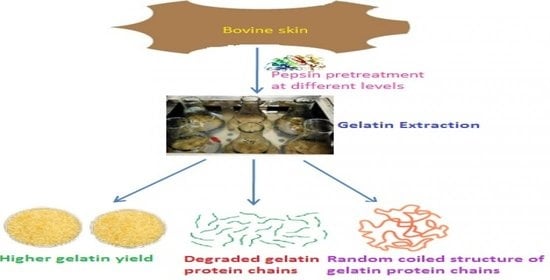
Recovery of Gelatin from Bovine Skin with the Aid of Pepsin and Its Effects on the Characteristics of the Extracted Gelatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Skin
2.3. Gelatin Recovery from Bovine Skin Using Pepsin Pretreatment
2.3.1. Removal of Non-Collagenous Proteins
2.3.2. Gelatin Extraction with the Aid of Pepsin
2.4. Analyses of Gelatin
2.4.1. Gelatin Yield
2.4.2. pH
2.4.3. Color
2.4.4. Amino Acid Profile
2.4.5. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.6. Free Amino Acid Content
2.4.7. Gel Strength
2.4.8. Turbidity
2.4.9. Viscosity
2.4.10. 1H NMR Measurements
2.4.11. FTIR Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Pepsin on Gelatin Recovery
3.2. pH
3.3. Color
3.4. Amino Acid Profile
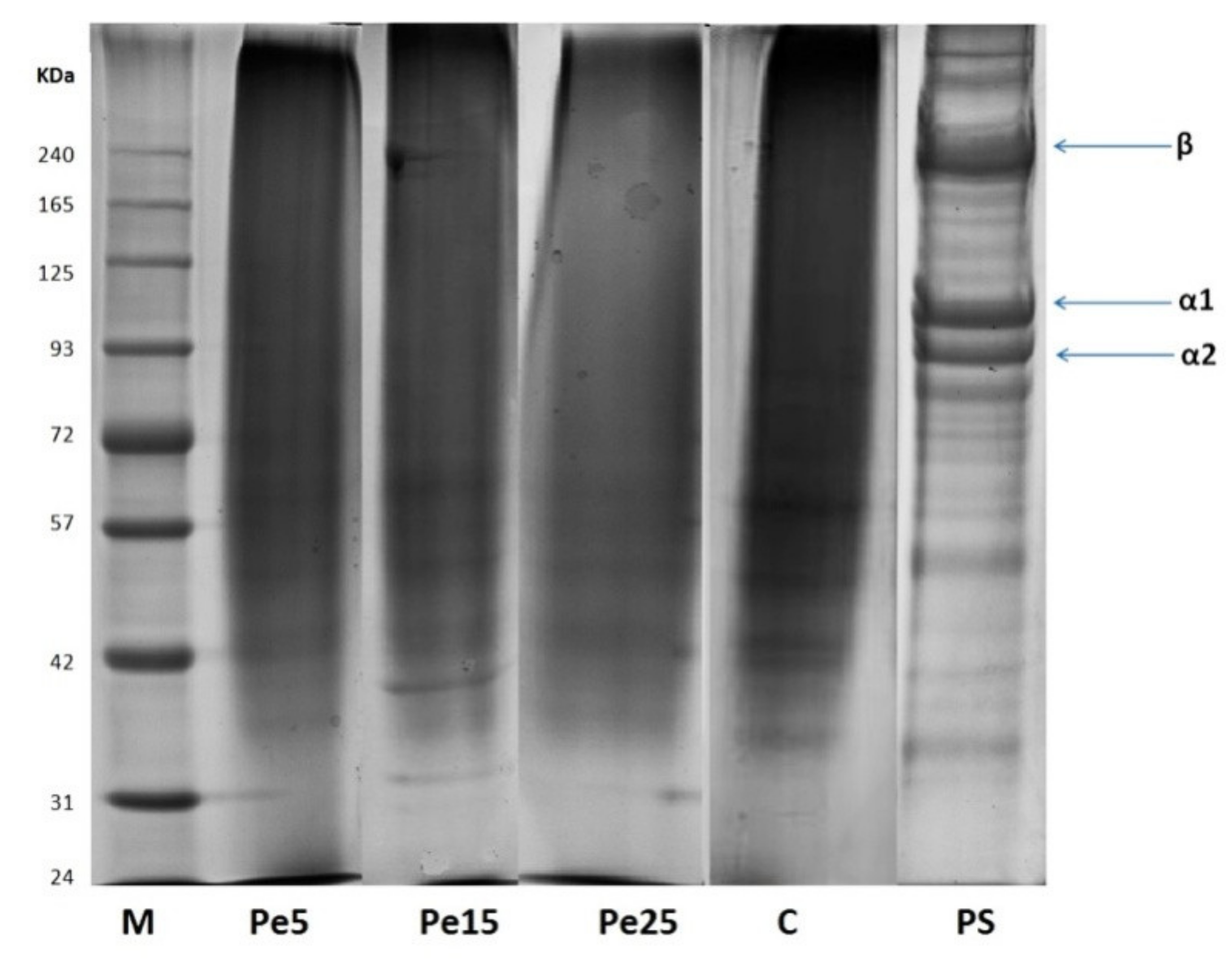
3.5. SDS-PAGE Analysis
3.6. Free Amino Acid Content
3.7. Gel Strength
3.8. Turbidity
3.9. Viscosity
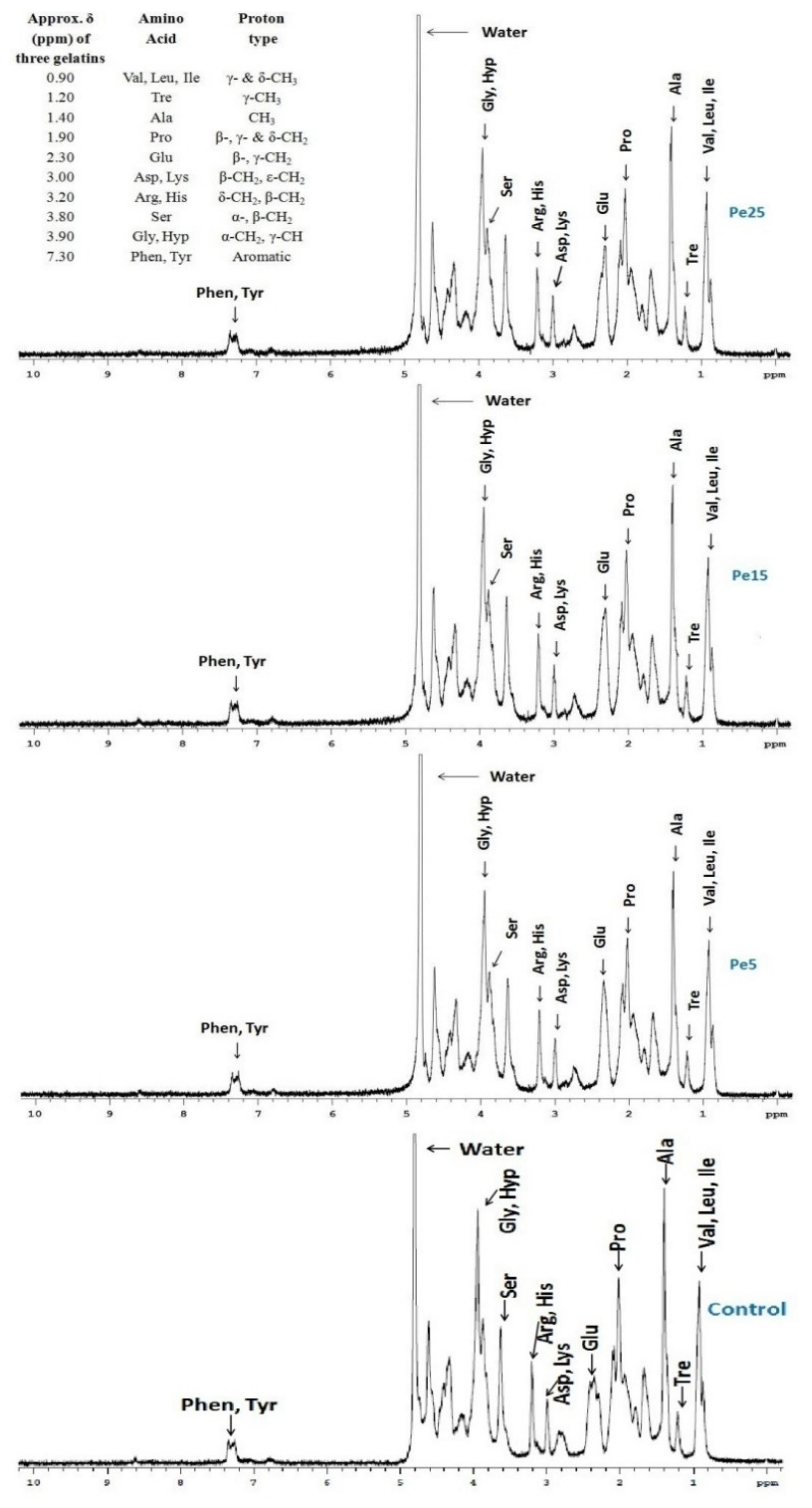
3.10. 1H NMR Measurements
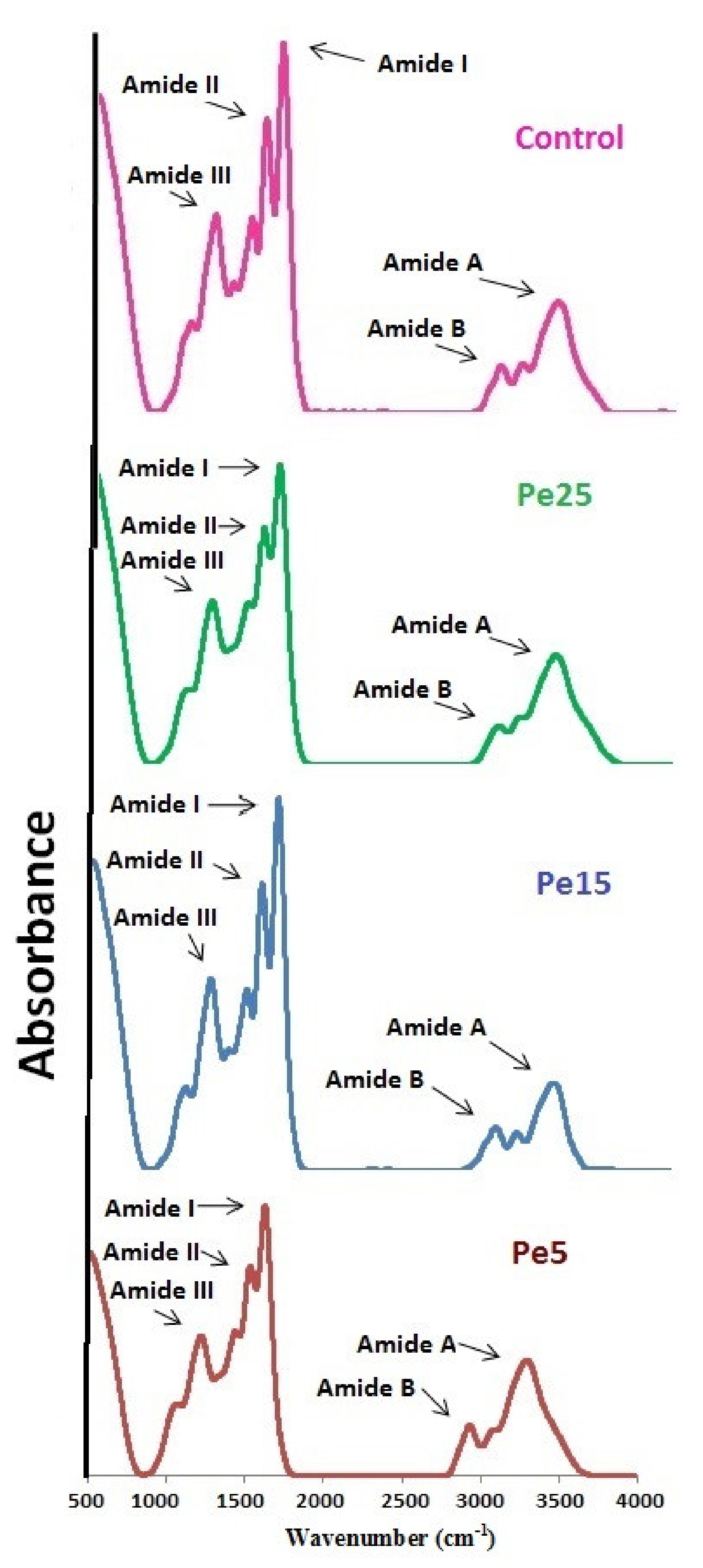
3.11. FTIR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Regenstein, J.M. Optimization of gelatin extraction from silver carp skin. J. Food Sci. 2009, 74, E432–E441. [Google Scholar] [CrossRef] [PubMed]
- Galea, C.A.; Dalrymple, B.P.; Kuypers, R.; Blakeley, R. Modification of the substrate specificity of porcine pepsin for the enzymatic production of bovine hide gelatin. Protein. Sci. 2000, 9, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008, 22, 615–622. [Google Scholar] [CrossRef]
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and biophysical properties of gelatins extracted from alkali-pretreated skin of cuttlefish (Sepia officinalis) using pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M.; Barkia, A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 309–318. [Google Scholar] [CrossRef]
- Chomarat, N.; Robert, L.; Seris, J.; Kern, P. Comparative efficiency of pepsin and proctase for the preparation of bovine skin gelatin. Enzyme Microb. Technol. 1994, 16, 756–760. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kee, L.T.; Awad, E.A.; Sazili, A.Q. Extraction, characterization and molecular structure of bovine skin gelatin extracted with plant enzymes bromelain and zingibain. J. Food Sci. Technol. 2020, 57, 3772–3781. [Google Scholar] [CrossRef]
- Ktari, N.; Bkhairia, I.; Jridi, M.; Hamza, I.; Riadh, B.S.; Nasri, M. Digestive acid protease from zebra blenny (Salaria basilisca): Characteristics and application in gelatin extraction. Food Res. Int. 2014, 57, 218–224. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S. Asian bullfrog (Rana tigerina) skin gelatin extracted by ultrasound-assisted process: Characteristics and in-vitro cytotoxicity. Int. J. Biol. Macromol. 2020, 148, 391–400. [Google Scholar] [CrossRef]
- Eastoe, J.; Leach, A. Chemical Constitution of Gelatin [from Mammals, Chicken Tendon, Calf Skin, Pig Skin]; The Science and Technology of Gelatin; Academic Press Inc.: London, UK, 1977; pp. 73–105. [Google Scholar]
- Awad, E.A.; Zulkifli, I.; Farjam, A.S.; Chwen, L.T. Amino acids fortification of low-protein diet for broilers under tropical climate. 2. Nonessential amino acids and increasing essential amino acids. Ital. J. Anim. Sci. 2014, 13, 3297. [Google Scholar] [CrossRef]
- Fernandez-Dıaz, M.; Montero, P.; Gomez-Guillen, M. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by the coenhancers magnesium sulphate, glycerol and transglutaminase. Food Chem. 2001, 74, 161–167. [Google Scholar] [CrossRef]
- Cho, S.; Kwak, K.; Park, D.; Gu, Y.; Ji, C.; Jang, D.; Lee, Y.; Kim, S. Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocoll. 2004, 18, 573–579. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Skin gelatin from bigeye snapper and brownstripe red snapper: Chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocoll. 2006, 20, 1216–1222. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Turnay, J.; Fernández-Dıaz, M.; Ulmo, N.; Lizarbe, M.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Bougatef, A.; Balti, R.; Sila, A.; Nasri, R.; Graiaa, G.; Nasri, M. Recovery and physicochemical properties of smooth hound (Mustelus mustelus) skin gelatin. LWT-Food Sci. Technol. 2012, 48, 248–254. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Koç, A.; Erbaş, M. Extraction and physicochemical characterization of broiler (Gallus gallus domesticus) skin gelatin compared to commercial bovine gelatin. Poult. Sci. 2017, 96, 4124–4131. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, N.F.; Perera, C.; Quek, S.-Y. Optimisation of gelatine extraction from hoki (Macruronus novaezelandiae) skins and measurement of gel strength and SDS–PAGE. Food Chem. 2010, 122, 307–313. [Google Scholar] [CrossRef]
- Mulyani, S.; Setyabudi, F.M.S.; Pranoto, Y.; Santoso, U. Physicochemical properties of gelatin extracted from buffalo hide pretreated with different acids. Korean J. Food Sci. Anim. Resour. 2017, 37, 708–715. [Google Scholar] [CrossRef]
- Zhou, P.; Regenstein, J. Determination of total protein content in gelatin solutions with the Lowry or Biuret assay. J. Food Sci. 2006, 71, C474–C479. [Google Scholar] [CrossRef]
- Al-Hassan, A.A. Gelatin from camel skins: Extraction and characterizations. Food Hydrocoll. 2020, 101, 105457. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Giménez, B.; Turnay, J.; Lizarbe, M.; Montero, P.; Gómez-Guillén, M. Use of lactic acid for extraction of fish skin gelatin. Food Hydrocoll. 2005, 19, 941–950. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Comparative study on characteristics of gelatin from the skins of brownbanded bamboo shark and blacktip shark as affected by extraction conditions. Food Hydrocoll. 2010, 24, 164–171. [Google Scholar] [CrossRef]
- Balti, R.; Jridi, M.; Sila, A.; Souissi, N.; Nedjar-Arroume, N.; Guillochon, D.; Nasri, M. Extraction and functional properties of gelatin from the skin of cuttlefish (Sepia officinalis) using smooth hound crude acid protease-aided process. Food Hydrocoll. 2011, 25, 943–950. [Google Scholar] [CrossRef]
- Pitpreecha, S.; Damrongsakkul, S. Hydrolysis of raw hide using proteolytic enzyme extracted from papaya latex. Korean J. Chem. Eng. 2006, 23, 972–976. [Google Scholar] [CrossRef]
- Karim, A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, H.; Su, W. Characterization of edible films based on tilapia (Tilapia zillii) scale gelatin with different extraction pH. Food Hydrocoll. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T.; Nalinanon, S. Physicochemical and functional properties of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as affected by extraction conditions. Food Biosci. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Tu, Z.-c.; Huang, T.; Wang, H.; Sha, X.-m.; Shi, Y.; Huang, X.-q.; Man, Z.-z.; Li, D.-j. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J. Food Sci. Technol. 2015, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Damrongsakkul, S.; Ratanathammapan, K.; Komolpis, K.; Tanthapanichakoon, W. Enzymatic hydrolysis of rawhide using papain and neutrase. J. Ind. Eng. Chem. Res. 2008, 14, 202–206. [Google Scholar] [CrossRef]
- Santana, J.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.; Llanos, S.A.; Heredia, J.A.; Zamuner, S.; Gamarra, F.; Farias, T. Valorization of chicken feet by-product of the poultry industry: High qualities of gelatin and biofilm from extraction of collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Norziah, M.; Kee, H.; Norita, M. Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Biosci. 2014, 5, 9–18. [Google Scholar] [CrossRef]
- Tabarestani, H.S.; Maghsoudlou, Y.; Motamedzadegan, A.; Mahoonak, A.S. Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresour. Technol. 2010, 101, 6207–6214. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.; Ninan, G.; Mathew, S.; Joshy, C.; Lakshmanan, P. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Jamilah, B.; Tan, K.W.; Umi Hartina, M.R.; Azizah, A. Gelatins from three cultured freshwater fish skins obtained by liming process. Food Hydrocoll. 2011, 25, 1256–1260. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Hafsteinsson, H. Gelatin from cod skins as affected by chemical treatments. J. Food Sci. 1997, 62, 37–39. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Santacruz-Ortega, H.; Cinco-Moroyoqui, F.J.; Rouzaud-Sández, O.; Plascencia-Jatomea, M.; Ezquerra-Brauer, J.M. Giant squid skin gelatin: Chemical composition and biophysical characterization. Food Res. Int. 2011, 44, 3243–3249. [Google Scholar] [CrossRef]
- Voron’ko, N.G.; Derkach, S.R.; Vovk, M.A.; Tolstoy, P.M. Complexation of κ-carrageenan with gelatin in the aqueous phase analysed by 1H NMR kinetics and relaxation. Carbohydr. Polym. 2017, 169, 117–126. [Google Scholar] [CrossRef]
- Fullerton, G.D.; Nes, E.; Amurao, M.; Rahal, A.; Krasnosselskaia, L.; Cameron, I. An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol. Int. 2006, 30, 66–73. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Arias-Moscoso, J.L.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.; Rouzaud-Sández, O.; Cardenas-Lopez, J.L.; Marquez-Rios, E.; Ezquerra-Brauer, J.M. Jumbo squid (Dosidicus gigas) mantle collagen: Extraction, characterization, and potential application in the preparation of chitosan–collagen biofilms. Bioresour. Technol. 2010, 101, 4212–4219. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Gelatin from clown featherback skin: Extraction conditions. LWT-Food Sci. Technol. 2016, 66, 186–192. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Awad, E.A.; Leo, T.K.; Imlan, J.C.; Sazili, A.Q. Characterization of gelatin from bovine skin extracted using ultrasound subsequent to bromelain pretreatment. Food Hydrocoll. 2018, 80, 264–273. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]

| Sample | Yield (%) | pH | Gel Strength (g) | Turbidity (ppm) | Viscosity (mPa·s) |
|---|---|---|---|---|---|
| Control | 17.90 ± 0.19 c | 2.16 ± 0.05 a | 283.35 ± 1.84 a | 12.53 ± 0.20 d | 12.10 ± 0.23 a |
| Pe5 | 18.17 ± 0.12 c | 2.26 ± 0.03 a | 215.49 ± 2.15 b | 18.97 ± 0.32 c | 9.17 ± 0.09 b |
| Pe15 | 22.79 ± 0.23 b | 2.23 ± 0.02 a | 78.58 ± 0.95 c | 21.07 ± 0.18 b | 8.10 ± 0.12 c |
| Pe25 | 24.67 ± 0.31 a | 2.27 ± 0.05 a | 56.06 ± 0.99 d | 24.00 ± 0.46 a | 8.17 ± 0.03 c |
| Sample | L* | a* | b* |
|---|---|---|---|
| Control | 68.19 ± 0.14 a | 1.10 ± 0.06 c | 9.52 ± 0.11 b |
| Pe5 | 47.57 ± 1.15 d | 1.19 ± 0.09 c | 5.65 ± 0.08 c |
| Pe15 | 52.28 ± 0.26 c | 2.19 ± 0.07 a | 12.12 ± 0.10 a |
| Pe25 | 58.71 ± 0.08 b | 1.92 ± 0.01 b | 10.86 ± 0.04 b |
| Amino Acids | Gelatin Samples | |||
|---|---|---|---|---|
| Control | Pe5 | Pe15 | Pe25 | |
| Hydroxyproline (Hyp) | 14.14 ± 1.10 | 14.87 ± 1.13 | 14.43 ± 1.13 | 14.76 ± 1.13 |
| Aspartic acid (Asp) | 4.06 ± 0.47 | 3.59 ± 0.44 | 3.60 ± 0.44 | 3.54 ± 0.36 |
| Serine (Ser) | 2.82 ± 0.58 | 3.25 ± 0.68 | 3.19 ± 0.68 | 3.20 ± 0.45 |
| Glutamic acid (Glu) | 7.81 ± 0.70 | 7.34 ± 0.58 | 7.28 ± 0.58 | 7.18 ± 0.70 |
| Glycine (Gly) | 19.87 ± 1.61 | 21.56 ± 1.52 | 21.44 ± 1.52 | 21.47 ± 1.61 |
| Histidine (His) | 0.82 ± 0.26 | 0.96 ± 0.16 | 0.89 ± 0.16 | 0.93 ± 0.23 |
| Arginine (Arg) | 6.87 ± 0.79 | 7.54 ± 0.79 | 7.24 ± 0.79 | 7.35 ± 0.68 |
| Threonine (Thr) | 1.63 ± 0.35 | 1.84 ± 0.34 | 1.77 ± 0.34 | 1.79 ± 0.45 |
| Alanine (Ala) | 6.50 ± 0.63 | 6.69 ± 0.70 | 6.73 ± 0.70 | 6.70 ± 0.40 |
| Proline (Pro) | 10.29 ± 0.94 | 10.46 ± 1.26 | 10.72 ± 1.26 | 10.51 ± 1.26 |
| Tyrosine (Tyr) | 0.62 ± 0.17 | 0.73 ± 0.12 | 0.69 ± 0.12 | 0.69 ± 0.14 |
| Valine (Val) | 2.11 ± 0.41 | 2.13 ± 0.48 | 2.07 ± 0.48 | 2.07 ± 0.50 |
| Lysine (Lys) | 3.13 ± 0.37 | 3.07 ± 0.74 | 3.02 ± 0.74 | 3.01 ± 0.70 |
| Isoleucine (Ile) | 1.30 ± 0.30 | 1.40 ± 0.31 | 1.34 ± 0.31 | 1.35 ± 0.39 |
| Leucine (Leu) | 2.66 ± 0.40 | 2.84 ± 0.50 | 2.73 ± 0.50 | 2.76 ± 0.38 |
| Phenylalanine (Phe) | 1.78 ± 0.32 | 1.91 ± 0.55 | 1.86 ± 0.55 | 1.89 ± 0.33 |
| Imino acids (Pro + Hyp) | 24.43 ± 2.03 | 25.33 ± 2.05 | 25.15 ± 0.13 | 25.27 ± 2.39 |
| Total amino acids | 86.41 ± 0.33 | 90.20 ± 2.72 | 88.99 ± 3.52 | 89.19 ± 1.45 |
| Region | Peak Wavenumber (cm−1) of Gelatins | |||
|---|---|---|---|---|
| Control | Pe5 | Pe15 | Pe25 | |
| Amide I | 1631.78 | 1631.83 | 1633.70 | 1632.92 |
| Amide II | 1537.27 | 1536.71 | 1534.61 | 1535.21 |
| Amide III | 1234.44 | 1226.02 | 1226.92 | 1224.26 |
| Amide A | 3294.42 | 3293.23 | 3289.73 | 3294.53 |
| Amide B | 2931.80 | 2930.84 | 2939.95 | 2947.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.; Ismail, A.; Ahmad, S.A.; Abdul Khalil, K.; Awad, E.A.; Akhtar, M.T.; Sazili, A.Q. Recovery of Gelatin from Bovine Skin with the Aid of Pepsin and Its Effects on the Characteristics of the Extracted Gelatin. Polymers 2021, 13, 1554. https://doi.org/10.3390/polym13101554
Ahmad T, Ismail A, Ahmad SA, Abdul Khalil K, Awad EA, Akhtar MT, Sazili AQ. Recovery of Gelatin from Bovine Skin with the Aid of Pepsin and Its Effects on the Characteristics of the Extracted Gelatin. Polymers. 2021; 13(10):1554. https://doi.org/10.3390/polym13101554
Chicago/Turabian StyleAhmad, Tanbir, Amin Ismail, Siti Aqlima Ahmad, Khalilah Abdul Khalil, Elmutaz Atta Awad, Muhammad Tayyab Akhtar, and Awis Qurni Sazili. 2021. "Recovery of Gelatin from Bovine Skin with the Aid of Pepsin and Its Effects on the Characteristics of the Extracted Gelatin" Polymers 13, no. 10: 1554. https://doi.org/10.3390/polym13101554
APA StyleAhmad, T., Ismail, A., Ahmad, S. A., Abdul Khalil, K., Awad, E. A., Akhtar, M. T., & Sazili, A. Q. (2021). Recovery of Gelatin from Bovine Skin with the Aid of Pepsin and Its Effects on the Characteristics of the Extracted Gelatin. Polymers, 13(10), 1554. https://doi.org/10.3390/polym13101554