Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications
Abstract
1. Introduction
2. Materials and Methods
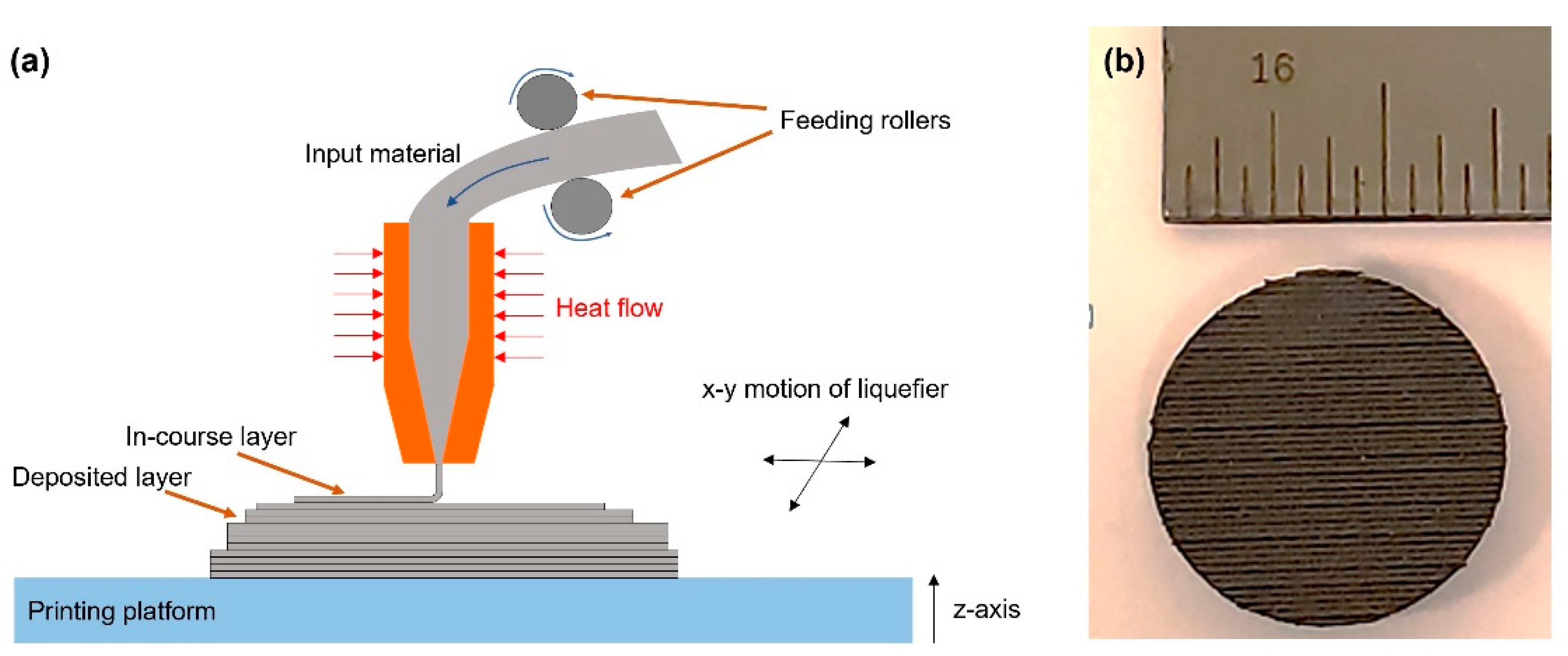
2.1. Materials and Processing
2.2. Structural Analysis
2.3. Thermal Analysis
2.4. Nanoindentation Testing
3. Results and Discussion
3.1. Structure Analysis
3.2. Thermal Analysis
3.3. Surface Morphology
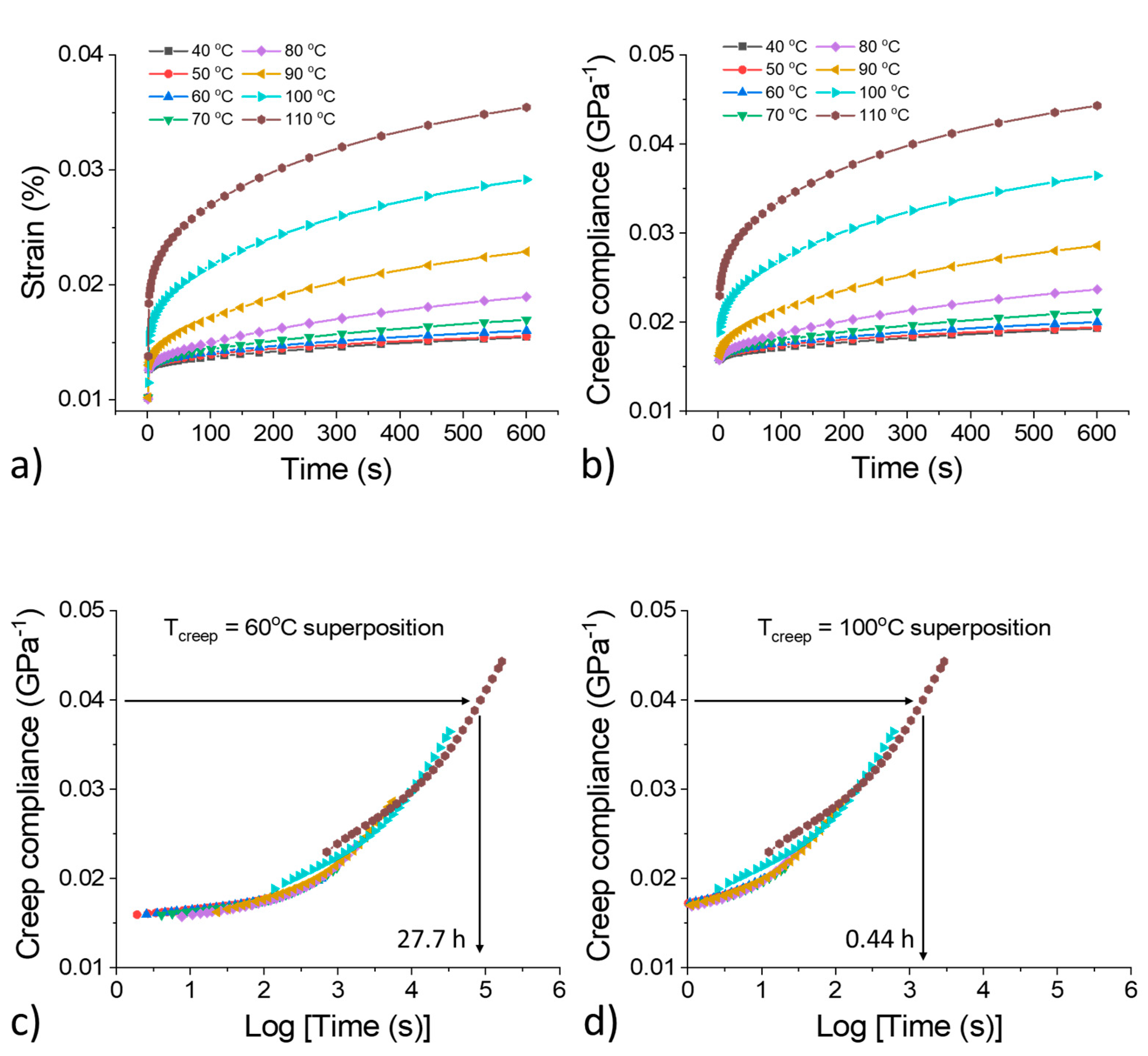
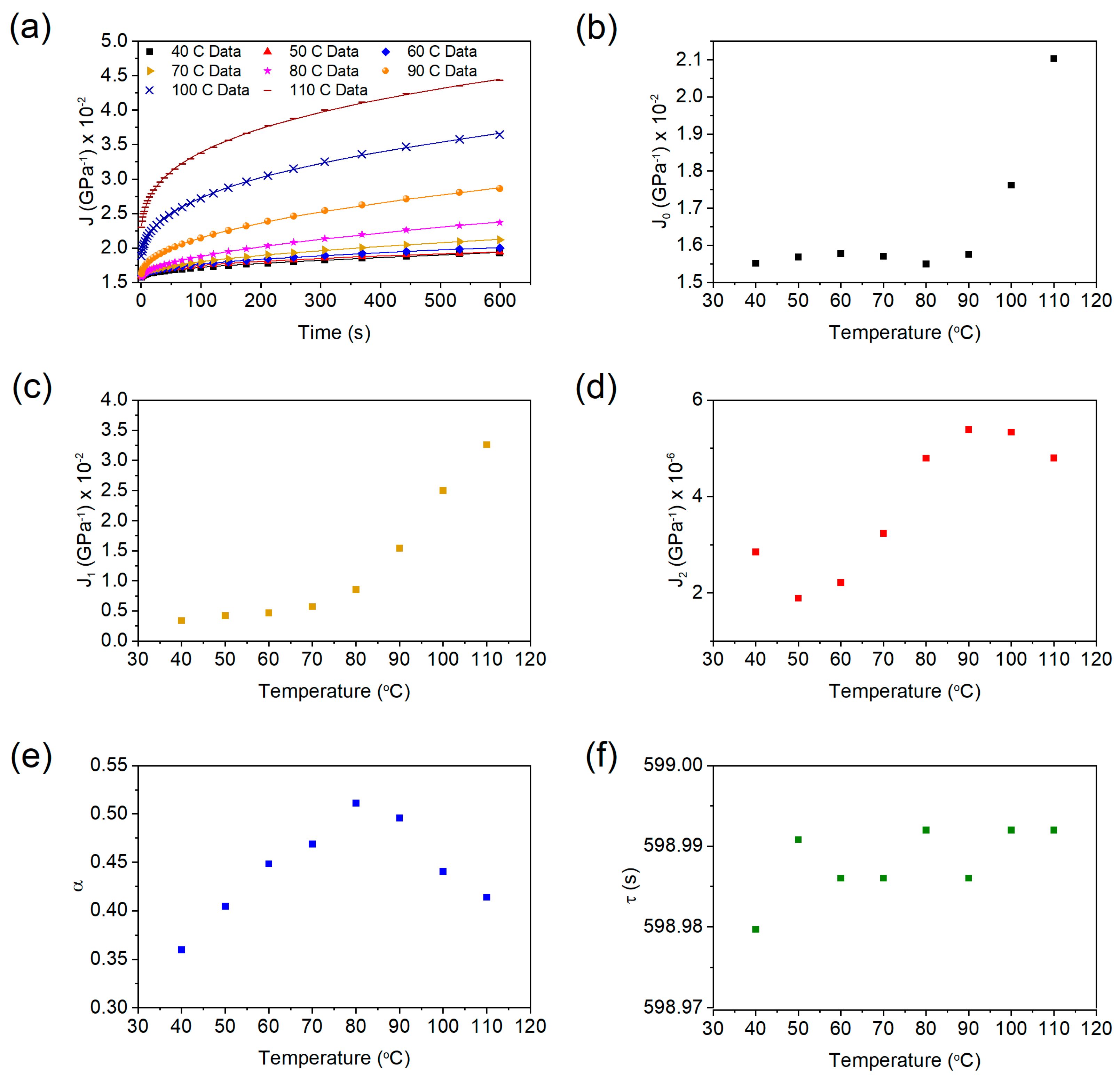
3.4. Creep Test and Modeling
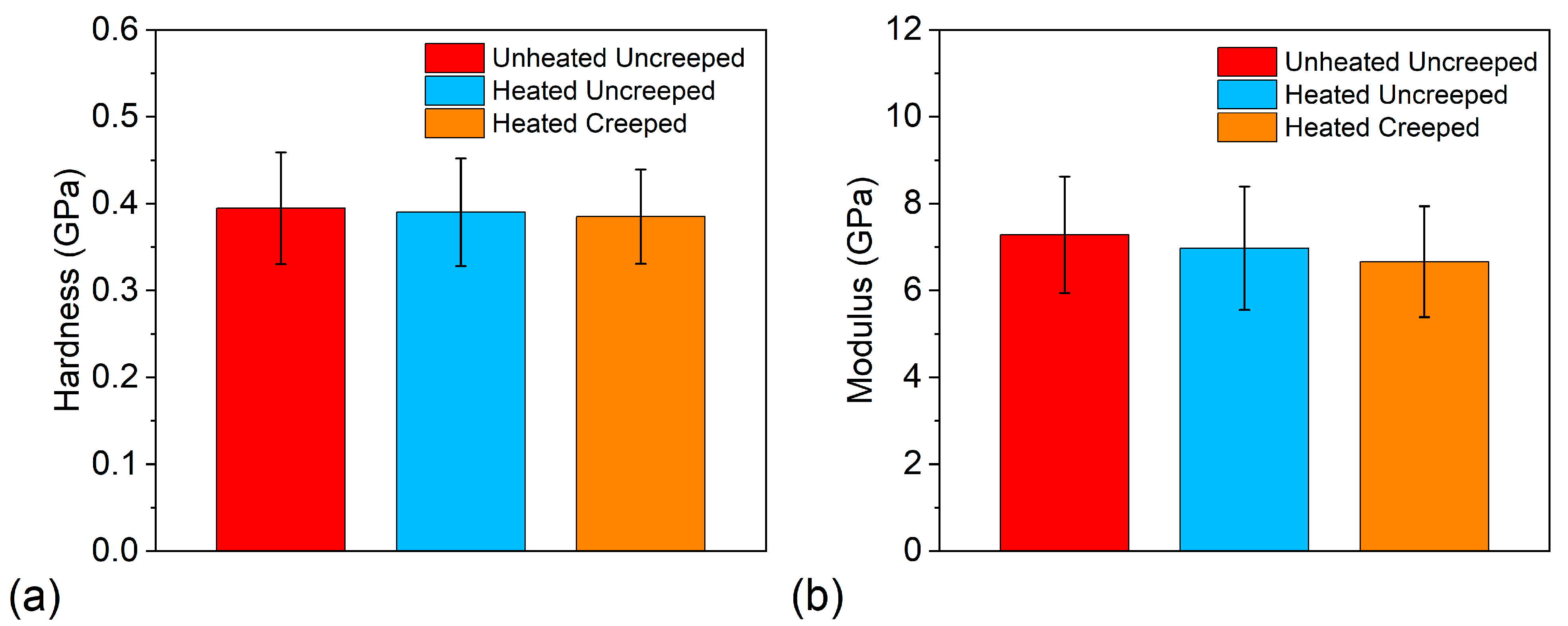
3.5. Nanoindentation Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jirawattanasomkul, T.; Minakawa, H.; Likitlersuang, S.; Ueda, T.; Dai, J.G.; Wuttiwannasak, N.; Kongwang, N. Use of water hyacinth waste to produce fibre-reinforced polymer composites for concrete confinement: Mechanical performance and environmental assessment. J. Clean Prod. 2021, 292, 13. [Google Scholar] [CrossRef]
- Torlova, A.; Vitkalova, I.; Pikalov, E.; Selivanov, O. Development of Polymer Composite Facing Material Using Anthropogenic Waste. In VIII International Scientific Siberian Transport Forum, Volume 2; Popovic, Z., Manakov, A., Breskich, V., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2020; Volume 1116, pp. 544–551. [Google Scholar]
- El-Salakawy, E.; Benmokrane, B.; Masmoudi, R.; Briere, F.; Breaumier, E. Concrete bridge barriers reinforced with glass fiber-reinforced polymer composite bars. ACI Struct. J. 2003, 100, 815–824. [Google Scholar]
- Lee, S.G.; Lee, H.; Gupta, A.; Chang, S.; Doyle, P.S. Site-Selective In Situ Grown Calcium Carbonate Micromodels with Tunable Geometry, Porosity, and Wettability. Adv. Funct. Mater. 2016, 26, 4896–4905. [Google Scholar] [CrossRef]
- Wang, Y.; Zanelotti, C.J.; Wang, X.E.; Kerr, R.; Jin, L.Y.; Kan, W.H.; Dingemans, T.J.; Forsyth, M.; Madsen, L.A. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 2021, 1–9. [Google Scholar] [CrossRef]
- Amith, V.; Sridhar, R.; Gangadhar, A.; Vishnumurthy, K.A. Design and synthesis of PVDF-cloisite-30B nanocomposite fibers for energy harvesting applications. Surf. Interfaces 2021, 22, 8. [Google Scholar] [CrossRef]
- Glasunov, A.; Bobryashov, V.; Bushuev, N. Building Platforms of Polymer Matrix Based Composite Construction Materials for Transportation Facilities. In International Scientific Conference Energy Management of Municipal Transportation Facilities and Transport, Emmft 2017; Murgul, V., Popovic, Z., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2018; Volume 692, pp. 801–807. [Google Scholar]
- Khalimovskyy, O.M.; Sokolskyy, O.L.; Ivitskyy, I.I.; Zaichenko, Y.M. Automation of Polymer Composite Materials Formation Process with a Specified Structure; IEEE: New York, NY, USA, 2019; pp. 606–609. [Google Scholar]
- Li, C.G.; Yin, X.L.; Wang, Y.J.; Zhang, L.; Zhang, Z.H.; Liu, Y.C.; Xian, G.J. Mechanical property evolution and service life prediction of pultruded carbon/glass hybrid rod exposed in harsh oil -well condition. Compos. Struct. 2020, 246, 12. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zhao, X.L.; Xian, G.J.; Wu, G.; Raman, R.K.S.; Al-Saadi, S. Effect of sustained load and seawater and sea sand concrete environment on durability of basalt- and glass-fibre reinforced polymer (B/GFRP) bars. Corros. Sci. 2018, 138, 200–218. [Google Scholar] [CrossRef]
- Li, C.G.; Guo, R.; Xian, G.J.; Li, H. Effects of elevated temperature, hydraulic pressure and fatigue loading on the property evolution of a carbon/glass fiber hybrid rod. Polym. Test. 2020, 90, 13. [Google Scholar] [CrossRef]
- Li, C.G.; Yin, X.L.; Liu, Y.C.; Guo, R.; Xian, G.J. Long-term service evaluation of a pultruded carbon/glass hybrid rod exposed to elevated temperature, hydraulic pressure and fatigue load coupling. Int. J. Fatigue 2020, 134, 15. [Google Scholar] [CrossRef]
- Kang, J.O.; Kim, S.C. Heat Transfer Characteristics of Heat Exchangers for Waste Heat Recovery from a Billet Casting Process. Energies 2019, 12, 2695. [Google Scholar] [CrossRef]
- Rajagopal, M.C.; Chang, H.C.; Man, T.; Kuntumalla, G.; Meng, Y.; Sundar, S.; Zhao, H.; Salapaka, S.; Shao, C.; Ferreira, P.; et al. Materials-to-device design of hybrid metal-polymer heat exchanger tubes for low temperature waste heat recovery. Int. J. Heat Mass Transf. 2019, 143, 118497. [Google Scholar] [CrossRef]
- Thekdi, A.; Nimbalkar, S.U. Industrial Waste Heat Recovery—Potential Applications, Available Technologies and Crosscutting R&D Opportunities; ORNL/TM-2014/622; Other: ED2701000; CEED063 United States 10.2172/1185778 Other: ED2701000; CEED063 ORNL English; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2015; 82p.
- Chen, X.J.; Su, Y.H.; Reay, D.; Riffat, S. Recent research developments in polymer heat exchangers—A review. Renew. Sustain. Energ. Rev. 2016, 60, 1367–1386. [Google Scholar] [CrossRef]
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M.J. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef]
- Zaheed, L.; Jachuck, R.J.J. Review of polymer compact heat exchangers, with special emphasis on a polymer film unit. Appl. Therm. Eng. 2004, 24, 2323–2358. [Google Scholar] [CrossRef]
- T’Joen, C.; Park, Y.; Wang, Q.; Sommers, A.; Han, X.; Jacobi, A. A review on polymer heat exchangers for HVAC&R applications. Int. J. Refrig. 2009, 32, 763–779. [Google Scholar] [CrossRef]
- Sinh, L.H.; Hong, J.M.; Son, B.T.; Trung, N.N.; Bae, J.Y. Thermal, dielectric, and rheological properties of aluminum nitride/liquid crystalline copoly(ester amide) composite for the application of thermal interface materials. Polym. Compos. 2012, 33, 2140–2146. [Google Scholar] [CrossRef]
- Brinson, H.F.; Brinson, L.C. Polymer Engineering Science and Viscoelasticity: An Introduction; Springer: New York, NY, USA, 2015. [Google Scholar]
- Jerez-Mesa, R.; Travieso-Rodriguez, J.A.; Corbella, X.; Busque, R.; Gomez-Gras, G. Finite element analysis of the thermal behavior of a RepRap 3D printer liquefier. Mechatronics 2016, 36, 119–126. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Bueche, F. Derivation of the WLF Equation for the Mobility of Molecules in Molten Glasses. J. Chem. Phys. 1956, 24, 418–419. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Sun, Z.; Li, Y.Q.; Huang, P.; Chen, Q.; Fu, S.Y. Tensile creep behavior of short-carbon-fiber reinforced polyetherimide composites. Compos. Part B Eng. 2021, 212, 8. [Google Scholar] [CrossRef]
- Xue, C.; Gao, H.Y.; Hu, Y.C.; Hu, G.X. Experimental test and curve fitting of creep recovery characteristics of modified graphene oxide natural rubber and its relationship with temperature. Polym. Test. 2020, 87, 12. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Akulichev, A.G.; Gagani, A.I.; Echtermeyer, A.T. Time-Temperature-Plasticization Superposition Principle: Predicting Creep of a Plasticized Epoxy. Polymers 2019, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Faraz, M.I.; Besseling, N.A.M.; Korobko, A.V.; Picken, S.J. Characterization and Modeling of Creep Behavior of a Thermoset Nanocomposite. Polym. Compos. 2015, 36, 322–329. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications; Elsevier Science: Ontario, ON, Canada, 2017. [Google Scholar]
- Vilcinskas, K.; Jansen, K.M.B.; Mulder, F.M.; Picken, S.J.; Koper, G.J.M. Composition dependent properties of graphene (oxide)-alginate biopolymer nanocomposites. Polym. Compos. 2018, 39, E236–E249. [Google Scholar] [CrossRef]
- Williams, G.; Watts, D.C. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 1970, 66, 80–85. [Google Scholar] [CrossRef]
- Xing, J.; Ni, Q.-Q.; Deng, B.; Liu, Q. Morphology and properties of polyphenylene sulfide (PPS)/polyvinylidene fluoride (PVDF) polymer alloys by melt blending. Compos. Sci. Technol. 2016, 134, 184–190. [Google Scholar] [CrossRef]
- Napolitano, R.; Pirozzi, B.; Salvione, A. Crystal Structure of Poly(p-phenylene sulfide): A Refinement by X-ray Measurements and Molecular Mechanics Calculations. Macromolecules 1999, 32, 7682–7687. [Google Scholar] [CrossRef]
- Lee, T.H.; Boey, F.Y.C.; Khor, K.A. X-ray diffraction analysis technique for determining the polymer crystallinity in a polyphenylene sulfide composite. Polym. Compos. 1995, 16, 481–488. [Google Scholar] [CrossRef]
- Fan, Z.-Z.; He, H.-W.; Yan, X.; Zhao, R.-H.; Long, Y.-Z.; Ning, X. Fabrication of Ultrafine PPS Fibers with High Strength and Tenacity via Melt Electrospinning. Polymers 2019, 11, 530. [Google Scholar] [CrossRef]
- Lu, S.X.; Cebe, P.; Capel, M. Effects of Molecular Weight on the Structure of Poly(phenylene sulfide) Crystallized at Low Temperatures. Macromolecules 1997, 30, 6243–6250. [Google Scholar] [CrossRef]
- Chukov, D.; Nematulloev, S.; Zadorozhnyy, M.; Tcherdyntsev, V.; Stepashkin, A.; Zherebtsov, D. Structure, Mechanical and Thermal Properties of Polyphenylene Sulfide and Polysulfone Impregnated Carbon Fiber Composites. Polymers 2019, 11, 684. [Google Scholar] [CrossRef]
- Khan, S.A.; Lazoglu, I. Development of additively manufacturable and electrically conductive graphite–polymer composites. Prog. Addit. Manuf. 2020, 5, 153–162. [Google Scholar] [CrossRef]
- Dubey, K.A.; Hassan, P.A.; Bhardwaj, Y.K. Chapter 5—High Performance Polymer Nanocomposites for Structural Applications. In Materials Under Extreme Conditions; Tyagi, A.K., Banerjee, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–194. [Google Scholar] [CrossRef]
- Jing, Y.; Nai, X.; Dang, L.; Zhu, D.; Wang, Y.; Dong, Y.; Li, W. Reinforcing polypropylene with calcium carbonate of different morphologies and polymorphs. Sci. Eng. Compos. Mater. 2018, 25, 745–751. [Google Scholar] [CrossRef]
- Eiras, D.; Pessan, L.A. Mechanical Properties of Polypropylene/Calcium Carbonate Nanocomposites. Mater. Res. Ibero-am. J. Mater. 2009, 12, 517–522. [Google Scholar] [CrossRef]
- Dai, Z.H.; Gao, Y.; Liu, L.Q.; Potschke, P.; Yang, J.L.; Zhang, Z. Creep-resistant behavior of MWCNT-polycarbonate melt spun nanocomposite fibers at elevated temperature. Polymer 2013, 54, 3723–3729. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhang, Z.; Schlarb, A.K.; Friedrich, K. On the characterization of tensile creep resistance of polyamide 66 nanocomposites. Part I. Experimental results and general discussions. Polymer 2006, 47, 2791–2801. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhang, Z.; Schlarb, A.K.; Friedrich, K. On the characterization of tensile creep resistance of polyamide 66 nanocomposites. Part II: Modeling and prediction of long-term performance. Polymer 2006, 47, 6745–6758. [Google Scholar] [CrossRef]
- Dean, G.D.; Broughton, W. A model for non-linear creep in polypropylene. Polym. Test. 2007, 26, 1068–1081. [Google Scholar] [CrossRef]

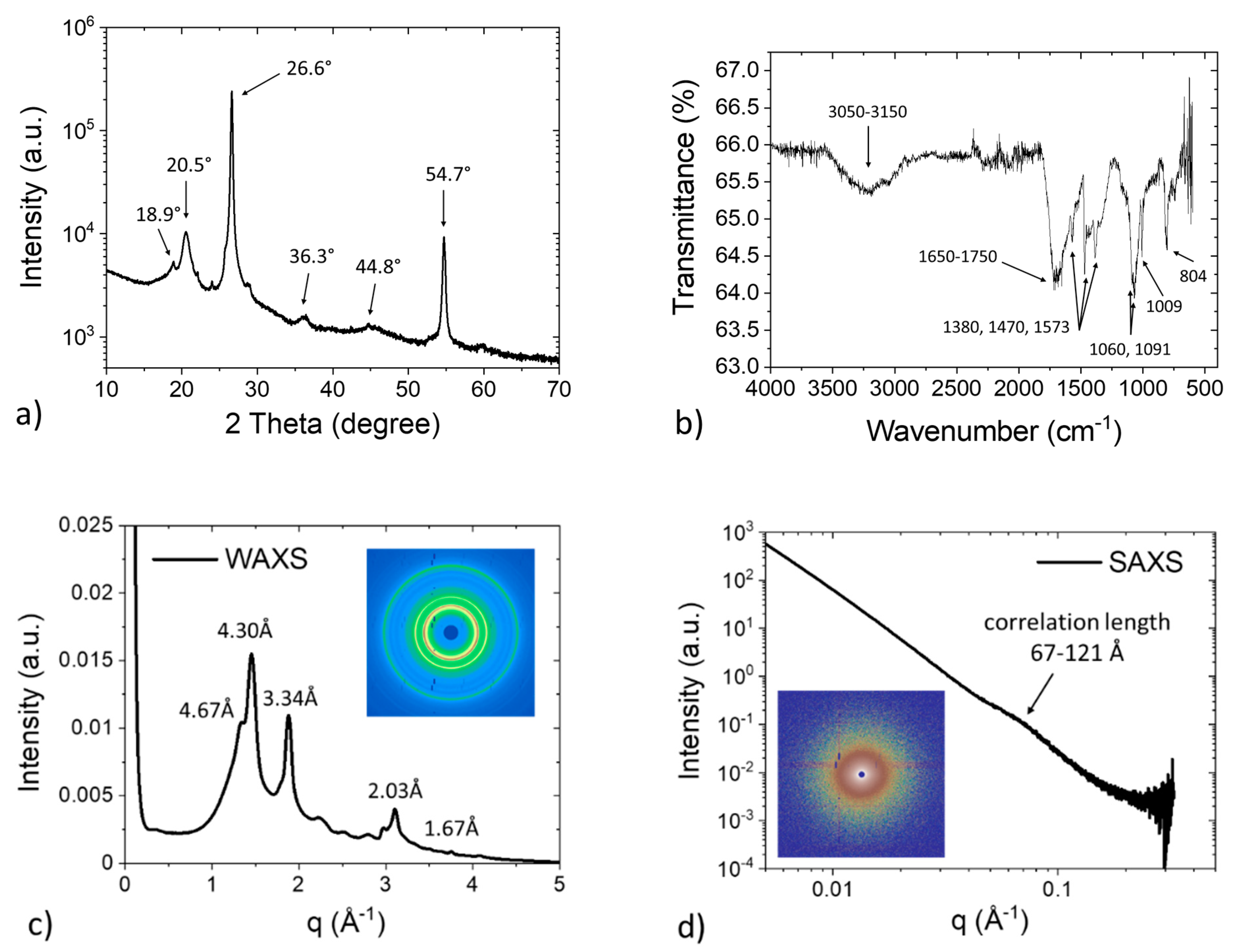




| XRD | ||
|---|---|---|
| 2 Theta Angle (°) | d-Spacing (Å) | Diffraction Plane |
| 18.9 | 4.69 | (110) of PPS |
| 20.5 | 4.33 | (102), (200), (111) of PPS |
| 26.6 | 3.35 | (002) of graphite |
| 36.3 | 2.47 | (004), (302), (311), (022), (104), (213) of PPS |
| 54.7 | 1.68 | (004) of graphite |
| FTIR | ||
| Wavenumber (cm−1) | IR Fingerprint | |
| 3050–3150 | Aromatic C-H stretching | |
| 1650–1750 | Aromatic C-H bending | |
| 1380, 1470, 1573 | Aromatic C=C stretching | |
| 1060,1091 | C-S stretching | |
| 1009, 804 | Aromatic C–H bending | |
| Sample | Hardness (GPa) | Young’s Modulus (GPa) |
|---|---|---|
| Unheated and uncrept | 0.40 ± 0.06 | 7.28 ± 1.34 |
| Heated and uncrept | 0.39 ± 0.06 | 6.97 ± 1.42 |
| Heated and crept | 0.39 ± 0.05 | 6.66 ± 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brechtl, J.; Li, Y.; Li, K.; Kearney, L.; Nawaz, K.; Flores-Betancourt, A.; Thompson, M.; Rios, O.; Momen, A.M. Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications. Polymers 2021, 13, 1970. https://doi.org/10.3390/polym13121970
Brechtl J, Li Y, Li K, Kearney L, Nawaz K, Flores-Betancourt A, Thompson M, Rios O, Momen AM. Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications. Polymers. 2021; 13(12):1970. https://doi.org/10.3390/polym13121970
Chicago/Turabian StyleBrechtl, Jamieson, Yuzhan Li, Kai Li, Logan Kearney, Kashif Nawaz, Alexis Flores-Betancourt, Michael Thompson, Orlando Rios, and Ayyoub M. Momen. 2021. "Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications" Polymers 13, no. 12: 1970. https://doi.org/10.3390/polym13121970
APA StyleBrechtl, J., Li, Y., Li, K., Kearney, L., Nawaz, K., Flores-Betancourt, A., Thompson, M., Rios, O., & Momen, A. M. (2021). Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications. Polymers, 13(12), 1970. https://doi.org/10.3390/polym13121970









