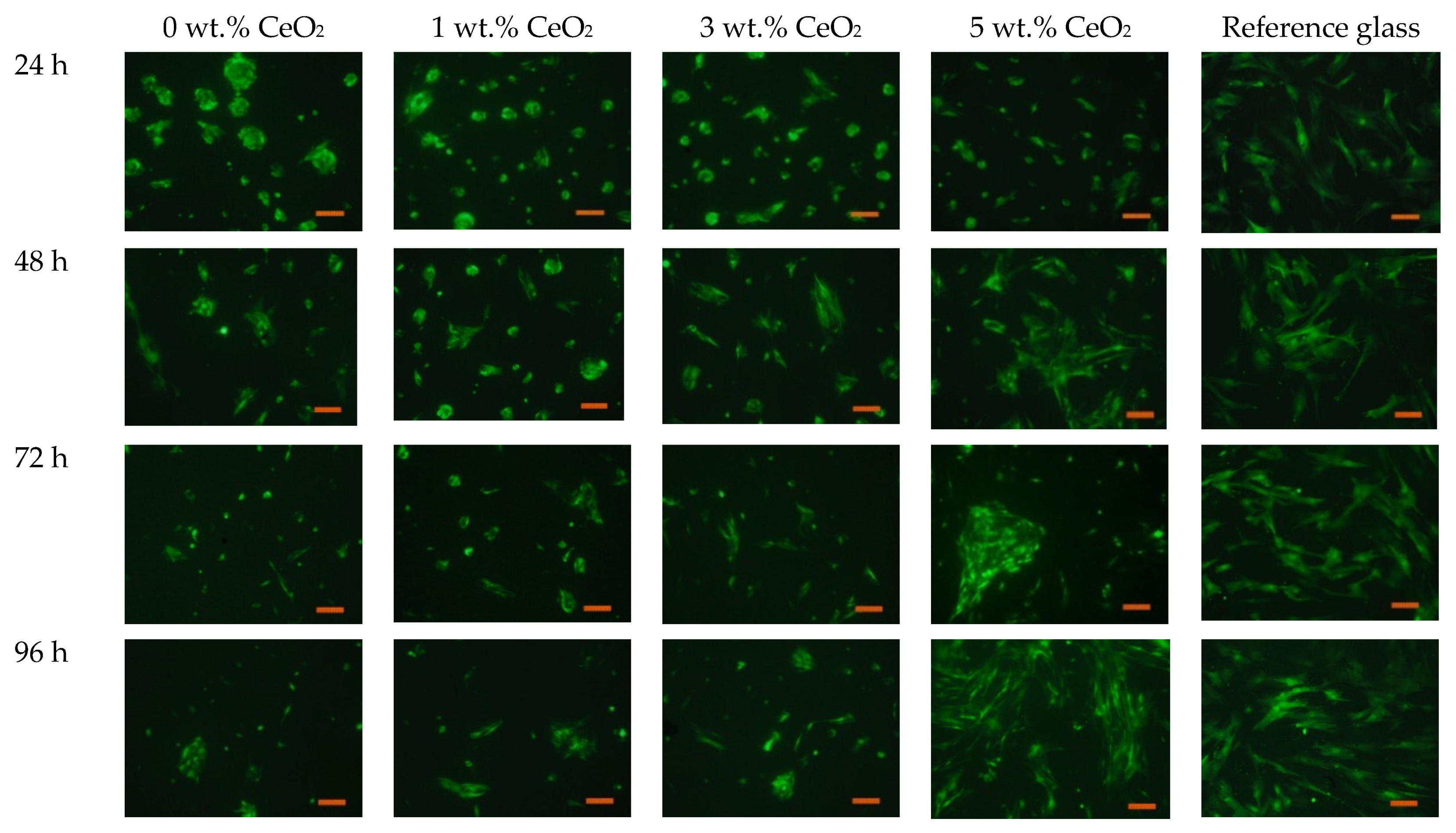
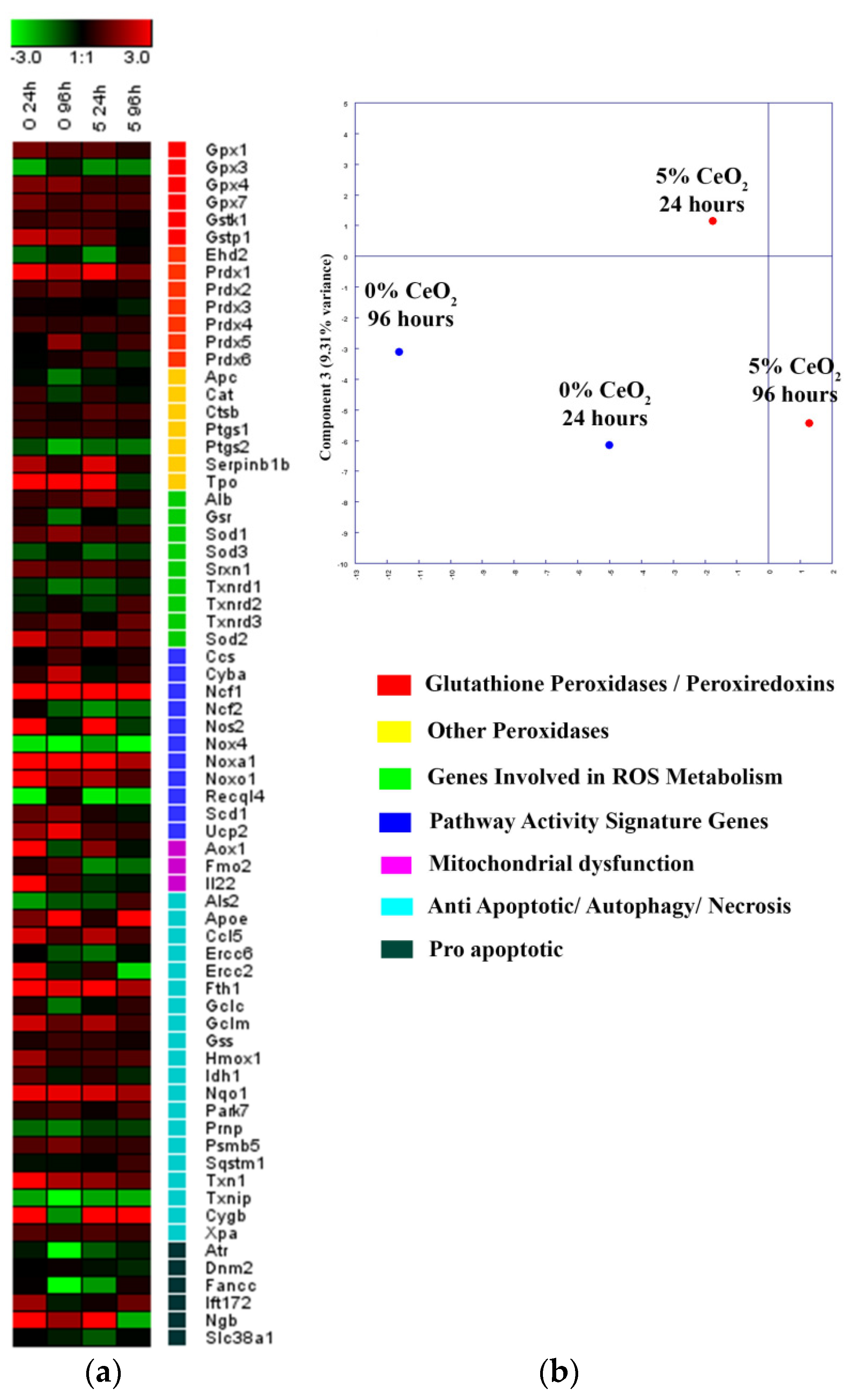
3.7. MSC Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, O.J. (Ed.) Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, 1st ed.; Springer: Cham, Switzerland, 2016; Volume 271, ISBN 978-3-319-79875-2. [Google Scholar]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Mitrofanov, R.Y.; Budaeva, V.V.; Sakovich, G.V. Preparation and properties of bacterial cellulose gel films. Chem. Sustain. Dev. 2010, 18, 503–508. [Google Scholar]
- Gromovykh, T.I.; Sadykova, V.S.; Lutcenko, S.V.; Dmitrenok, A.S.; Feldman, N.B.; Danilchuk, T.N.; Kashirin, V.V. Bacterial cellulose synthesized by Gluconacetobacter hansenii for medical applications. Appl. Biochem. Microbiol. 2017, 53, 60–67. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Feng, X.; Ullah, N.; Wang, X.; Sun, X.; Li, C.; Bai, Y.; Chen, L.; Li, Z. Characterization of Bacterial Cellulose by Gluconacetobacter hanseniiCGMCC 3917. J. Food Sci. 2015, 80, E2217–E2227. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Nainggolan, H.; Gea, S.; Bilotti, E.; Peijs, T.; Hutagalung, S.D. Mechanical and thermal properties of bacterial-cellulose-fibre-reinforced Mater-Bi® bionanocomposite. Beilstein J. Nanotechnol. 2013, 4, 325–329. [Google Scholar] [CrossRef]
- Buyanov, A.; Gofman, I.; Revel’Skaya, L.; Khripunov, A.; Tkachenko, A. Anisotropic swelling and mechanical behavior of composite bacterial cellulose–poly(acrylamide or acrylamide–sodium acrylate) hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 102–111. [Google Scholar] [CrossRef]
- Smyslov, R.Y.; Ezdakova, K.V.; Kopitsa, G.P.; Khripunov, A.K.; Bugrov, A.N.; Tkachenko, A.; Angelov, B.; Pipich, V.; Szekely, N.K.; Baranchikov, A.; et al. Morphological structure of Gluconacetobacter xylinus cellulose and cellulose-based organic-inorganic composite materials. J. Phys. Conf. Ser. 2017, 848, 12017. [Google Scholar] [CrossRef]
- Shah, P. Advancement in Packaging Film Using Microcrystalline Cellulose and TiO2. Am. J. Polym. Sci. Technol. 2017, 3, 97. [Google Scholar] [CrossRef]
- Bouadjela, S.; Abdoune, F.Z.; Benmoussa, N.; Mechernene, L.; Rahmoun, K.; Maschke, U. Effect of titanium dioxide nanoparticles on polymer network formation. Spectrosc. Lett. 2017, 50, 522–527. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Mallakpour, S.; Darvishzadeh, M. Ultrasonic treatment as recent and environmentally friendly route for the synthesis and characterization of polymer nanocomposite having PVA and biosafe BSA-modified ZnO nanoparticles. Polym. Adv. Technol. 2018, 29, 2174–2183. [Google Scholar] [CrossRef]
- Shcherbakov, A.; Reukov, V.; Yakimansky, A.; Krasnopeeva, E.; Ivanova, O.; Popov, A.; Ivanov, V. CeO2 Nanoparticle-Containing Polymers for Biomedical Applications: A Review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Mao, C.; Meng, M.; Liu, S.; Tian, Y.; Yu, L.; Sun, B.; Li, C.M. Fabrication of CeO2 nanoparticle-modified silk for UV protection and antibacterial applications. J. Colloid Interface Sci. 2014, 435, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-Q.; Kuang, C.-X.; Zhong, M.-Q.; Shi, Y.-Q.; Chen, F. Synthesis, characterization and UV-shielding property of polystyrene-embedded CeO2 nanoparticles. Opt. Mater. 2013, 35, 2710–2715. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Tiefensee, F.; Becker-Willinger, C.; Heppe, G.; Herbeck-Engel, P.; Jakob, A. Nanocomposite cerium oxide polymer matching layers with adjustable acoustic impedance between 4 MRayl and 7 MRayl. Ultrasonics 2010, 50, 363–366. [Google Scholar] [CrossRef]
- Jia, R.-P.; Wang, C.-F.; Zheng, K.-S.; He, X.-Y.; Huang, M.-S. Preparation, characterization, and properties of CeO2/thermoplastic polyurethane nanocomposites. J. Reinf. Plast. Compos. 2015, 34, 1090–1098. [Google Scholar] [CrossRef]
- Shang, Z.; Lü, C.; Lü, X.; Gao, L. Studies on syntheses and properties of novel CeO2/polyimide nanocomposite films from Ce(Phen)3 complex. Polymers 2007, 48, 4041–4046. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B. Chapter 12—Interaction of nanoceria with microorganisms. In Nanobiomaterials in Antimicrobial Therapy; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 419–450. ISBN 978-0-323-42864-4. [Google Scholar]
- Zholobak, N.M.; Shcherbakov, A.B.; Vitukova, E.O.; Yegorova, A.V.; Scripinets, Y.V.; Leonenko, I.I.; Baranchikov, A.; Antonovich, V.P.; Ivanov, V. Direct monitoring of the interaction between ROS and cerium dioxide nanoparticles in living cells. RSC Adv. 2014, 4, 51703–51710. [Google Scholar] [CrossRef]
- Jiao, X.; Song, H.; Zhao, H.; Bai, W.; Zhang, L.; Lv, Y. Well-redispersed ceria nanoparticles: Promising peroxidase mimetics for H2O2 and glucose detection. Anal. Methods 2012, 4, 3261–3267. [Google Scholar] [CrossRef]
- Popov, A.L.; Zaichkina, S.I.; Popova, N.R.; Rozanova, O.M.; Romanchenko, S.P.; Ivanova, O.S.; Smirnov, A.A.; Mironova, E.V.; Selezneva, I.I.; Ivanov, V.K. Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv. 2016, 6, 106141–106149. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Spivak, M.; Ivanov, V. Advances and prospects of using nanocrystalline ceria in cancer theranostics. Russ. J. Inorg. Chem. 2014, 59, 1556–1575. [Google Scholar] [CrossRef]
- Selvaraj, V.; Nepal, N.; Rogers, S.; Manne, N.D.P.K.; Arvapalli, R.; Rice, K.M.; Asano, S.; Fankhanel, E.; Ma, J.J.; Shokuhfar, T.; et al. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials 2015, 59, 160–171. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, D.-Y.; Yin, J.-H.; Xu, H.; Zhang, C.-Q.; Ke, Q.-F.; Gao, Y.-S.; Guo, Y.-P. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication 2019, 11, 025012. [Google Scholar] [CrossRef]
- You, M.; Li, K.; Xie, Y.; Huang, L.; Zhen, X. The Effects of Cerium Valence States at Cerium Oxide Coatings on the Responses of Bone Mesenchymal Stem Cells and Macrophages. Biol. Trace Elem. Res. 2017, 179, 259–270. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. Cerium and cerium oxide: A brief introduction. In Cerium Oxide (CeO₂): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Ikram, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Engineered regenerated bacterial cellulose scaffolds for application in in vitro tissue regeneration. RSC Adv. 2015, 5, 84565–84573. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef]
- Zhong, M.; Li, J.; Tang, A.; Zhang, Q.; Ji, D.; Peng, M.; Zhang, R.; Xiong, G.; Wan, Y.; Fan, H. A facile green approach for fabricating bacterial cellulose scaffold with macroporous structure and cell affinity. J. Bioact. Compat. Polym. 2019, 34, 442–452. [Google Scholar] [CrossRef]
- Buyanov, A.L.; Revel’Skaya, L.G.; Kuznetzov, Y.P.; Shestakova, A.S. Cellulose-poly(acrylamide or acrylic acid) interpenetrating polymer network membranes for the pervaporation of water-ethanol mixtures. J. Appl. Polym. Sci. 1998, 69, 761–769. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’Yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Kriegner, D.; Matěj, Z.; Kužel, R.; Holý, V. Powder diffraction in Bragg–Brentano geometry with straight linear detectors. J. Appl. Crystallogr. 2015, 48, 613–618. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Ustinovich, K.B.; Khamova, T.V.; Eneyskaya, E.V.; Gorshkova, Y.E.; Tsvigun, N.V.; Burdakov, V.S.; Verlov, N.A.; Zinovev, E.V.; Asadulaev, M.S.; et al. Crystal and Supramolecular Structure of Bacterial Cellulose Hydrolyzed by Cellobiohydrolase from Scytalidium Candidum 3C: A Basis for Development of Biodegradable Wound Dressings. Materials 2020, 13, 2087. [Google Scholar] [CrossRef]
- Balta-Calleja, F.J.; Vonk, C.G. X-ray Scattering of Synthetic Polymers; Polymer Science Library 8; Elsevier: Amsterdam, The Netherlands, 1989; 317p. [Google Scholar]
- Carltonbird, M.; Eaimsumang, S.; Pongstabodee, S.; Boonyuen, S.; Smith, S.M.; Luengnaruemitchai, A. Effect of the exposed ceria morphology on the catalytic activity of gold/ceria catalysts for the preferential oxidation of carbon monoxide. Chem. Eng. J. 2018, 344, 545–555. [Google Scholar] [CrossRef]
- Gao, M.; Wu, W.H.; Wu, F.C. Thermal degradation and smoke suspension of cotton cellulose modified with THPC and its lanthanide metal complexes. J. Therm. Anal. Calorim. 2009, 98, 245–251. [Google Scholar] [CrossRef]
- Silva, M.; Lopes, O.; Colodette, J.L.; Porto, A.; Rieumont, J.; Chaussy, D.; Belgacem, M.N.; Silva, G.G. Characterization of three non-product materials from a bleached eucalyptus kraft pulp mill, in view of valorising them as a source of cellulose fibres. Ind. Crop. Prod. 2008, 27, 288–295. [Google Scholar] [CrossRef]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-Charge-Dependent Cell Localization and Cytotoxicity of Cerium Oxide Nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. Biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Metal Oxides Series; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; IntechOpen: London, UK, 2011. [Google Scholar]

| Sample | τ5, °C | τ10, °C |
|---|---|---|
| BC | 286 | 302 |
| BC + 1 wt.%CeO2 | 283 | 298 |
| BC + 3 wt.%CeO2 | 272 | 291 |
| BC + 5 wt.% CeO2 | 261 | 281 |
| Composition | E, GPa | Eswollen/Edry 1 | σb, MPa | εb, % |
|---|---|---|---|---|
| BC | 5.5 ± 0.3 | 101 ± 5 | 3.6 ± 0.2 | |
| BC, swollen | 0.17 ± 0.02 | 0.032 | 4.2 ± 0.4 | 2.9 ± 0.2 |
| BC + 1 wt.% CeO2 | 5.8 ± 0.3 | 96 ± 3 | 2.7 ± 0.2 | |
| BC + 1 wt.% CeO2, swollen | 0.20 ± 0.02 | 0.034 | 3.6 ± 0.3 | 2.6 ± 0.3 |
| BC + 3 wt.% CeO2 | 6.3 ± 0.3 | 107 ± 3 | 3.3 ± 0.2 | |
| BC + 3 wt.% CeO2, swollen | 0.24 ± 0.02 | 0.038 | 4.4 ± 0.4 | 2.2 ± 0.1 |
| BC + 5 wt.% CeO2 | 6.9 ± 0.4 | 113 ± 5 | 3.3 ± 0.2 | |
| BC + 5 wt.% CeO2, swollen | 0.30 ± 0.03 | 0.044 | 5.8 ± 0.3 | 2.8 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gofman, I.V.; Nikolaeva, A.L.; Khripunov, A.K.; Ivan’kova, E.M.; Shabunin, A.S.; Yakimansky, A.V.; Romanov, D.P.; Popov, A.L.; Ermakov, A.M.; Solomevich, S.O.; et al. Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation. Polymers 2021, 13, 1999. https://doi.org/10.3390/polym13121999
Gofman IV, Nikolaeva AL, Khripunov AK, Ivan’kova EM, Shabunin AS, Yakimansky AV, Romanov DP, Popov AL, Ermakov AM, Solomevich SO, et al. Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation. Polymers. 2021; 13(12):1999. https://doi.org/10.3390/polym13121999
Chicago/Turabian StyleGofman, Iosif V., Alexandra L. Nikolaeva, Albert K. Khripunov, Elena M. Ivan’kova, Anton S. Shabunin, Alexander V. Yakimansky, Dmitriy P. Romanov, Anton L. Popov, Artem M. Ermakov, Sergey O. Solomevich, and et al. 2021. "Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation" Polymers 13, no. 12: 1999. https://doi.org/10.3390/polym13121999
APA StyleGofman, I. V., Nikolaeva, A. L., Khripunov, A. K., Ivan’kova, E. M., Shabunin, A. S., Yakimansky, A. V., Romanov, D. P., Popov, A. L., Ermakov, A. M., Solomevich, S. O., Bychkovsky, P. M., Baranchikov, A. E., & Ivanov, V. K. (2021). Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation. Polymers, 13(12), 1999. https://doi.org/10.3390/polym13121999