In Situ Surface-Initiated Atom-Transfer Radical Polymerization Utilizing the Nonvolatile Nature of Ionic Liquids: A First Attempt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
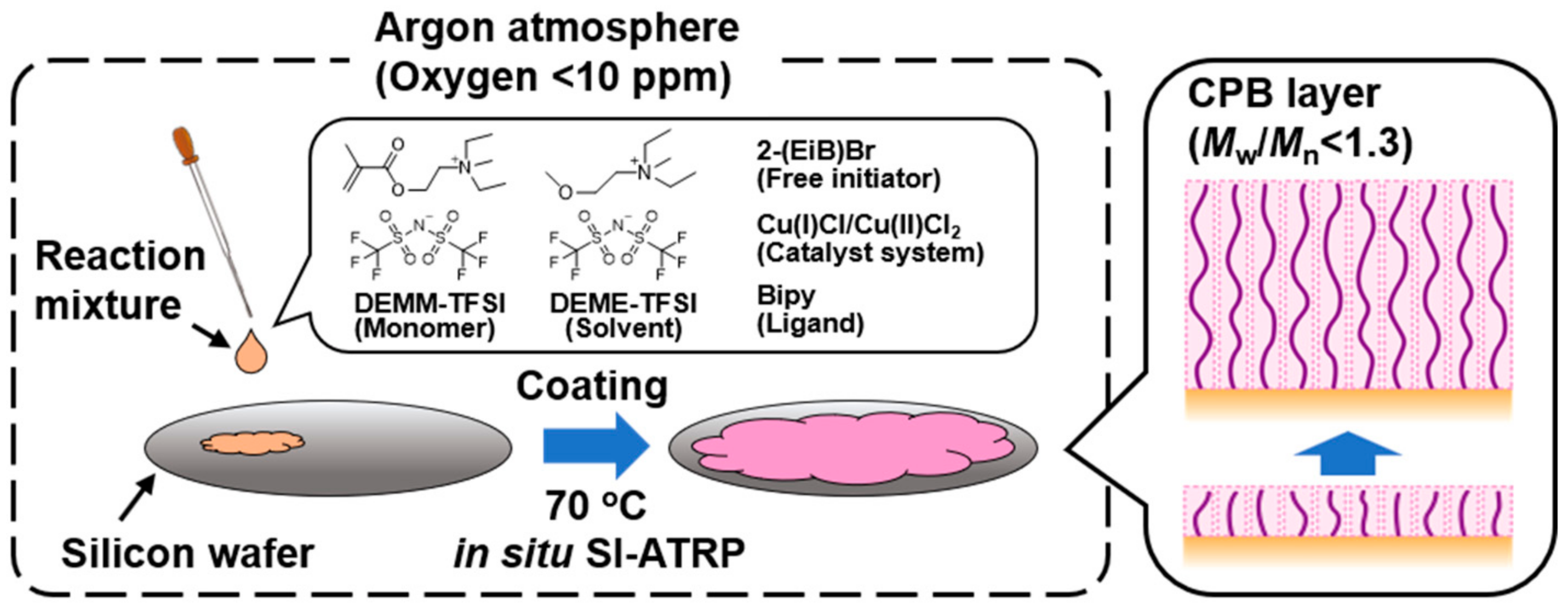
2.2. In Situ Preparation of CPBs of Poly(DEMM–TFSI) by SI-ATRP
2.3. Characterization of Poly(DEMM–TFSI) Obtained by In Situ SI-ATRP
2.4. Friction Force Measurements
3. Results and Discussion
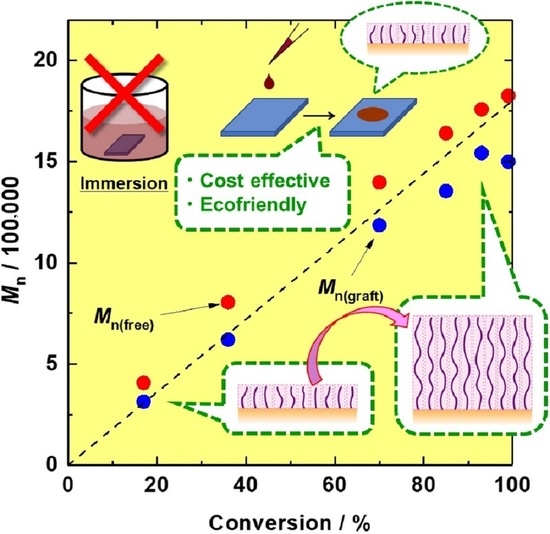
3.1. In Situ SI-ATRP of Polymerizable IL on a Silicon Wafer
3.2. Characterization of Poly(DEMM–TFSI) on a Silicon Wafer by ATR/FTIR
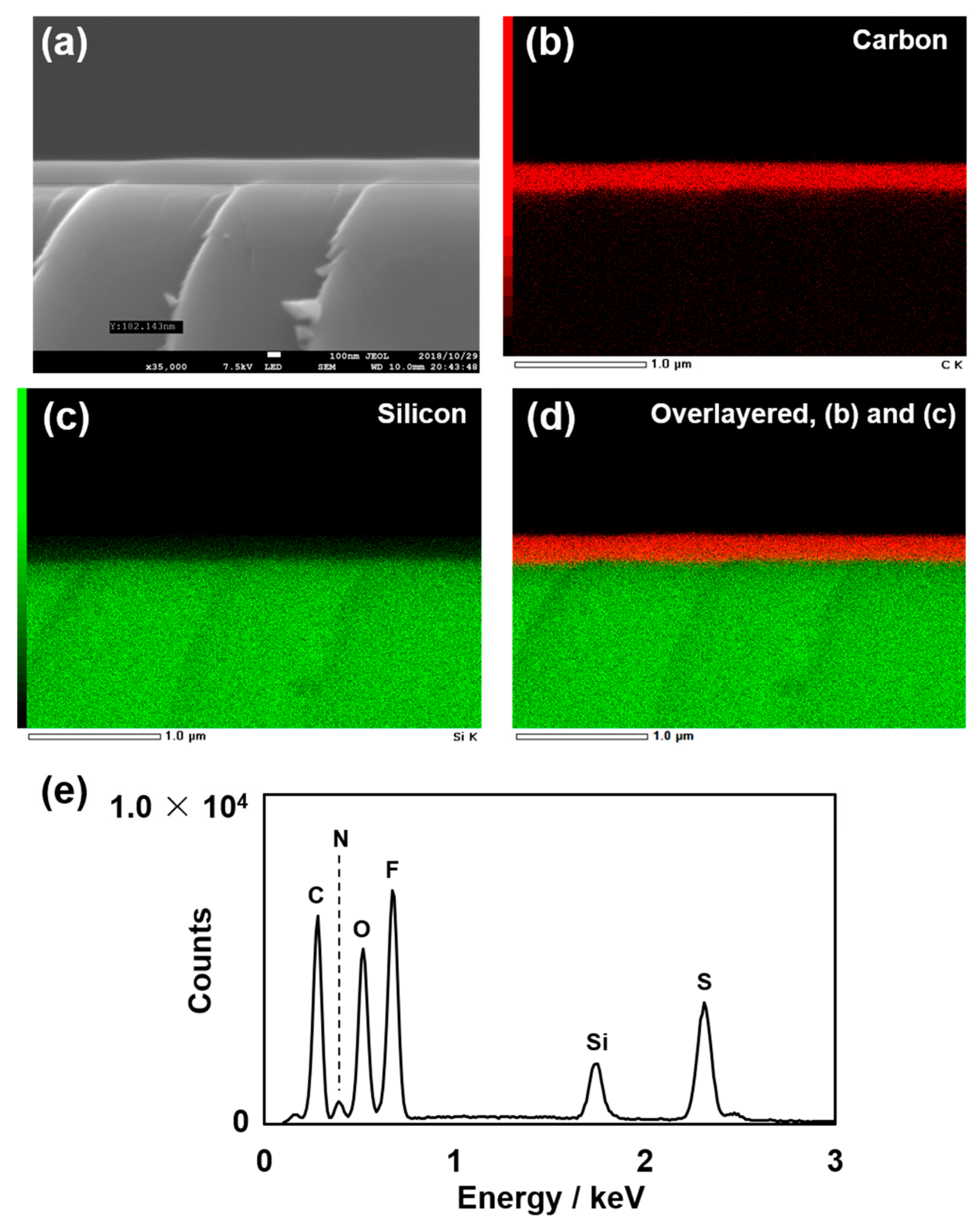
3.3. Cross-Sectional Observation of the Poly(DEMM–TFSI) Grafted on the Silicon Wafer and Elemental Analysis Using SEM and EDX
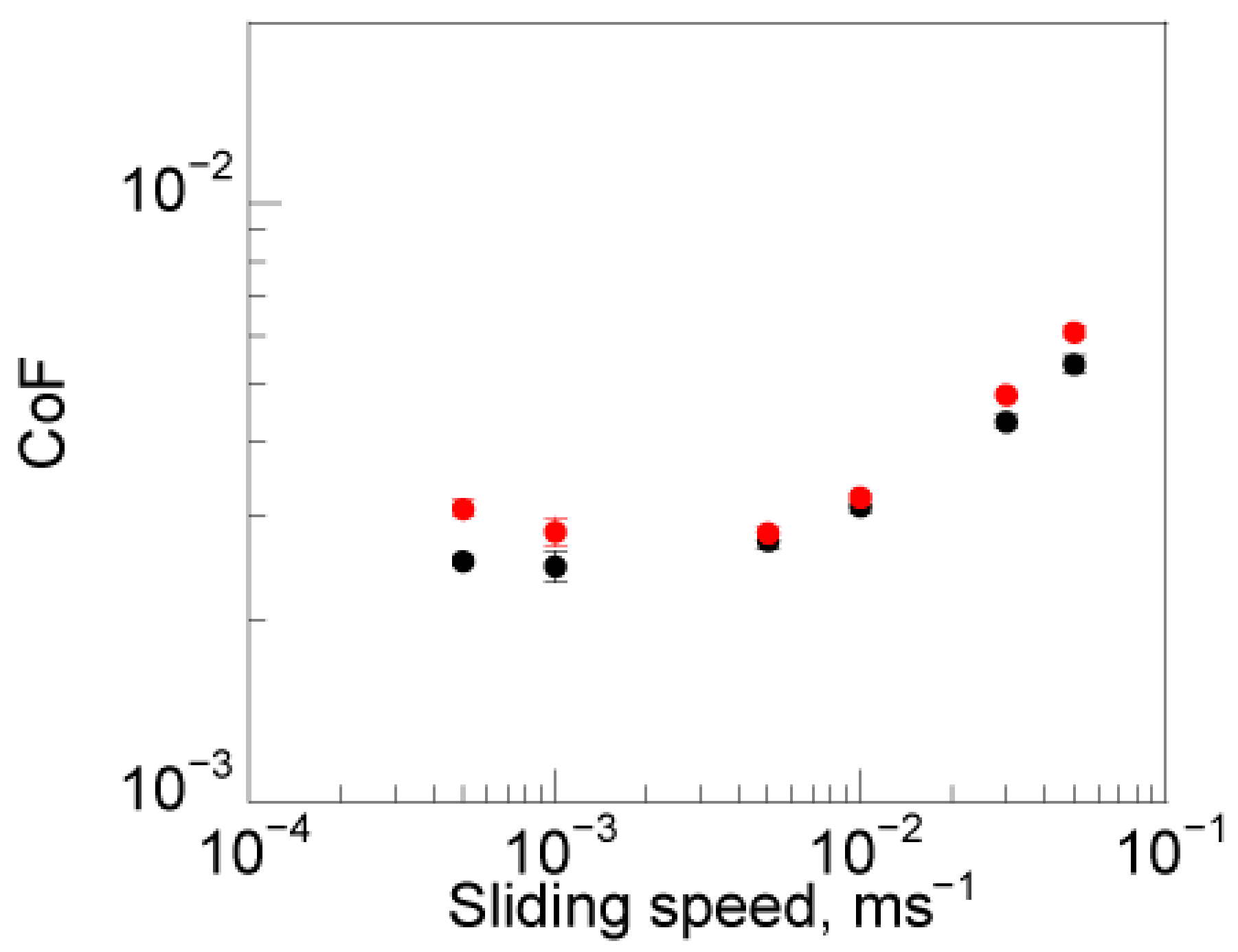
3.4. Lubrication Properties Using IL Polymer Brushes Formed by In Situ Synthesis
3.5. Future Perspective
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflectance |
| ATRP | atom-transfer radical polymerization |
| Bipy | 2,2′-bipyridine |
| CoF | coefficient of friction |
| CPB | concentrated polymer brush |
| EDX | energy-dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| DEME | N,N-diethylmethyl(2-methoxyethyl)ammonium |
| DEMM | N,N-diethyl-N-(2-methacryloylethyl)-N-methylammonium |
| dn/dc | refractive index increment |
| GPC | gel permeation chromatography |
| HF | hydrofluoric acid |
| IL | ionic liquid |
| MALLS | multi-angle laser light scattering |
| Mn | number average molecular weight |
| Mw | weight average molecular weight |
| Mw/Mn | dispersity |
| NMR | nuclear magnetic resonance |
| SEM | scanning electron microscopy |
| SI-ATRP | surface-initiated atom-transfer radical polymerization |
| TFSI | bis(trifluoromethylsulfonyl)imide |
| 2-(EiB)Br | ethyl 2-bromoisobutyrate |
References
- Ejaz, M.; Yamamoto, S.; Ohno, K.; Tsujii, Y.; Fukuda, T. Controlled Graft Polymerization of Methyl Methacrylate on Silicon Substrate by the Combined Use of the Langmuir–Blodgett and Atom Transfer Radical Polymerization Techniques. Macromolecules 1998, 31, 5934–5936. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Goto, A.; Tsujii, Y.; Fukuda, T.; Kimura, T.; Yamamoto, K.; Kishida, A. Protein repellency of well-defined, concentrated poly(2-hydroxyethyl methacrylate) brushes by the size-exclusion effect. Macromolecules 2006, 39, 2284–2290. [Google Scholar] [CrossRef]
- Nomura, A.; Ohno, K.; Fukuda, T.; Sato, T.; Tsujii, Y. Lubrication mechanism of concentrated polymer brushes in solvents: Effect of solvent viscosity. Polym. Chem. 2012, 3, 148. [Google Scholar] [CrossRef]
- Arafune, H.; Kamijo, T.; Morinaga, T.; Honma, S.; Sato, T.; Tsujii, Y. A robust lubrication system using an ionic liquid polymer brush. Adv. Mater. Interfaces 2015, 2, 1500187. [Google Scholar] [CrossRef]
- Tsujii, Y.; Sakakibara, K.; Watanabe, H.; Kurihara, K.; Sato, T.; Nakano, K. Lubricant and SRT material. World Intellectual Property Organization Patent WO2017171071A1, 5 October 2017. [Google Scholar]
- Bieleck, R.M.; Benetti, E.M.; Spencer, N.D. Lubrication with Oil-Compatible Polymer Brushes. World Intellectual Property Organization Patent WO20121525-12A8, 25 April 2013. [Google Scholar]
- Sato, T.; Marukane, S.; Narutomi, T.; Akao, T. High rate performance of a lithium polymer battery using a novel ionic liquid polymer composite. J. Power Sources 2007, 164, 390–396. [Google Scholar] [CrossRef]
- Sato, T.; Morinaga, T.; Marukane, S.; Narutomi, T.; Igarashi, T.; Kawano, Y.; Ohno, K.; Fukuda, T.; Tsujii, Y. Novel solid-state polymer electrolyte of colloidal crystal decorated with ionic-liquid polymer brush. Adv. Mater. 2011, 23, 4868–4872. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Honma, S.; Ishizuka, T.; Kamijo, T.; Sato, T.; Tsujii, Y. Synthesis of monodisperse silica particles grafted with concentrated ionic liquid-type polymer brushes by surface-initiated atom transfer radical polymerization for use as a solid state polymer electrolyte. Polymers 2016, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Arafune, H.; Honma, S.; Morinaga, T.; Kamijo, T.; Miura, M.; Furukawa, H.; Sato, T. Highly robust and low frictional double-network ion gel. Adv. Mater. Interfaces 2017, 4, 1700074. [Google Scholar] [CrossRef]
- Belin, M.; Arafune, H.; Kamijo, T.; Perret-Liaudet, J.; Morinaga, T.; Honma, S.; Sato, T. Low friction, lubricity, and durability of polymer brush coatings, characterized using the relaxation tribometer technique. Lubricants 2018, 6, 52. [Google Scholar] [CrossRef]
- Shipp, D.A.; Matyjaszewski, K. Kinetic analysis of controlled/“living” radical polymerizations by simulations. 1. The importance of diffusion-controlled reac-tions. Macromolecules 2000, 33, 1553–1559. [Google Scholar] [CrossRef]
- Nomura, A.; Okayasu, K.; Ohno, K.; Fukuda, T.; Tsujii, Y. Lubrication mechanism of concentrated polymer brushes in solvents: Effect of solvent quality and thereby swelling state. Macromolecules 2011, 44, 5013–5019. [Google Scholar] [CrossRef]
- Sato, T.; Arafune, H.; Kamijo, T.; Morinaga, T.; Honma, S.; Nakano, K.; Tsujii, Y. Method for forming polymer brush layer. World Intellectual Property Organization Patent WO2018225693A1, 13 December 2018. [Google Scholar]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: from process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, A.J.; Haddleton, D.M.; Bona, S.A.F.; Seddon, K.R. Copper(I) mediated living radical polymerization in an ionic liquid. Chem. Commun. 2000, 1237–1238. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of monodisperse silica particles coated with well-defined, high-density polymer brushes by surface-initiated atom transfer radical polymerization. Macromolecules 2005, 38, 2137–2142. [Google Scholar] [CrossRef]
- Morinaga, T.; Ohkura, M.; Ohno, K.; Tsujii, Y.; Fukuda, T. Monodisperse silica particles grafted with concentrated oxetane-carrying polymer brushes: Their synthesis by surface-initiated atom transfer radical polymerization and use for fabrication of hollow spheres. Macromolecules 2007, 40, 1159–1164. [Google Scholar] [CrossRef]
- Rey, I.; Johansson, P.; Lindgren, J.; Lassègues, J.C.; Grondin, J.; Servant, L. Spectroscopic and theoretical study of (CF3SO2)2N− (TFSI−) and (CF3SO2)2NH (HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, B.; Bhattacharya, S. Electroactive phase nucleation and non-isothermal crystallization kinetics study in [DEMM][TFSI] ionic liquid incorporated P(VDF-HFP) co-polymer membranes. J. Mater. Sci. 2016, 51, 7814–7830. [Google Scholar] [CrossRef]
- Yan, W.; Dadashi-Silab, S.; Matyjaszewski, K.; Spencer, N.D.; Benetti, E.M. Surface-initiated photoinduced ATRP: Mechanism, oxygen tolerance, and temporal control during the synthesis of polymer brushes. Macromolecules 2020, 53, 2801–2810. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, R.; Honma, S.; Arafune, H.; Shomura, R.; Kamijo, T.; Morinaga, T.; Sato, T. In Situ Surface-Initiated Atom-Transfer Radical Polymerization Utilizing the Nonvolatile Nature of Ionic Liquids: A First Attempt. Polymers 2021, 13, 61. https://doi.org/10.3390/polym13010061
Satoh R, Honma S, Arafune H, Shomura R, Kamijo T, Morinaga T, Sato T. In Situ Surface-Initiated Atom-Transfer Radical Polymerization Utilizing the Nonvolatile Nature of Ionic Liquids: A First Attempt. Polymers. 2021; 13(1):61. https://doi.org/10.3390/polym13010061
Chicago/Turabian StyleSatoh, Ryo, Saika Honma, Hiroyuki Arafune, Ryo Shomura, Toshio Kamijo, Takashi Morinaga, and Takaya Sato. 2021. "In Situ Surface-Initiated Atom-Transfer Radical Polymerization Utilizing the Nonvolatile Nature of Ionic Liquids: A First Attempt" Polymers 13, no. 1: 61. https://doi.org/10.3390/polym13010061
APA StyleSatoh, R., Honma, S., Arafune, H., Shomura, R., Kamijo, T., Morinaga, T., & Sato, T. (2021). In Situ Surface-Initiated Atom-Transfer Radical Polymerization Utilizing the Nonvolatile Nature of Ionic Liquids: A First Attempt. Polymers, 13(1), 61. https://doi.org/10.3390/polym13010061