The Dual Effect of Ionic Liquid Pretreatment on the Eucalyptus Kraft Pulp during Oxygen Delignification Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory Scale Pulp Preparation
2.3. Ionic Liquid Pretreatment
2.4. Oxygen Delignification
2.5. Pulp Analysis
2.6. Pulp Hand-Sheet Preparation
2.7. Extraction of Lignin from Kraft Pulp
2.8. Characterization Analysis
2.8.1. Ultraviolet Spectroscopy (UV) Analysis
2.8.2. Scanning Electron Microscopy (SEM) Analysis
2.8.3. X-ray Photoelectron Spectrometer (XPS) Analysis
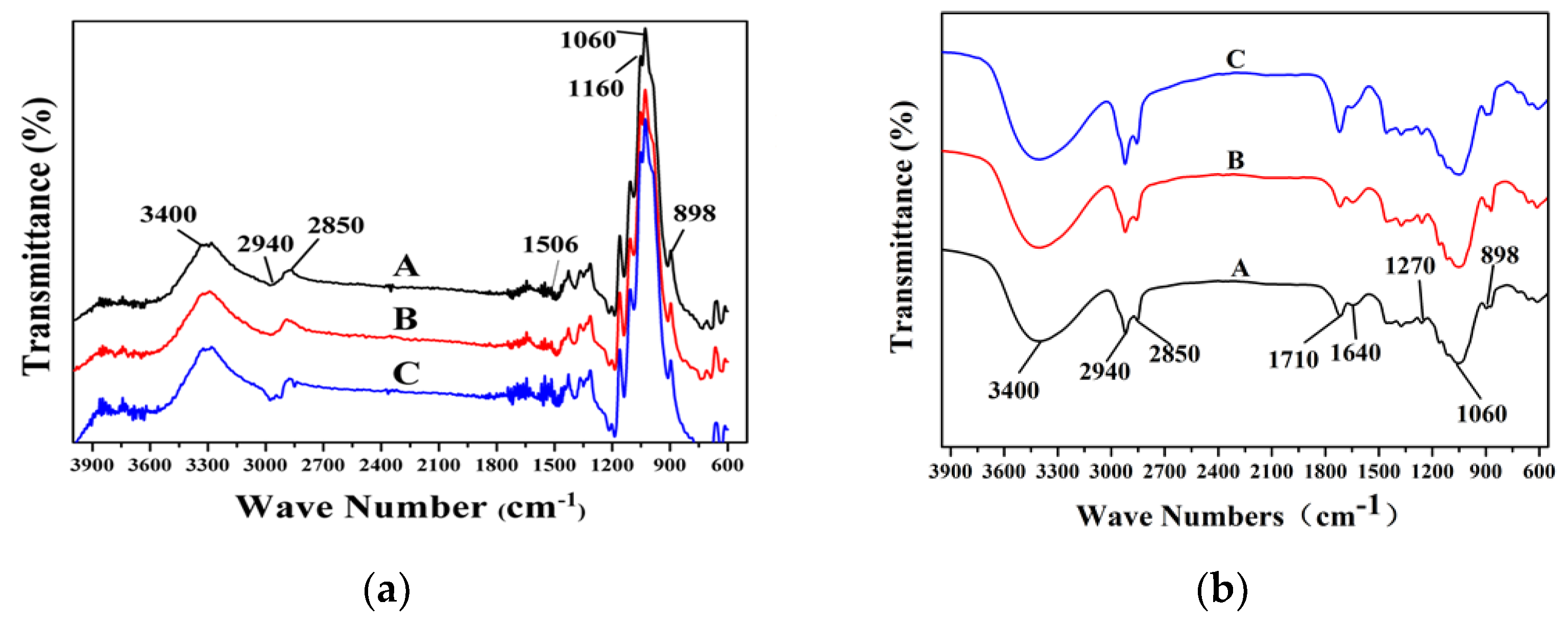
2.8.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
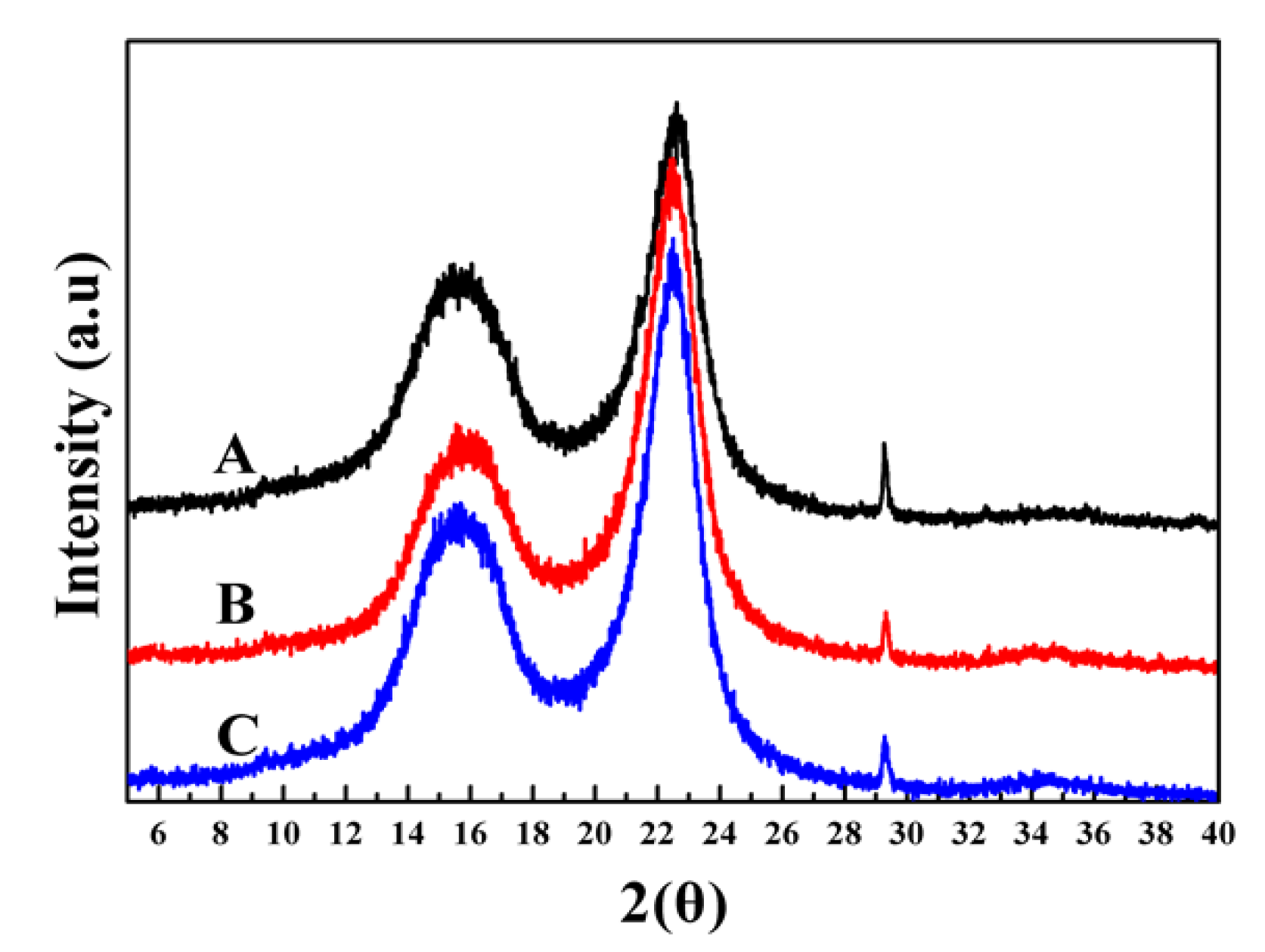
2.8.5. X-ray Diffraction (XRD) Analysis
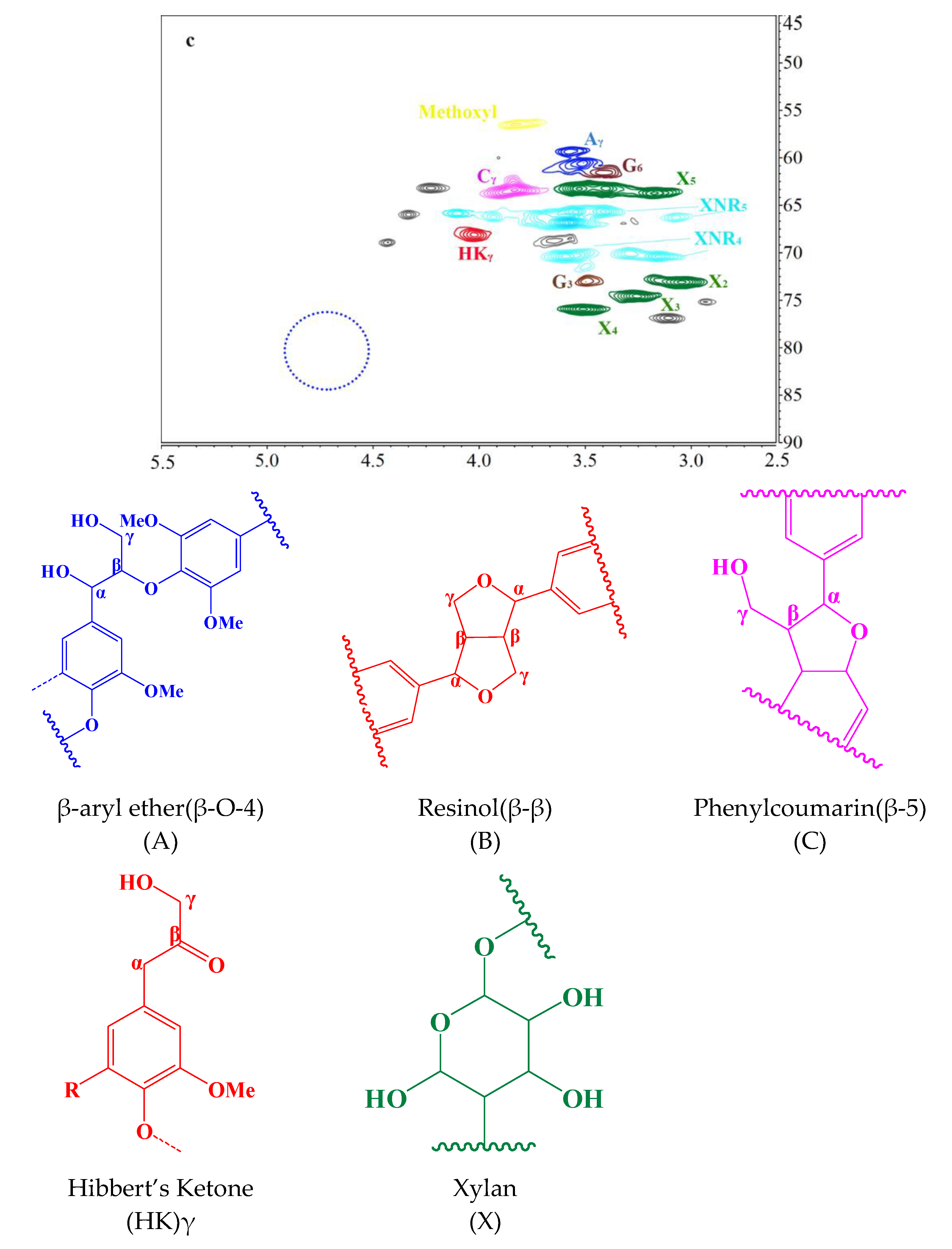
2.8.6. HSQC NMR Analysis
3. Results
3.1. Effect of IL Pretreatments on Pulp Bleachability
3.2. Effect of ILs Pretreatment on Pulp Properties
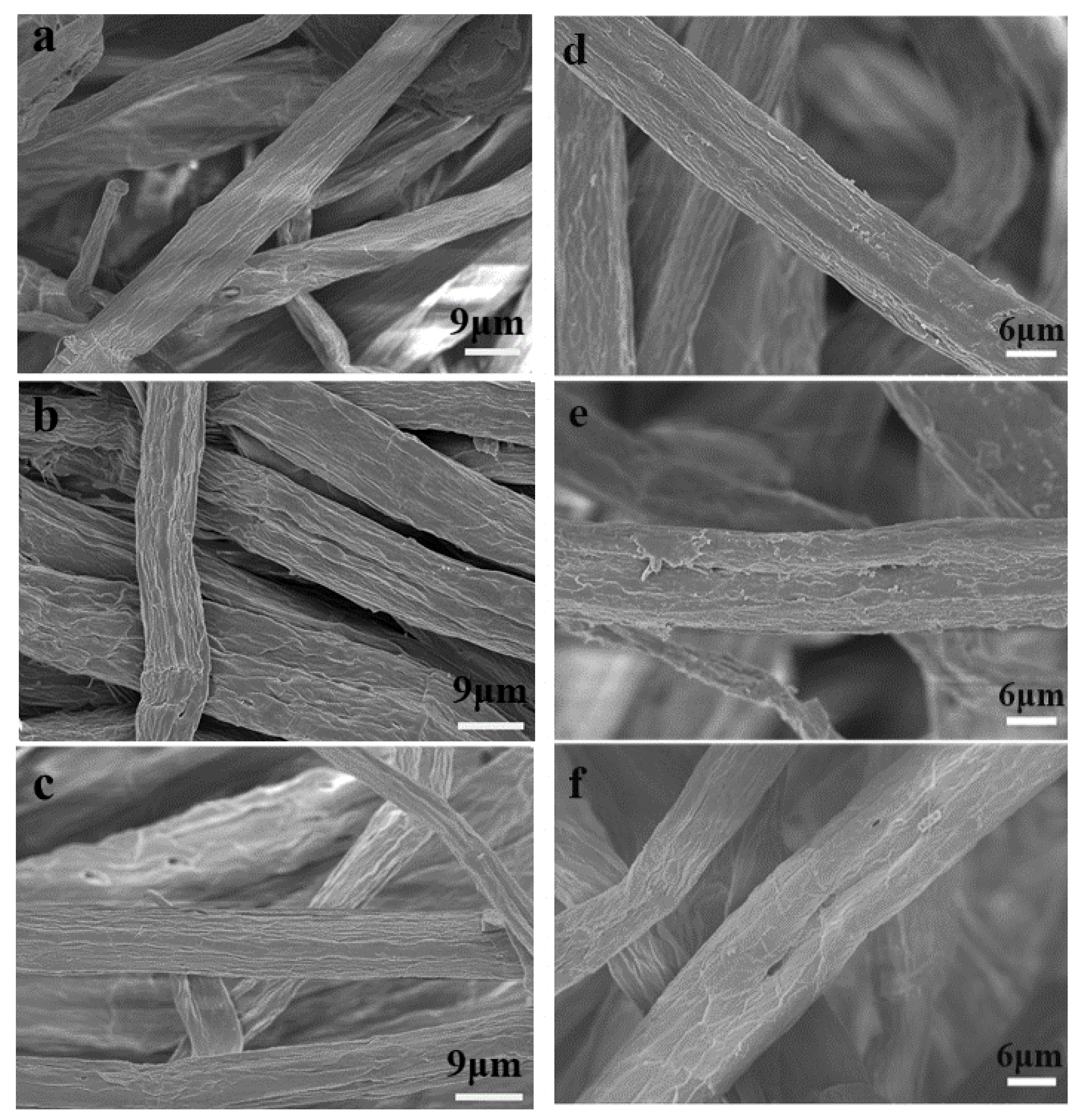
3.3. Pulp Fiber Surface Morphology and Characterization
3.4. Characterization of Oxygen Bleached Fibers
3.5. HSQC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lucia, L.; Ragauskas, A.J.; Jameel, H. Oxygen Delignification Chemistry and Its Impact on Pulp Fibers. J. Wood Chem. Technol. 2003, 23, 13–29. [Google Scholar] [CrossRef]
- Esteves, C.S.V.G.; Brännvall, E.; Östlund, S.; Sevastyanova, O. Evaluating the Potential to Modify Pulp and Paper Properties through Oxygen Delignification. ACS Omega 2020, 5, 13703–13711. [Google Scholar] [CrossRef]
- Esteves, C.S.V.G.; Sevastyanova, O.; Östlund, S.; Brännvall, E. Differences and similarities between kraft and oxygen delignification of softwood fibers: Effects on chemical and physical properties. Cellulose 2021, 28, 3149–3167. [Google Scholar] [CrossRef]
- Ding, N.; Liu, H.; Sun, Y.; Tang, X.; Lei, T.; Xu, F.; Zeng, X.; Lin, L. Lignin degradation in cooking with active oxygen and solid Alkali process: A mechanism study. J. Clean. Prod. 2021, 278, 123984. [Google Scholar] [CrossRef]
- Akim, L.G.; Colodette, J.L.; Argyropoulos, D.S. Factors limiting oxygen delignification of kraft pulp. Can. J. Chem. 2001, 79, 201–210. [Google Scholar] [CrossRef]
- Zhou, X. A one-pot catalysis combining laccase with Cu(salen) for selective removal of refractory lignin units during oxygen delignification of bamboo kraft pulp. Sci. For. 2020, 48, e3393. [Google Scholar] [CrossRef]
- Donaldson, L. Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci. Technol. 2007, 41, 443–460. [Google Scholar] [CrossRef]
- Jafari, V.; Nieminen, K.; Sixta, H.; van Heiningen, A. Delignification and cellulose degradation kinetics models for high lignin content softwood Kraft pulp during flow-through oxygen delignification. Cellulose 2015, 22, 2055–2066. [Google Scholar] [CrossRef]
- Gaspar, A.R.; Evtuguin, A.D.V.; Neto, C.P. Polyoxometalate-Catalyzed Oxygen Delignification of Kraft Pulp: A Pilot-Plant Experience. Ind. Eng. Chem. Res. 2004, 43, 7754–7761. [Google Scholar] [CrossRef]
- Koc, B.O.; Gümükaya, E.; Erisir, E.; Peman, E.; Krc, H. Comparison of reinforced oxygen delignification methods for old corrugated board (occ) fibres. Drewno 2017, 60, 1677–3985. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, X. Fundamental understanding of distracted oxygen delignification efficiency by dissolved lignin during biorefinery process of eucalyptus. Bioresour. Technol. 2018, 258, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gümüşkaya, E.; Peşman, E.; Kirci, H.; Uçar, M.B. Influence of plum gum and sodium perborate addition on spruce kraft pulp properties during oxygen delignification. Wood Sci. Technol. 2011, 45, 573–582. [Google Scholar] [CrossRef]
- Ji, X.; Chen, J.; Wang, Q.; Tian, Z.; Yang, G.; Liu, S. Boosting Oxygen Delignification of Poplar Kraft Pulp by Xylanase Pretreatment. Bioresources 2015, 10, 2518–2525. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.L.; Brennecke, J.F.; Holbrey, J.D.; Maginn, E.J.; Mantz, R.; Rogers, R.D.; Trulove, P.C.; Visser, A.E.; Welton, T. Physicochemical Properties of Ionic Liquids. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH Co. KGaA: Weinheim, Germany, 2003; pp. 41–126. [Google Scholar]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, T.; Sher, F.; Rasheed, T.; Zafar, F.; Zhang, S.; Murugesan, T. Evaluation of current and future solvents for selective lignin dissolution—A review. J. Mol. Liq. 2021, 321, 114577. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef]
- Lopes, A.M.D.C.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-L.; Chen, X.-M.; Han, Y.-J.; Wang, X.-Q.; Potprommanee, L.; Ning, X.-A.; Liu, J.-Y.; Sun, J.; Peng, Y.-P.; Sun, S.-Y.; et al. Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour. Technol. 2016, 214, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Uju; Goto, M.; Kamiya, N. Powerful peracetic acid–ionic liquid pretreatment process for the efficient chemical hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2016, 214, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, B.; Hou, H.; Hu, J. Pretreatment of eucalyptus with recycled ionic liquids for low-cost biorefinery. Bioresour. Technol. 2017, 234, 406–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Pang, Z.; Lucia, L.A.; Li, F.; Yang, G. Ionic Liquids as a New Platform for Fiber Brittleness Removal. Bioresources 2015, 10, 6565–6575. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Jiang, Q.; Yang, G.; Wang, Q.; Fatehi, P. Ultrasonic-assisted ionic liquid treatment of chemithermomechanical pulp fibers. Cellulose 2017, 24, 1483–1491. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Dong, C.; Yang, G. Highly selective and efficient extraction of lignin in kraft pulp by aqueous ionic liquids for enhanced bleaching properties. RSC Adv. 2014, 4, 29897–29900. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef] [Green Version]
- Asim, A.M.; Uroos, M.; Naz, S.; Muhammad, N. Pyridinium protic ionic liquids: Effective solvents for delignification of wheat straw. J. Mol. Liq. 2021, 325, 115013. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Peng, J.; Qi, L.; Yang, G.; He, M.; Xue, Y.; Chen, J. Effect of Ultrasonic-assisted Ionic Liquid Pretreatment on the Bleachability and Properties of Eucalyptus Kraft Pulp. J. Korea Tech. Assoc. Pulp Pap. Ind. 2019, 51, 16–25. [Google Scholar] [CrossRef]
- Peng, J.; Yang, G.; Qi, L.; Liu, J.; Li, F.; Chen, J.; Lucia, L.A. Enhancement of Delignification by Ionic Liquids Pre-treatment and Modification of Hardwood Kraft Pulp in Preparation for Bleaching. BioResources 2020, 15, 6299–6308. [Google Scholar] [CrossRef]
- Mazumder, B.B.; Ohtani, Y.; Cheng, Z.; Sameshima, K. Combination treatment of kenaf bast fiber for high viscosity pulp. J. Wood Sci. 2000, 46, 364–370. [Google Scholar] [CrossRef]
- Liu, J.; Qi, L.; Yang, G.; Xue, Y.; He, M.; Lucia, L.; Chen, J. Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content. Polymers 2020, 12, 1599. [Google Scholar] [CrossRef]
- Johansson, L.-S.; Campbell, J.; Koljonen, K.; Stenius, P. Evaluation of surface lignin on cellulose fibers with XPS. Appl. Surf. Sci. 1999, 144–145, 92–95. [Google Scholar] [CrossRef]
- Ju, X.; Engelhard, M.; Zhang, X. An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2013, 132, 137–145. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Peng, J. Effects of Ionic Liquid Assisted Ultrasonic Pretreatment on the Separation of Broad-Leaf Kraft Pulps. Master’s Thesis, Qilu University of Technology, Jinan, China, 2020. [Google Scholar]
- Brandt, A.; Welton, T.; Chen, L.; Van Dongen, B.E.; Hallett, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, Y.; Yoo, C.G.; Yang, X.; Lin, X.; Ralph, J.; Pan, X. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Miles-Barrett, D.M.; Neal, A.R.; Hand, C.; Montgomery, J.R.D.; Panovic, I.; Ojo, O.S.; Lancefield, C.S.; Cordes, D.B.; Slawin, A.M.Z.; Lebl, T.; et al. The synthesis and analysis of lignin-bound Hibbert ketone structures in technical lignins. Org. Biomol. Chem. 2016, 14, 10023–10030. [Google Scholar] [CrossRef] [Green Version]
- Stärk, K.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Oxidative Depolymerization of Lignin in Ionic Liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef]
- Kim, A.-R.; Choi, K.-H.; Cho, B.-U. Effects of Kneading Treatment on the Properties of Various Pulp Fibers. J. Korea Tech. Assoc. Pulp Pap. Ind. 2015, 47, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.; Liu, M.; Jameel, H.; Zhang, S.; Zhang, Z. Hydrothermal Pretreatment of Hardwood Chips Prior to Alkaline Pulping and D0(EP)D1 Bleaching. BioResources 2014, 9, 6193–6204. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, J.; Douek, M. Structural and Concentration Effects on the Diffuse Reflectance Ftir Spectra of Cellulose, Lignin and Pulp. J. Wood Chem. Technol. 1993, 13, 481–499. [Google Scholar] [CrossRef]
- Sun, R.; Lawther, J.; Banks, W. Fractional and structural characterization of wheat straw hemicelluloses. Carbohydr. Polym. 1996, 29, 325–331. [Google Scholar] [CrossRef]
- Roberts, J.C. The Chemistry of Paper; The Royal Society of Chemistry/Information Services: Cambridge, UK, 1996; ISBN 0-85404-518-X. [Google Scholar] [CrossRef]


| Pulp | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| KP | 81.77 ± 0.15 | 3.52 ± 0.06 | 15.30 ± 0.22 |
| Control | 93.11 ± 0.09 | 1.28 ± 0.07 | 6.76 ± 0.21 |
| [BMIM][HSO4] | 94.14 ± 0.17 | 0.52 ± 0.10 | 5.96 ± 0.15 |
| [TEA][HSO4] | 94.22 ± 0.11 | 2.05 ± 0.11 | 3.52 ± 0.17 |
| Pulp | Brightness (%ISO) | Kappa Number | Viscosity (mL/g) | DP | Pulp Yield (%) | WRV (g/g) | Quinone Compounds (D450) 1 |
|---|---|---|---|---|---|---|---|
| KP | 28.24 ± 0.20 | 20.04 ± 0.08 | 1015 ± 3 | 1527 | 100.0 | 1.32 ± 0.02 | -- |
| Control | 56.07 ± 0.22 | 8.06 ± 0.08 | 863 ± 2 | 1277 | 99.3 | 1.46 ± 0.07 | 0.212 |
| [BMIM][HSO4] | 54.27 ± 0.17 | 7.47 ± 0.08 | 905 ± 4 | 1346 | 98.6 | 1.45 ± 0.03 | 0.269 |
| [TEA][HSO4] | 42.28 ± 0.09 | 7.04 ± 0.08 | 999 ± 3 | 1501 | 98.2 | 1.62 ± 0.02 | 0.334 |
| Pulps | Fiber Length (mm) | Fiber Width (μm) | Curl Index (%) | Fines Content (%) | |
|---|---|---|---|---|---|
| Lc(n) | Lc(w) | ||||
| KP | 0.679 | 0.858 | 14.29 | 9.12 | 7.57 |
| Control | 0.647 | 0.800 | 13.46 | 14.48 | 6.02 |
| [BMIM][HSO4] | 0.666 | 0.840 | 13.79 | 14.81 | 5.99 |
| [TEA][HSO4] | 0.670 | 0.829 | 13.94 | 15.66 | 6.28 |
| Pulps | Tensile Index (N·m·g−1) | Tear Index (mN·m2·g−1) | Folding Endurance (times) |
|---|---|---|---|
| Control | 73.25 ± 0.13 | 8.47 ± 0.19 | 166 ± 2 |
| [BMIM][HSO4] | 75.50 ± 0.38 | 9.32 ± 0.09 | 275 ± 4 |
| [TEA][HSO4] | 81.00 ± 0.38 | 9.81 ± 0.21 | 348 ± 3 |
| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3400 | O–H stretching vibration |
| 2940, 2850 | C–H stretching vibration in methyl |
| 1710 | unconjugated C = O stretching vibration |
| 1640 | Aromatic ring skeleton vibration |
| 1270 | C–O stretching vibration of guaiacyl units |
| 1050 | C–H bending vibration of guaiacyl units |
| 898 | β-type glycosidic bond |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Liu, J.; Peng, J.; Yang, G.; Li, F.; Xue, Y.; Chen, J. The Dual Effect of Ionic Liquid Pretreatment on the Eucalyptus Kraft Pulp during Oxygen Delignification Process. Polymers 2021, 13, 1600. https://doi.org/10.3390/polym13101600
Qi L, Liu J, Peng J, Yang G, Li F, Xue Y, Chen J. The Dual Effect of Ionic Liquid Pretreatment on the Eucalyptus Kraft Pulp during Oxygen Delignification Process. Polymers. 2021; 13(10):1600. https://doi.org/10.3390/polym13101600
Chicago/Turabian StyleQi, Letian, Jinke Liu, Jianmin Peng, Guihua Yang, Fengfeng Li, Yu Xue, and Jiachuan Chen. 2021. "The Dual Effect of Ionic Liquid Pretreatment on the Eucalyptus Kraft Pulp during Oxygen Delignification Process" Polymers 13, no. 10: 1600. https://doi.org/10.3390/polym13101600
APA StyleQi, L., Liu, J., Peng, J., Yang, G., Li, F., Xue, Y., & Chen, J. (2021). The Dual Effect of Ionic Liquid Pretreatment on the Eucalyptus Kraft Pulp during Oxygen Delignification Process. Polymers, 13(10), 1600. https://doi.org/10.3390/polym13101600






