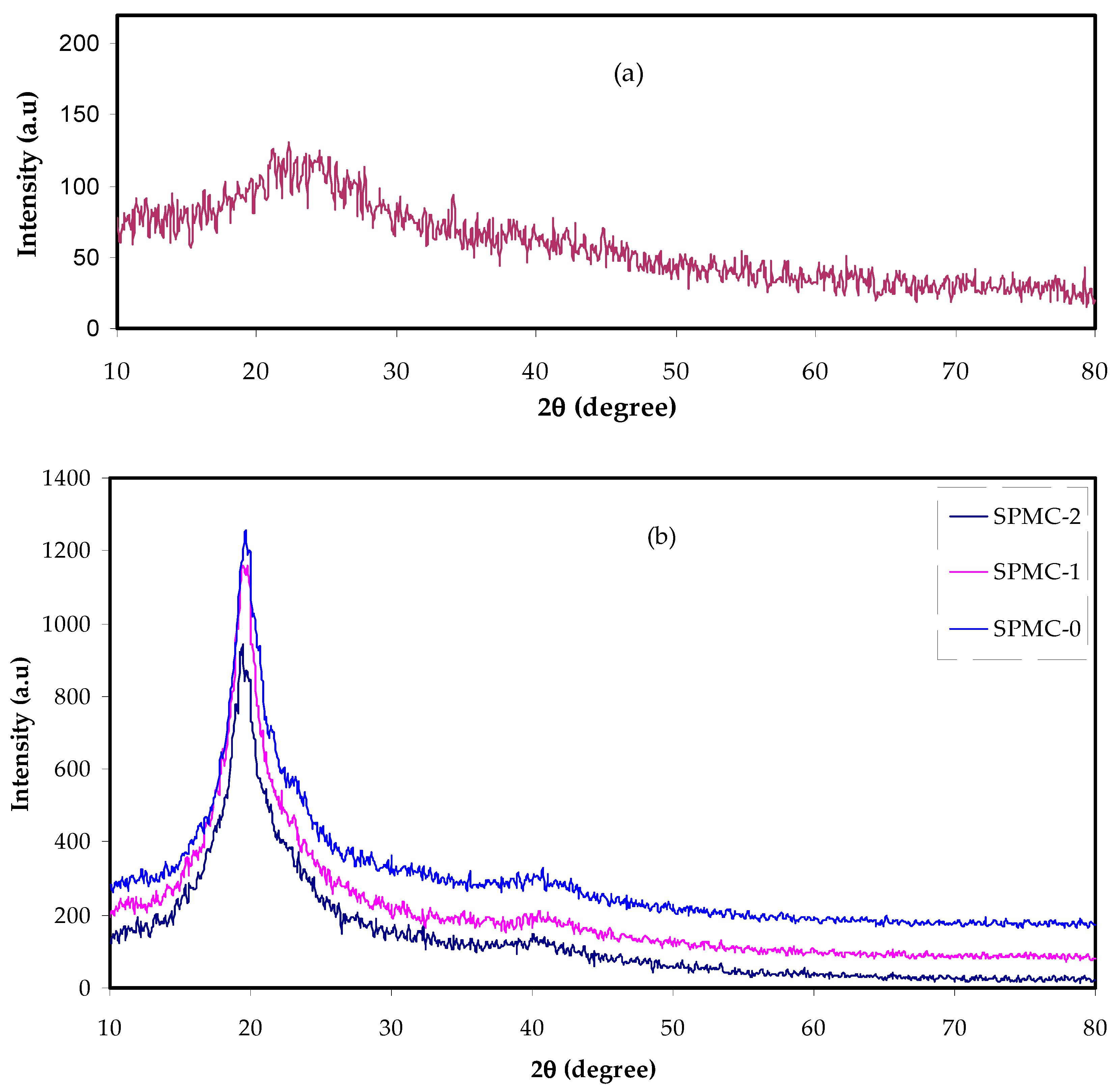
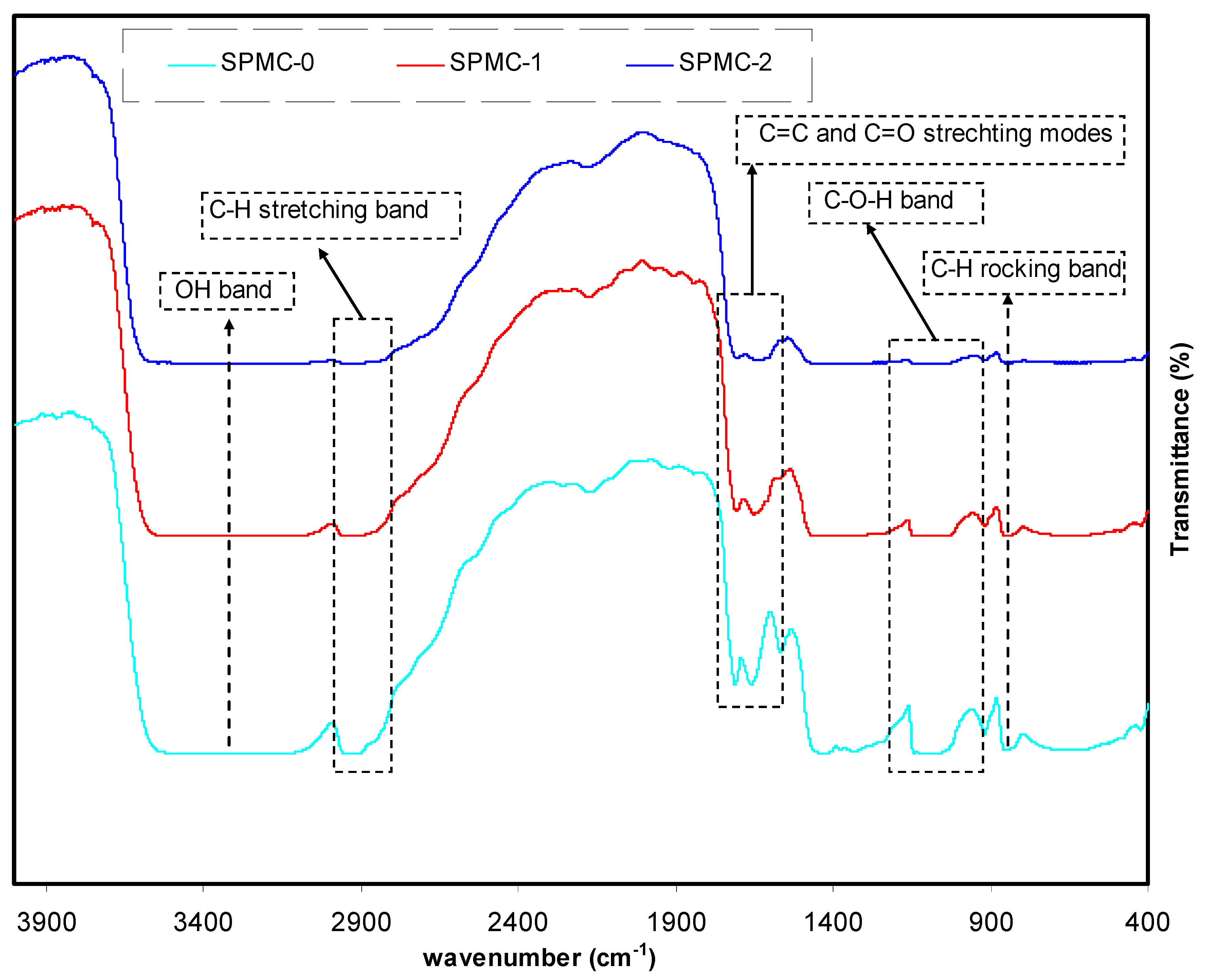
3.4.1. Transmittance and Absorbance Analysis
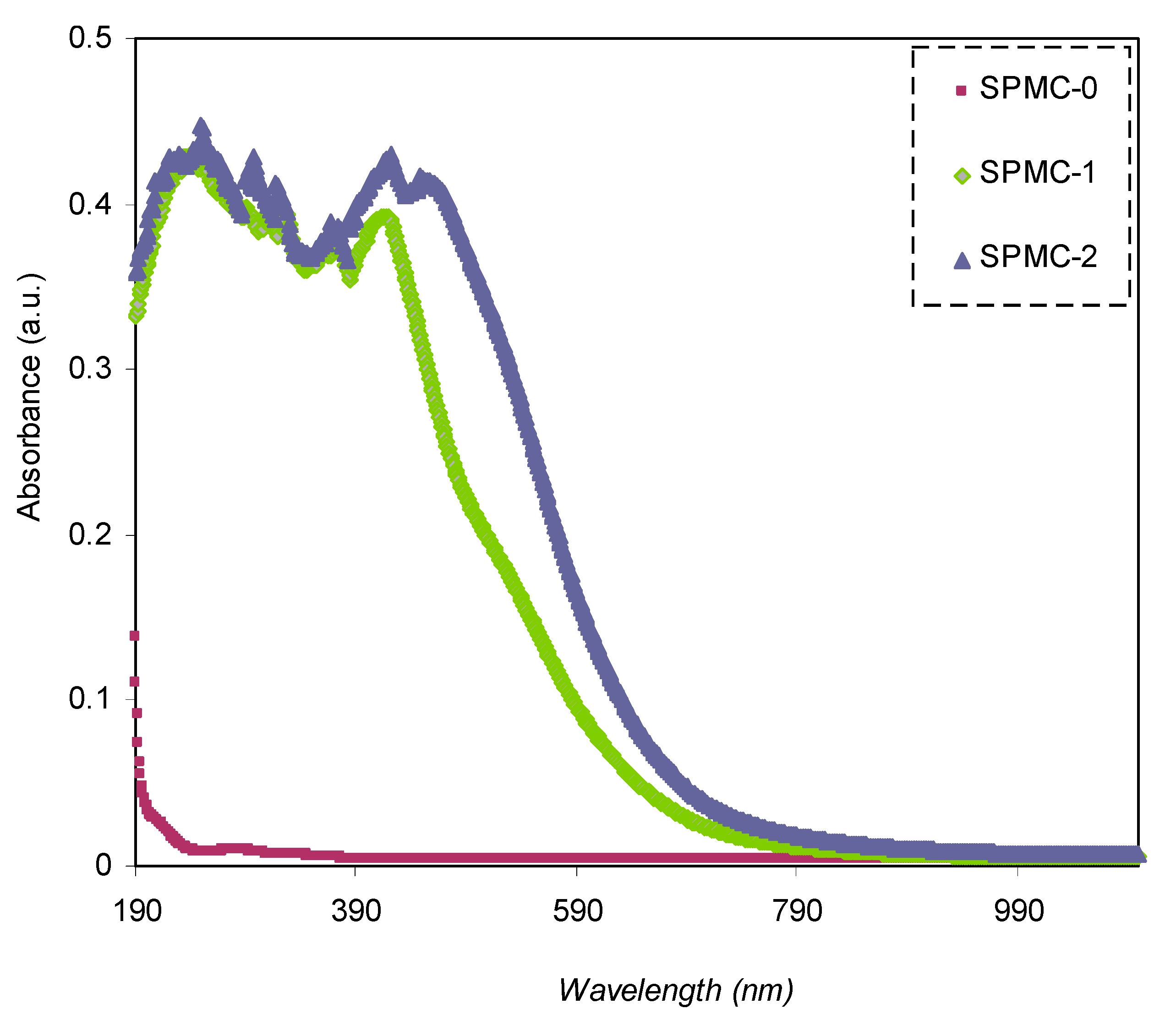
The transmittance of parent and doped PVA with various quantities of Co
2+-polyphenol complex is presented in
Figure 7. Pure PVA possesses relatively high transparency beyond the visible region, i.e., over 98%, whereas below the visible region, the transparency drops due to the high absorption of the films. PVA doped with 30 and 60 mL Co
2+-polyphenol complex exhibited lower transparency, reaching almost 45% at the UV region, while transparency increased to 0.98% in the visible region. This may be attributed to the scattering and relatively high refractive index of highly doped films at lower λ (nm). It was noted that the spectrum possessed a shoulder in the parent PVA (UV region); on the other side, this shoulder did not exist. This indicates strong interactions between the functional groups within the polymer chains and the added Co
2+-polyphenol complex, causing a drop in the transparency of the doped films. Molkenova et al. [
55] reported on Europium metal thin films doped with titanium oxide (TiO
2). Their findings revealed a transmittance percent of 83.3% for Eu doped film in the visible range. Also, the transparency value for the blend polymers of poly (propylene carbonate) PPC and poly (ethyl cyanoacrylate) PECA with caffeic acid was found to be around 80%, as documented by Quilez-Molina et al. [
56].
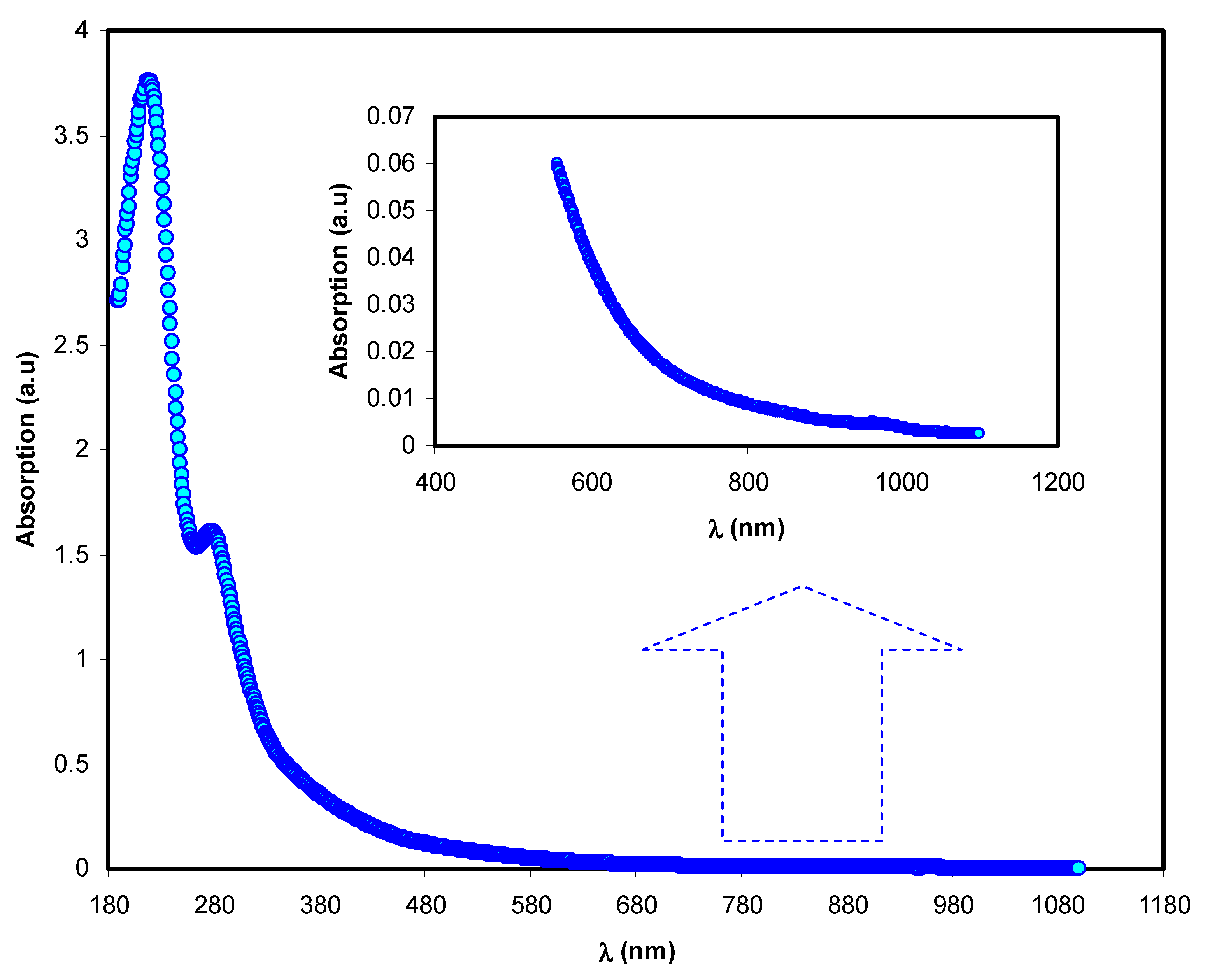
The absorption spectra of both parent PVA and doped PVA are shown in
Figure 8. It may be seen that the responses of the absorption spectra of the PVA doped samples cover almost all UV-visible regions, extending to the near-infrared range. Pure PVA has no absorption response in the visible to near-infrared range because of the absence of free electrons, causing it to be almost transparent. Furthermore, the relatively high-energy (UV region) photons result in electron transport across the bandgap between the valence and conduction bands.
From both the absorption spectra of pure PVA and doped PVA samples, it is seen that composite samples containing Co
2+-polyphenol complex display extreme absorption throughout the entire UV-Visible range. It may also be seen that as the content of the Co
2+-polyphenol complex increased in the PVA, and the absorption band position shifted to a greater wavelength (i.e., lesser energy). It is vital technologically that the synthesized polymer composites have the desired optical characteristics for applications in various industries, for instance, photonics, solar cells, and optoelectronic instruments [
57]. To achieve high-power conversion efficiency in solar cells, the light-harvesting potential needs to be manipulated via the absorption band widening and shifting the position of absorption bands to near IR region responses, which, in turn, increase the extinction coefficient [
58].
3.4.2. Absorption Edge Study
At this stage, it is worthwhile to discuss optical absorption, including both the structure and shift of the absorption edge. It is also helpful to have a comprehensive understanding of the potential mechanism of crystalline and noncrystalline materials that are optically induced. Analyses can further verify improvements due to the structures of energy band changes [
59]; despite intensive studies on the optical characteristics of polymer composites. [
60,
61], there is still work to be done on polymer composites based on the Co
2+-polyphenol metal complex.
The optical absorption spectra help determine and estimate the optical bandgap of the film. The absorption coefficient can be calculated using the following relationship based on the transmittance and reflectance results [
54]:
where
α is the absorption coefficient,
T is the transmittance, and
t and
R the thickness and reflectance. To determine
T, Beer’s law (
T = 10
−A) can be used. In general, responses to the transmission light from one medium to another include reflection and diffraction, light-transmitting from air to a solid medium, and absorption. As the beam strikes the second medium surface, the responses must be proportional to the summation of the absorption beam
IA, reflected beam intensity
IR, and transmitted beam
IT, as shown in the following relationship:
The intensity of radiation is designated as W m
−2. Energy transfer over time and area units indicated the propagation direction at a right angle. As a result of Equation (2), the following relationship is obtained:
where
R represents the reflectivity (
IR/
Io),
A is the absorptivity (
IA/
Io), and
T is the transmissivity (
IT/
Io). In other words,
R,
A, and
T refer to reflection, absorption and transmittance, respectively [
62]. Thus, reflectance (
R), as a crucial parameter, can be determined from the refractive index using Equation (3). From an analysis of the optical absorption, the absorption edge is shown to be a decisive parameter in specifying the electronic structure within the material. Optical absorption spectra denote the response of the indirect and direct transition within the bandgap [
63].
Figure 9 shows the absorption coefficient for a variety of photon energies; the absorption edge values are summarized in
Table 2. The absorption edge for pure PVA was found to be about 6.3 eV. A broad shift toward a lower photon energy range was observed after incorporating the Co
2+-polyphenol complex into the system.
A modification of the absorption edge resulted from the dispersion of the complex of Co
2+-polyphenol within the PVA matrix. In the present study, the value of the absorption edge was in good agreement with that reported previously [
62].
Throughout the literature, it has been confirmed that narrowed optical band gaps can be obtained by introducing localized levels inside the energy gap as a consequence of trapping charged species [
64]. There is a similarity between this shifting and broadening of the absorption edge to a lower energy range, as in the current results, and those documented for organic and inorganic semiconductors. Organic semiconductors are used extensively in photonic and electronic instruments [
65].
3.4.3. Study of the Refractive Index
The refractive index value (
n) of optical materials is one of the key properties, showing the extent of light-speed changes from one medium to another. For application in optoelectronic instruments, the
n value of the material of interest must be known. Fundamentally, at constant temperature and pressure, the refractive index (
n) is dependent upon both density and polarizability of the medium [
66]. Therefore, the refractive index of a material is a decisive parameter in determining its optical efficiency. The representation of the complex refractive index of a sample may be summarized as follows:
The relationship between
n and
k is formulated as follows [
54]:
In Equations (4) and (5), K is the extinction coefficient equal to αλ/4πt, and t is the film thickness.
Variable
n can be determined using different techniques. Based on the type of synthesis, two methodologies are widely applied; heavy atoms, such as the polymer matrices, are doped with sulfur and/or halogens [
67], and the synthesis of materials of relatively large
n values from the incorporation of metal or inorganic nanoparticle into polymers [
68,
69,
70]. Two significant challenges are faced in both cases. Firstly, two obstacles exist in the first methodology: the complexity and cost of incorporating heavy atoms into matrices of polymers [
71]. Secondly, the aggregation phenomenon occurs with the incorporation of nanofillers comprising inorganic nanoparticles into polymer matrices, for instance, ZrO
2 (zirconium oxide) [
68], TiO
2 (titanium oxide) [
69], and Au (gold) [
72]. This leads to the formation of high surface energy and low compatibility with the polymer host. Therefore, in the present research, to change the (
n) value, a dopant of the Co
2+-polyphenol complex was inserted into the PVA polymer.
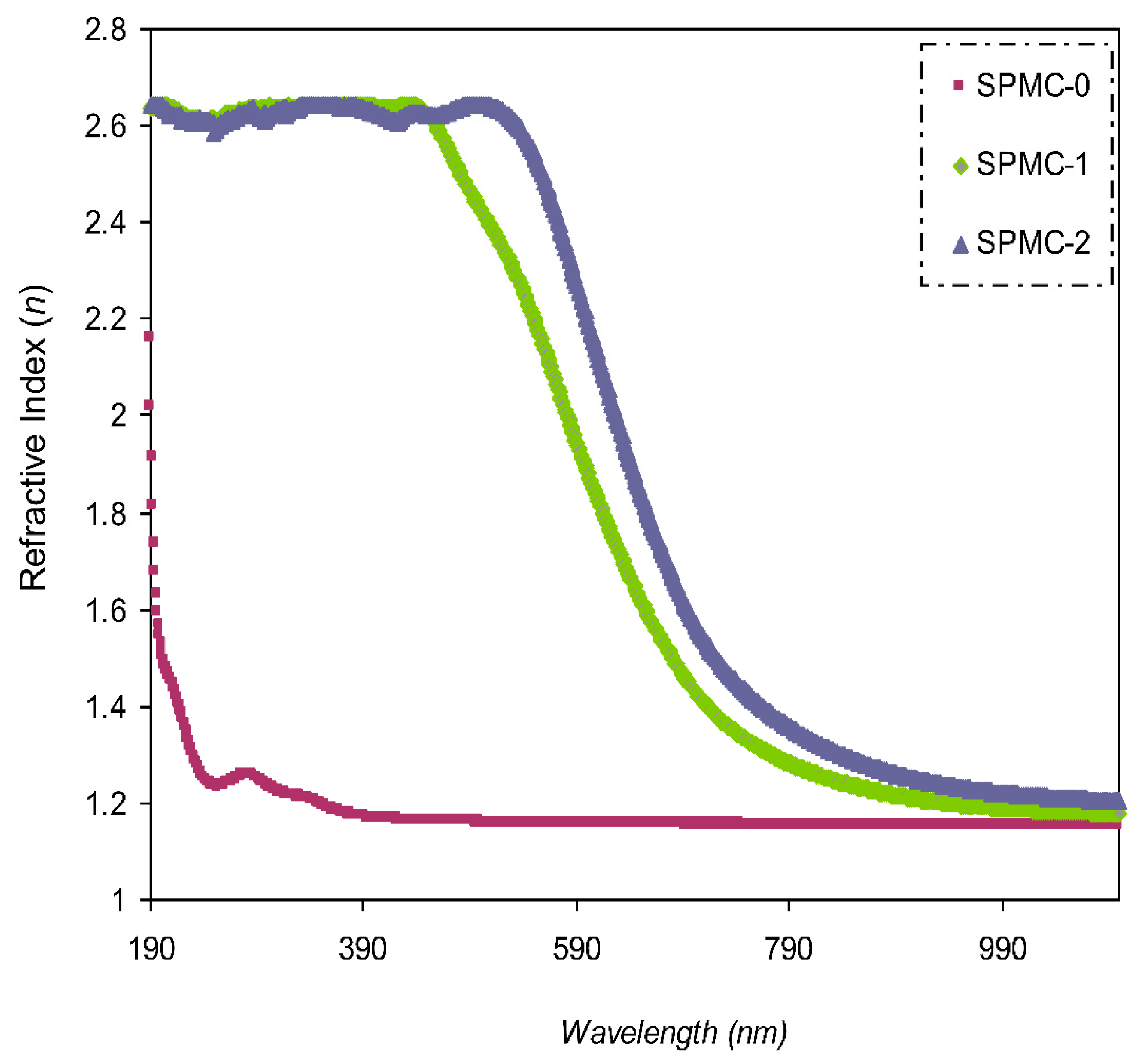
The value of (
n) compared to the wavelength is shown in
Figure 10. It was confirmed that larger
n values can be achieved with incorporated films, indicating considerable dopant dispersion.
Figure 10 also shows that as the Co
2+-polyphenol complex concentration is increased, as is the value of
n.
Polymers and polymer composites of relatively high
n are desirable, and have been the focus of many research groups, as their mass, large formability, and flexibility are superior to those of inorganic materials. Many applications of polymers with high (
n) value have been noted, including in optical storage devices [
73], lenses [
74], antireflective coatings [
75], and optical immersion lithography [
76].
3.4.4. Study of the Dielectric Constant
The advantages of polymers, such as their mass, low cost, and minimal dielectric loss, make them ideal for use in energy storage devices. Polymer materials must have a high dielectric constant (
εr) for use in energy storage applications (i.e., if a large specific capacitance is to be achieved). Composite polymeric materials are among the most reliable methods to modify the
εr value of polymers; however, several other approaches have been applied. Effectively, nanoparticles can be incorporated into polymers to make polymer nanocomposites. Therefore, polymer nanocomposites (NCs) have been utilized in energy storage capacitors [
77,
78,
79,
80] as solid alternatives to battery management systems in microgrids in order to reduce pollution [
81,
82].
The real and imaginary responses of
εr are well-established in a relationship in which both values of (
n) and (
k) are involved, as shown below [
83]:
The
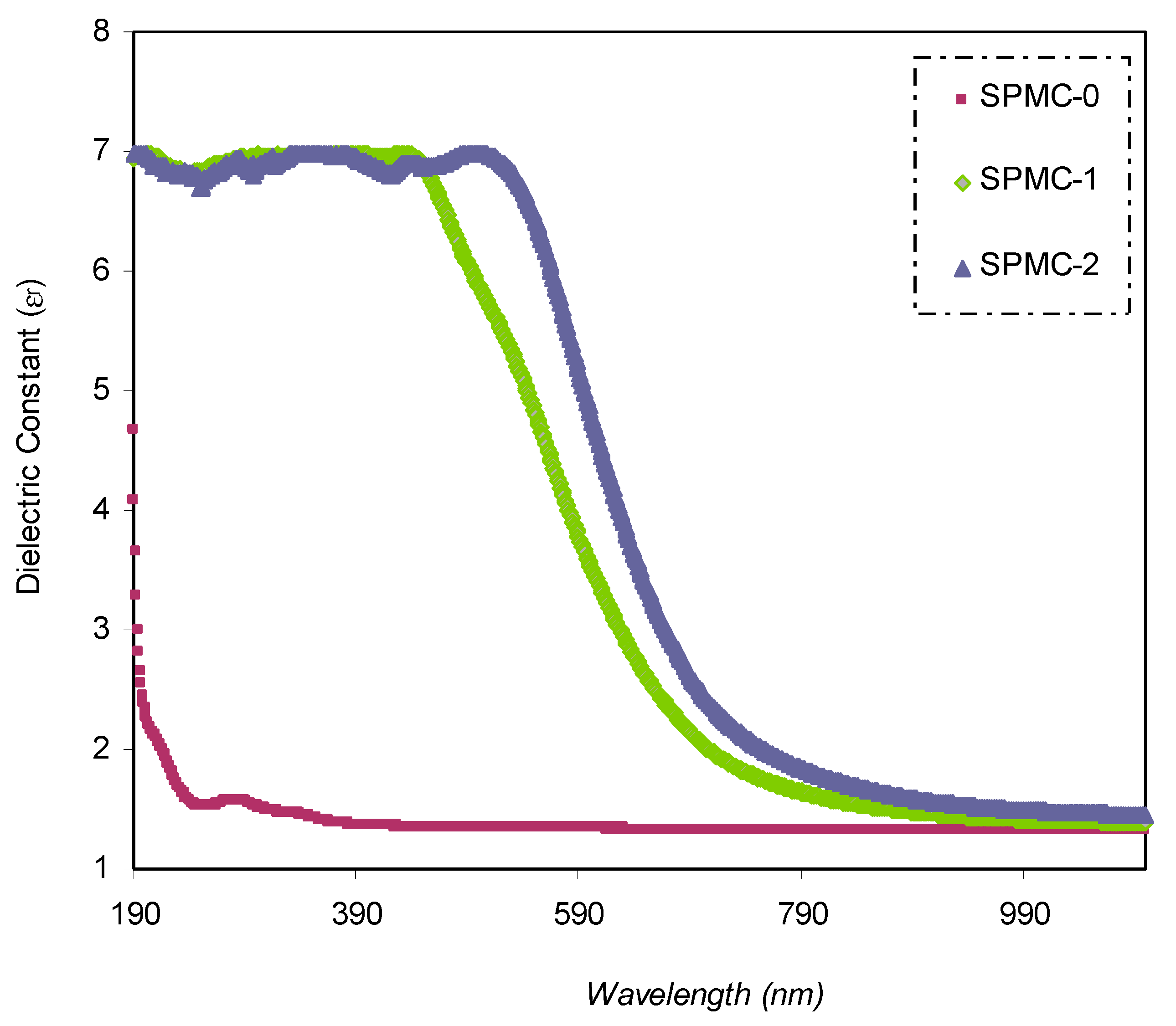
εr spectra against wavelength for pure PVA and composite samples are shown in
Figure 11. It is seen that as the quantity of Co
2+-polyphenol complexes increases,
εr increases as well. This is related to the formation of the density of states within the polymeric film forbidden gap [
31].
It is worth noting that modifications in the optical dielectric constant are responsible for the fundamental optical transition in polymer composites. This may be divided into two parts: real, (εr) and imaginary, (εi). The optical dielectric constant is the measure of the feasibility of losing energy by an electron as it travels through the surface of the bulk material. The real (εr) and imaginary (εi) parts of the spectra reflect this property. On the one hand, the real part measures the ability of a specific material to attenuate the speed of the electromagnetic wave. On the other hand, the imaginary part shows the energy absorption efficiency due to polarization.
The value of εr is obtained from the refractive index (n) of the medium (), while εi is derived from the extinction coefficient (k) ().
The dielectric response (
ε∞) of the materials at high frequency (short wavelengths) can be determined from the relationship between the wavelength and refractive index, based on the Spitzer–Fan model [
84,
85]:
where
εo denotes the dielectric constant of free space,
N is the number of charge carriers,
m* is the effective mass (assumed to be 1.16
me), and
e, and
c have their standard meanings [
85,
86].
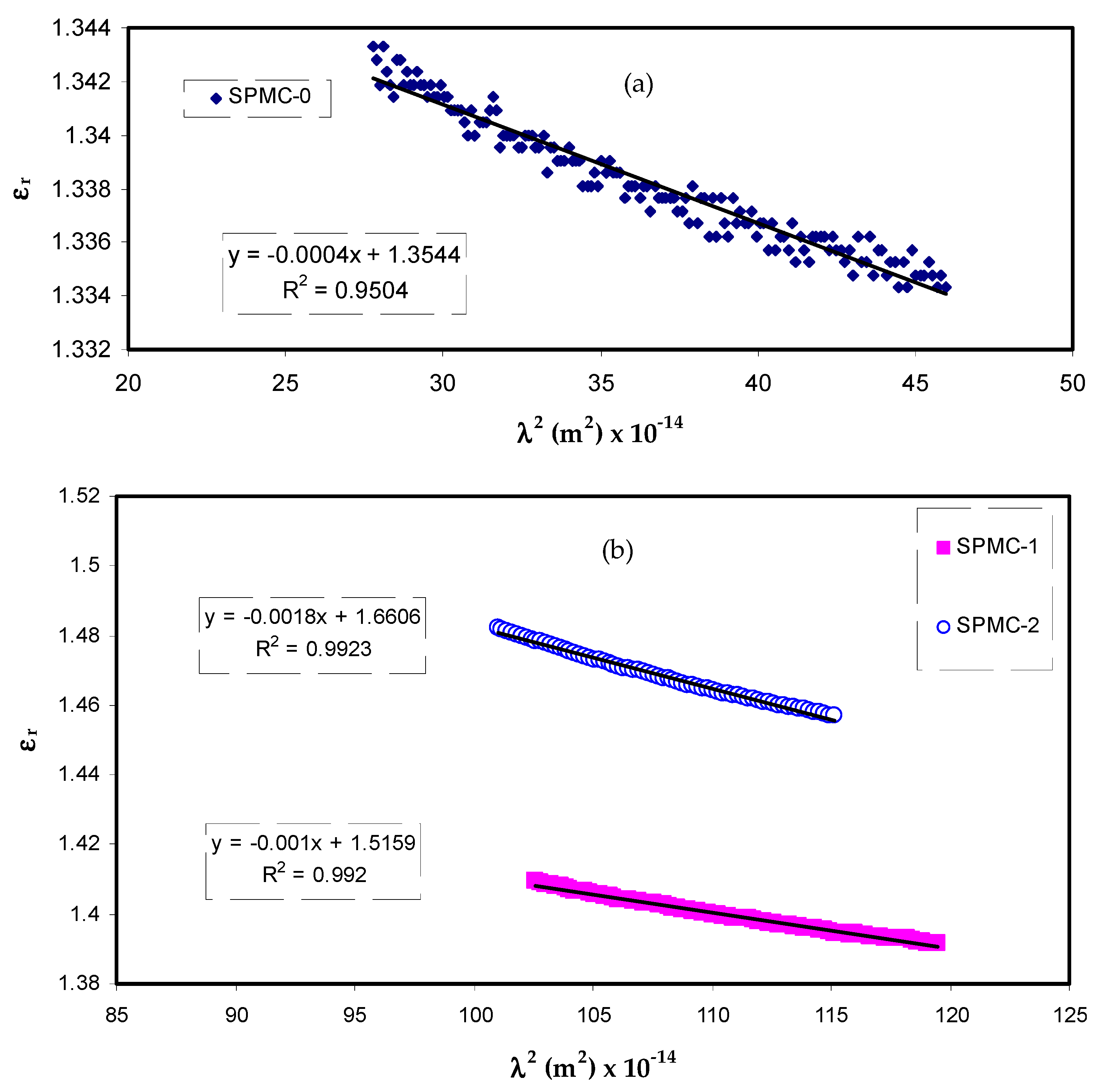
The relationship between the values of
εr and
λ2 in the visible wavelength region is a straight line, as shown in
Figure 12. Herein, one can determine the values of
ε∞ and
N/m* from the slope and intercept of the line in the y axis, respectively, using the parameters presented in
Table 3. The values of
ε∞,
N/m*, and
N can be estimated from Equation (7), as summarized in
Table 4.
From
Table 4, it can be seen that as the filler concentration increases, the values of charge carriers/m* of parent PVA film increase 20 fold, i.e., from 3.68 × 10
55 to 109 × 10
55 atoms/m
3, and the value of
ε∞ increases from 1.4 to 3.6. These increasing values of both charge carriers/m* and the
ε∞ can be considered indicators of increasing free charge carriers that vigorously participate in the polarization process. In the current study, the values that were estimated for the localized density of states (
N/m*) are in good accordance with those reported in the literature using Equation (7) [
87,
88].
3.4.5. Band Gap Study
Bandgap analyses, i.e., optical band gap energy (
Eg), are informative, based on the optical dielectric loss (
εi). It is necessary to determine the electronic transition behavior using Tauc’s model, as the material’s band structure does not have a significant effect on the optical dielectric function. Indeed, the material band structure and optical dielectric function (
ε*) exhibit a strong relationship. Thus, the band structures of materials can be clarified from the determination of
ε* using UV-vis spectroscopy [
31,
46,
71,
89].
The imaginary part of the
ε* relies on
n and
k values, as shown in the following relationship [
27]:
It is essential to mention that the appearance of the peak in the
εi spectra is the result of interband transitions [
90,
91,
92]. From the analysis of the
εi spectra, one can determine the real
Eg from the intersection of linear portions of the (
s) and horizontal axis (
hv).
In the meantime, complex dielectric function
ε* will provide a better understanding of the optical properties of a material. It characterizes the linear reaction of the substance to electromagnetic radiation. The
ε* value reflects the essence of the medium in response to electromagnetic wave transmissions. The real transitions between the occupied
and unoccupied
wave functions are represented by the imaginary part
ε2, which is given by [
93]:
Equation (9) shows an apparent direct proportionality between ε2 or εi and the band structure from the QM perspective. The CDF can be evaluated using simple equations, and intercorrelated to other optical parameters (n and extinction coefficient).
Figure 13 exhibits the plots of
εi versus
hν for parent PVA and composite films. It is seen that there are distinct peaks for all the films. The appearance of the peak in the
ε2 part of the dielectric function can be directly related to interband transitions [
94,
95]. Therefore, the intercept of linear parts below the peaks on the
hν axis can be regarded as an accurate bandgap value. The electron transition processes between the bands of a solid are well-defined by the interband absorption process. Furthermore, the absorption edge is caused by the onset of optical transitions through the fundamental bandgaps of a solid material [
96]. Yu et al. [
97] recently reported that the fundamental absorption edge from dielectric loss against photon energy should be equal, or somewhat similar, to that obtained from Tauc’s relationship.
The optical characteristics of solids can be preliminarily estimated from the CDF, which can be linked to the detectable optical quantities using straightforward equations [
88]. Previous studies have emphasized the existence of a strong relationship between the optical dielectric functions (
εr and
εi) and the density of localized electronic states within the forbidden gap of the composite films [
72,
98,
99]. Many other decisive parameters, for instance, relaxation time (
τ), plasma frequency (
ωp) and electrical resistivity (
ρ), can easily be estimated from the Drude free electron model using the
εi parameter with the help of (
N/m*) values:
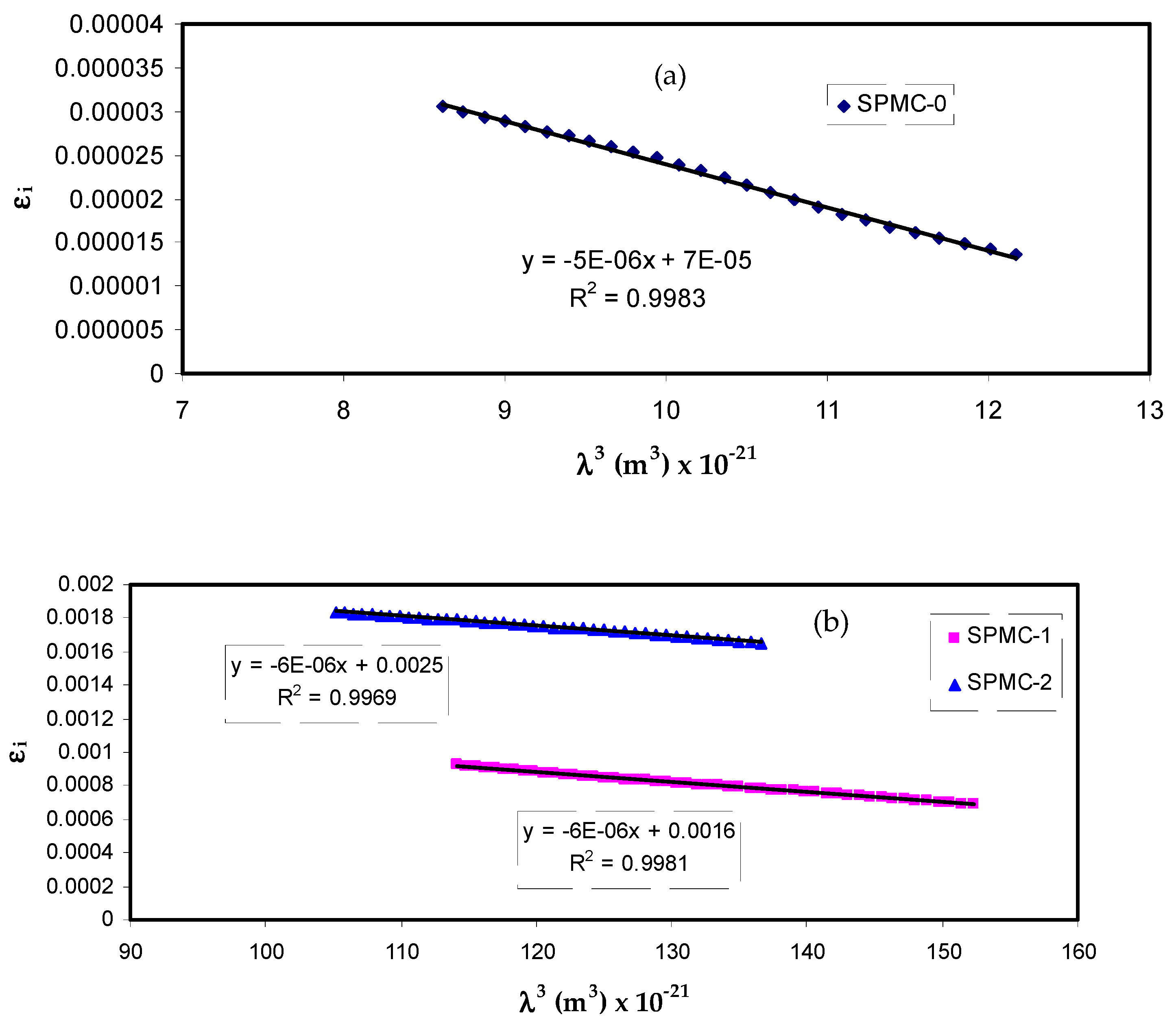
Figure 14 shows that variation of
εi corresponds to
λ3 for parent PVA films at different Co
2+ metal complex quantities in the region where linear behavior is achieved. By inserting the value of
N/m* obtained from Equation (10) and the slope of
εi versus
λ3, the relaxation time (
τ) values may be calculated. In addition, all other optical properties, such as the optical mobility (
µopt), electrical resistivity (
ρopt), and plasma angular frequency of the electron, can be computed from the following relations [
100]:
The values of
τ and
ωp are presented in
Table 5. It should also be noted that adding the Co
2+ metal complex to parent PVA reduces the relaxation time (
τ), optical mobility (
µopt), and optical resistivity (
ρopt), resulting in a faster relaxation response of the nanocomposites to the incident optical electric field in comparison to the unfilled one.
These low
τ,
µopt and
ρopt values are linked to an increase in
n; in other words, the velocity of light decreases in the medium with a higher refractive index. The addition of the Co
2+ metal complex also results in an up to 20-fold amplification of the plasma frequency (
ωp) of the electron, i.e., from 0.32 × 10
15 to 1.77 × 10
15 Hz. Fortunately, this is in accordance with data documented for polymer nanocomposites and the verified impact of a strong local electric field in the dipole moment of the nanofillers, enhancing the polarization of the material being exposed to an incident electric field [
87,
98]. This is not the only means by which to estimate the bandgap from optical dielectric loss function; other optical parameters can be determined, as mentioned previously. All these are crucial for choosing suitable materials for optoelectronic applications.
Tauc’s equation can be used to determine the
Eg of pure PVA, as well as that of PVA doped with Co
2+-polyphenol metal complex [
25,
26]:
In Equation (14), the
B value relies on the interband transition probability,(
hv) denotes the incident photon energy, and (
γ) is an exponent that specifies the electronic transition type [
31].
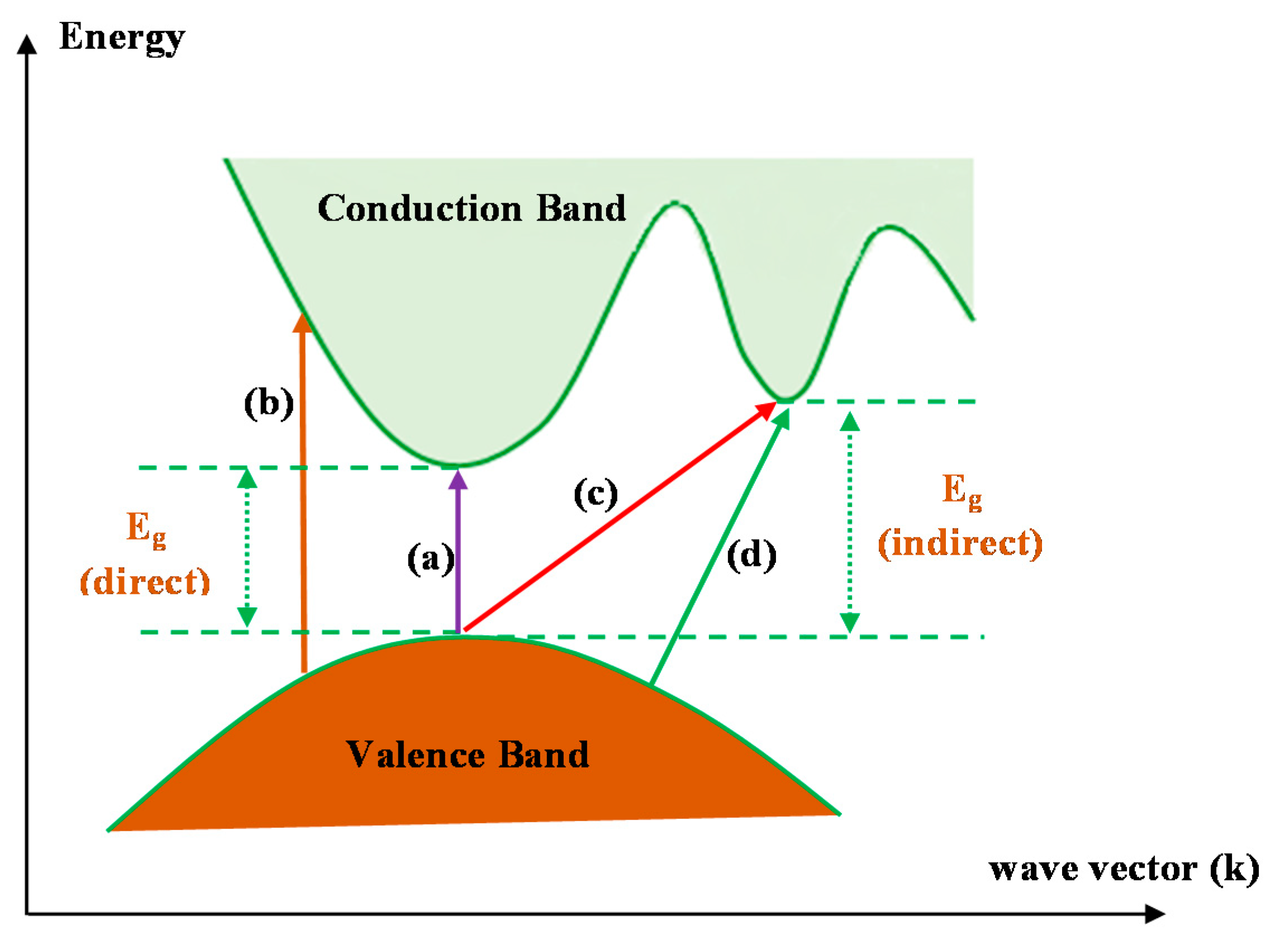
Figure 15 shows several electronic transitions which occur between the conduction band (CB) and valence band (VB) that are Tauc’s model-dependent [
101]. Indeed, when
γ is 0.5 or 2, direct electron and indirect transitions, respectively, may occur. If
γ is 1.5 or 3, direct and indirect transitions, respectively, cannot occur [
31].
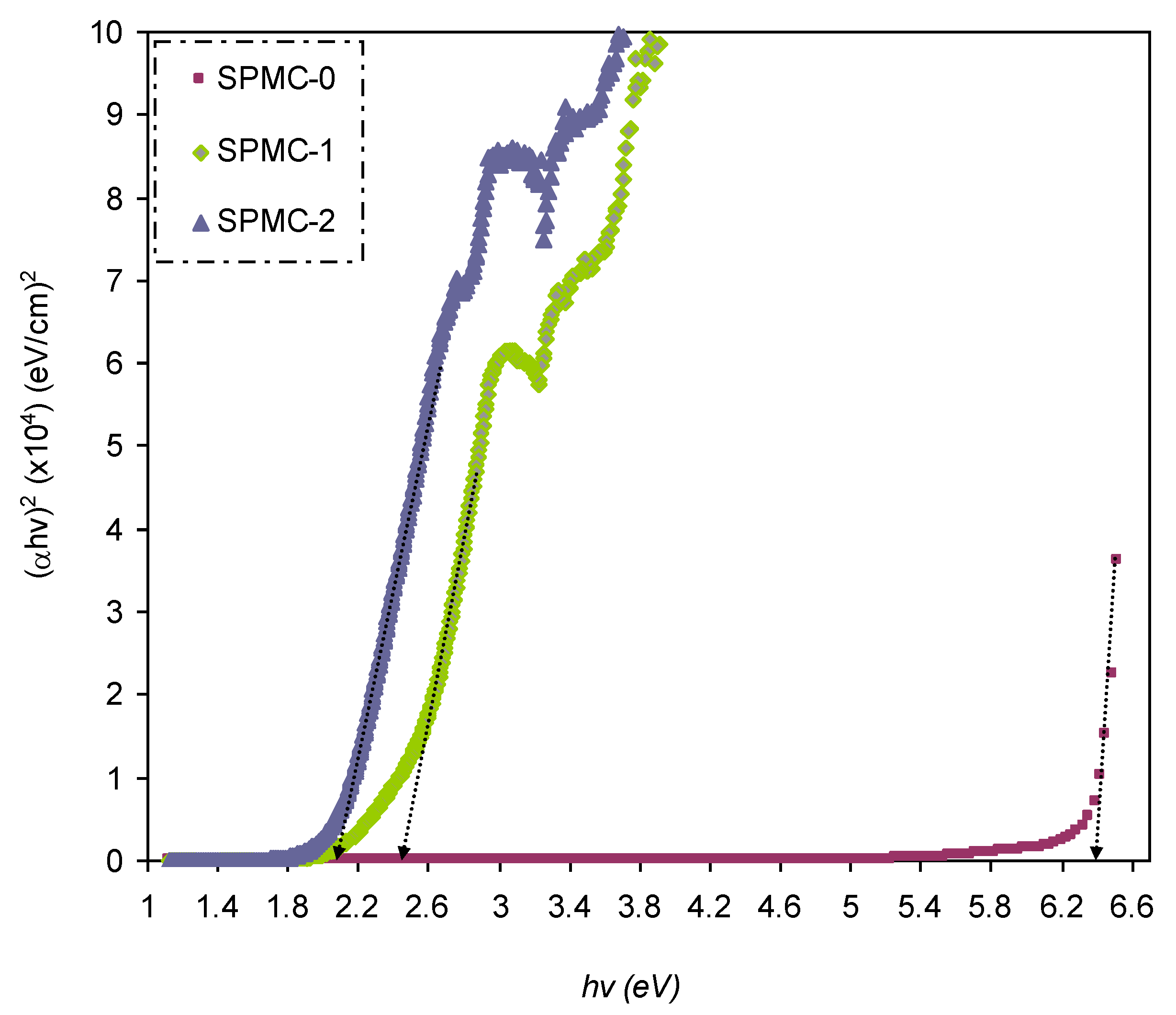
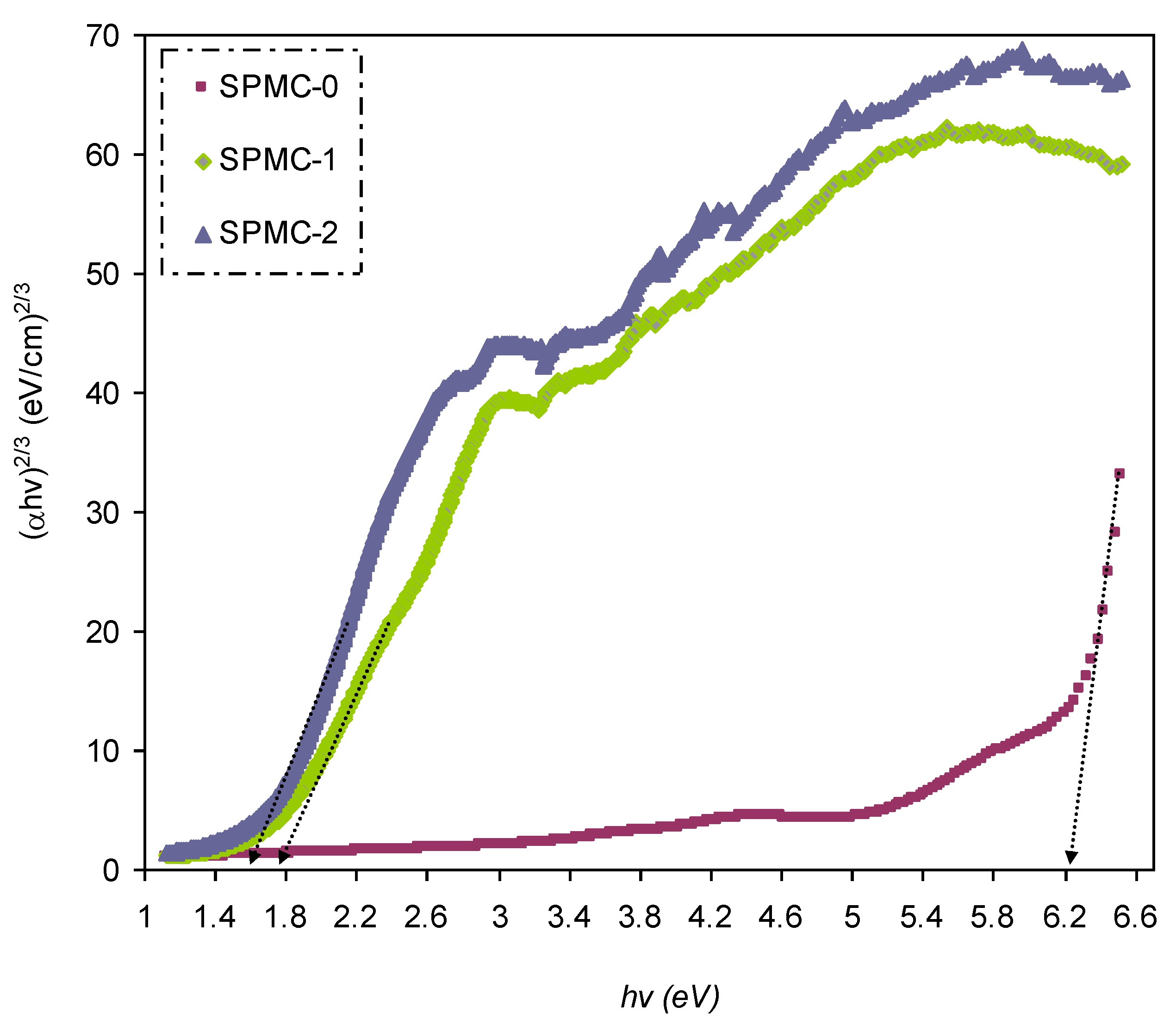
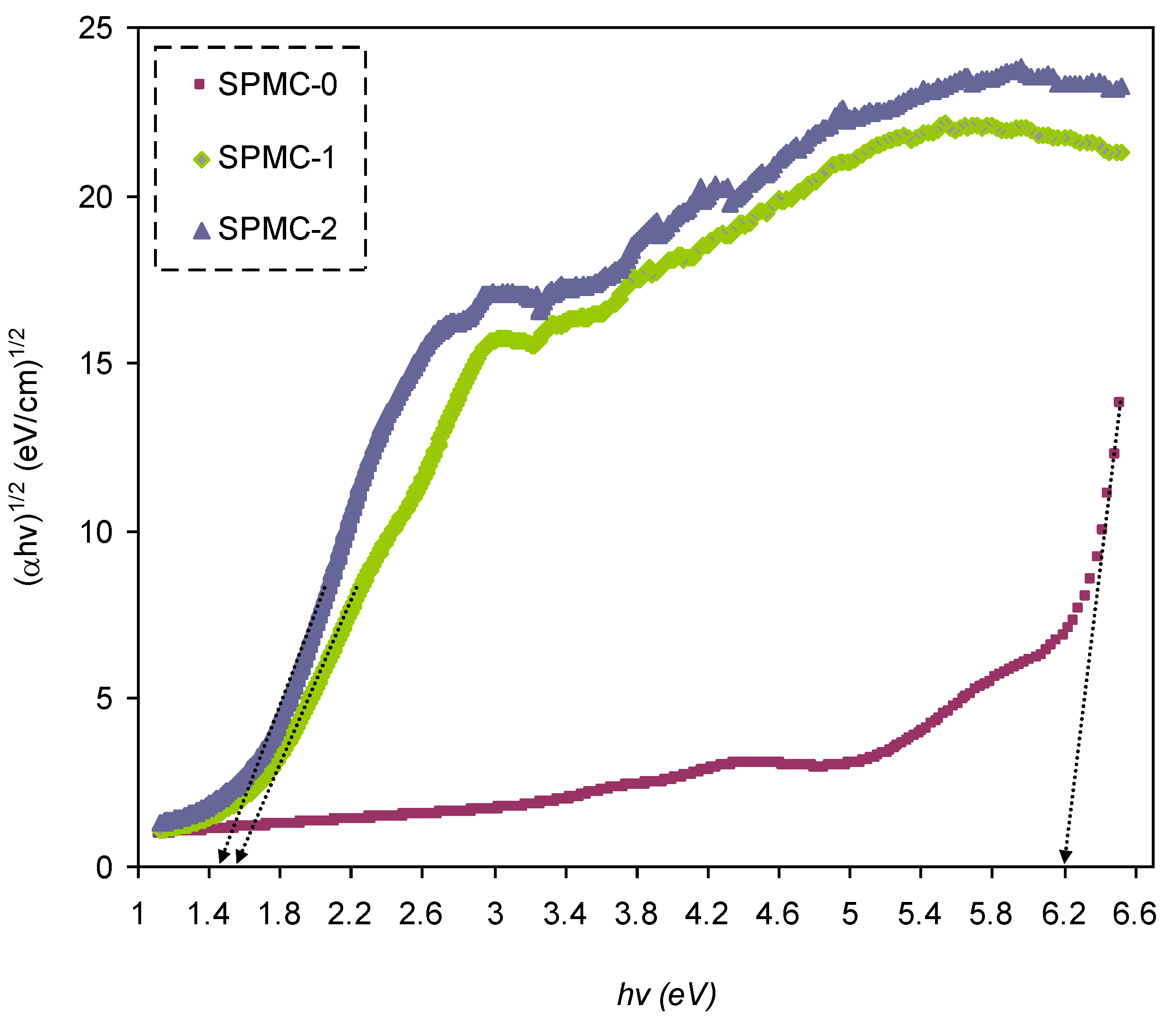
In
Figure 16,
Figure 17 and
Figure 18, from the extrapolated intersections of the linear part of the (
αhυ)
1/γ plots, in contradiction with
hυ on the horizontal axis, it is straightforward to determine the value of
Eg [
31]. It has previously been confirmed that the decrease in
Eg results from the introduction of several located states (i.e., trap states) into the forbidden bandgap by the insertion of fillers into the polymer matrix [
31,
102].
Table 6 presents the values of
Eg, which decrease when Co
2+-polyphenol complex insertion is increased. This may be due to the electronic structure rearrangement of the PVA polymer after addition of Co
2+-polyphenol complex, leading to imperfections in the PVA polymer [
104]. Consequently, the level trapping phenomenon occurs within the bandgap, mediating electron transitions from VB to CB [
102]. The two decisive parameters, i.e.,
Eg and the cut-off energy, can be obtained from Tauc’s model (see
Figure 15,
Figure 16 and
Figure 17) and
εi (see
Figure 13). Based on the values of both parameters, one can determine the electron transition types in the materials [
31]. The most likely types of transitions in parent PVA and PVA doped with Co
2+-polyphenol complex films are direct allowed (
γ = 1/2) and forbidden (
γ = 2/3) transitions. The material of choice must have a dominant direct bandgap if it is to be used in light-emitting diodes (LEDs), laser diodes, and photovoltaic cells.
Three materials effectively lower the
Eg, i.e., copper nanoparticles, copper powder, and Co
2+-polyphenol complex. Aziz et al. [
60] prepared a polystyrene composite system based on PS-Cu using up to 6 wt.%. copper powder dissolved in PS. It was shown that that the optimum Cu powder is 6 wt.%, in which the
Eg value can be decreased from 4.05 eV to around 3.65 eV. Aziz et al. [
61] also reported a nanocomposite system based on methylcellulose (MC) as a host polymer and doped with copper (II) sulfide nanoparticles. It was documented that the
Eg of MC could be manipulated, dropping from 6.2 eV to 2.3 eV when a quantity of 0.08 M of the (CuS) nanoparticles was used as a dopant.
In the present study, the Eg of PVA was lowered from 6.3 to 1.6 eV when 60 mL Co2+-polyphenol complex was added. Interestingly, it was shown that the Co2+-polyphenol complex was effective at lowering the Eg and superior to both copper nanoparticles and powder incorporations. Furthermore, bandgap manipulation was made possible due to the incorporation of the cobalt complex, giving rise to a low value of Eg, and an environmentally sustainable approach for the synthesis of polymer composites.
The refractive index dispersion of the prepared films was analyzed using experimental data fitting based upon the Wemple–DiDomenico (WDD) single oscillator model. The refractive index and its dispersion behavior are among the key optical material properties to be studied. The refractive index dispersion is crucial in evaluating the optical communication for spectral dispersion [
105].
In the normal region, the single oscillator model presented by WD can be implemented to examine the refractive index dispersion [
106,
107]. This is carried out by introducing a dispersion energy parameter (
Ed) to gauge the force of interband optical transition. This parameter combines both the coordination number and the charge allocation in each unit cell, and correlates with chemical bonding [
108,
109]. However, a single oscillator parameter (
Eo) is directly proportional to the oscillator energy (i.e., the average energy bandgap). The refractive index and the photon energy below the interband absorption edge may be related, as shown in the following semiempirical equation:
where
Eo and
Ed are constants denoting the single-oscillator and dispersive energies, respectively. Parameters
Eo and
Ed relate to the average excitation energy and structure disorder, enhancing the optical transition within the material’s band structure.
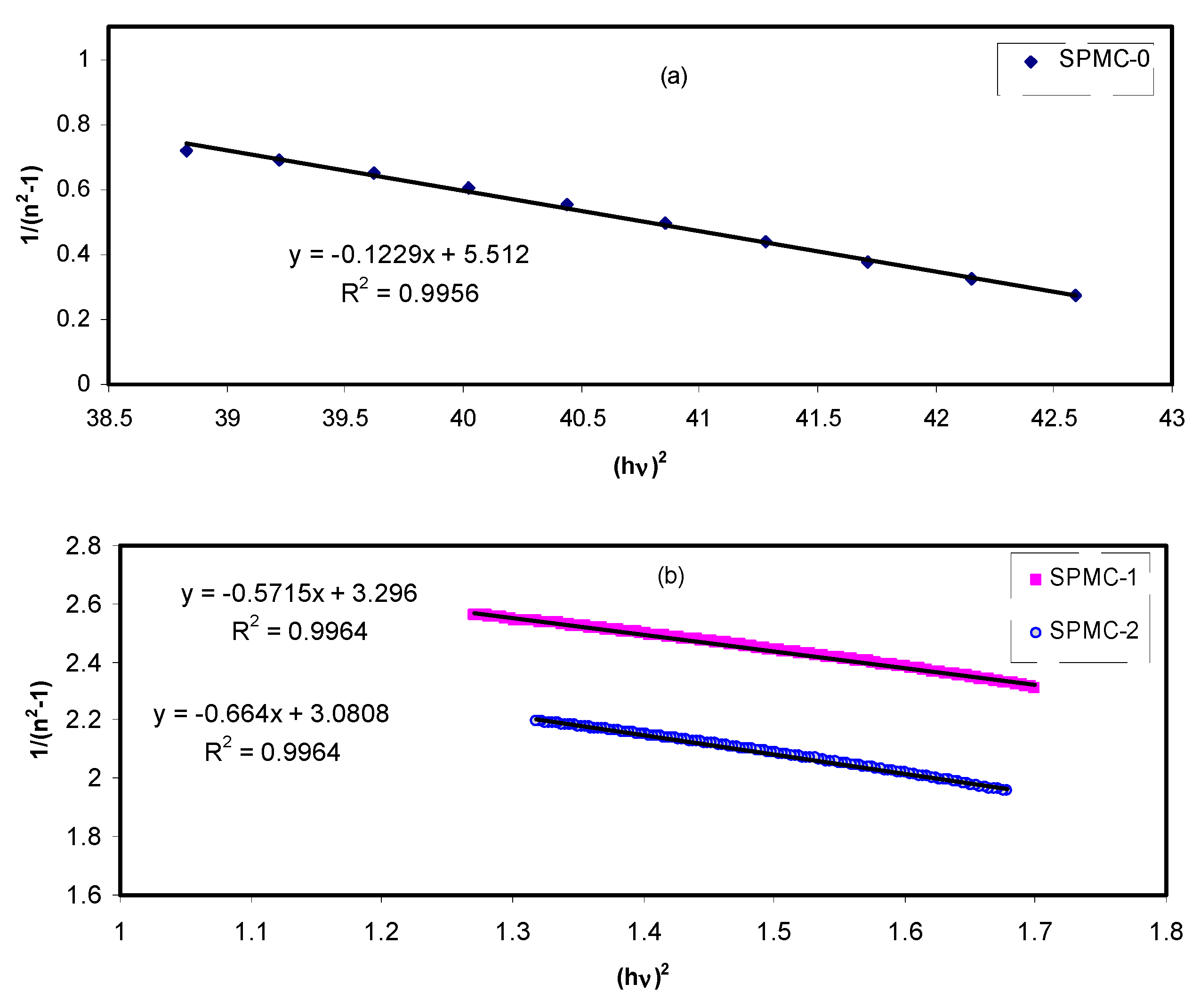
The
values are obtained from the slope of the linear portion of
verses
. The
values are determined from the intersection of the graph with the y-axis, as shown in
Figure 19.
The values of
Eo,
Ed, and parameters determined from the Wemple-Didomenic model are summarized in
Table 7.