Antibacterial Activity of N,O-Acylated Chitosan Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CH_LA
2.3. Dynamic Light Scattering
2.4. Polymer—Mucin Interactions
2.5. Bacteria Optimal Cultivation Conditions
2.6. Antibacterial Activity of Chitosan and Chitosan Derivatives
2.6.1. Disc Diffusion Method
2.6.2. Well Diffusion Method
2.6.3. Agar Impregnation Method
2.7. Microdilution Method
2.8. Statistical Analysis
3. Results
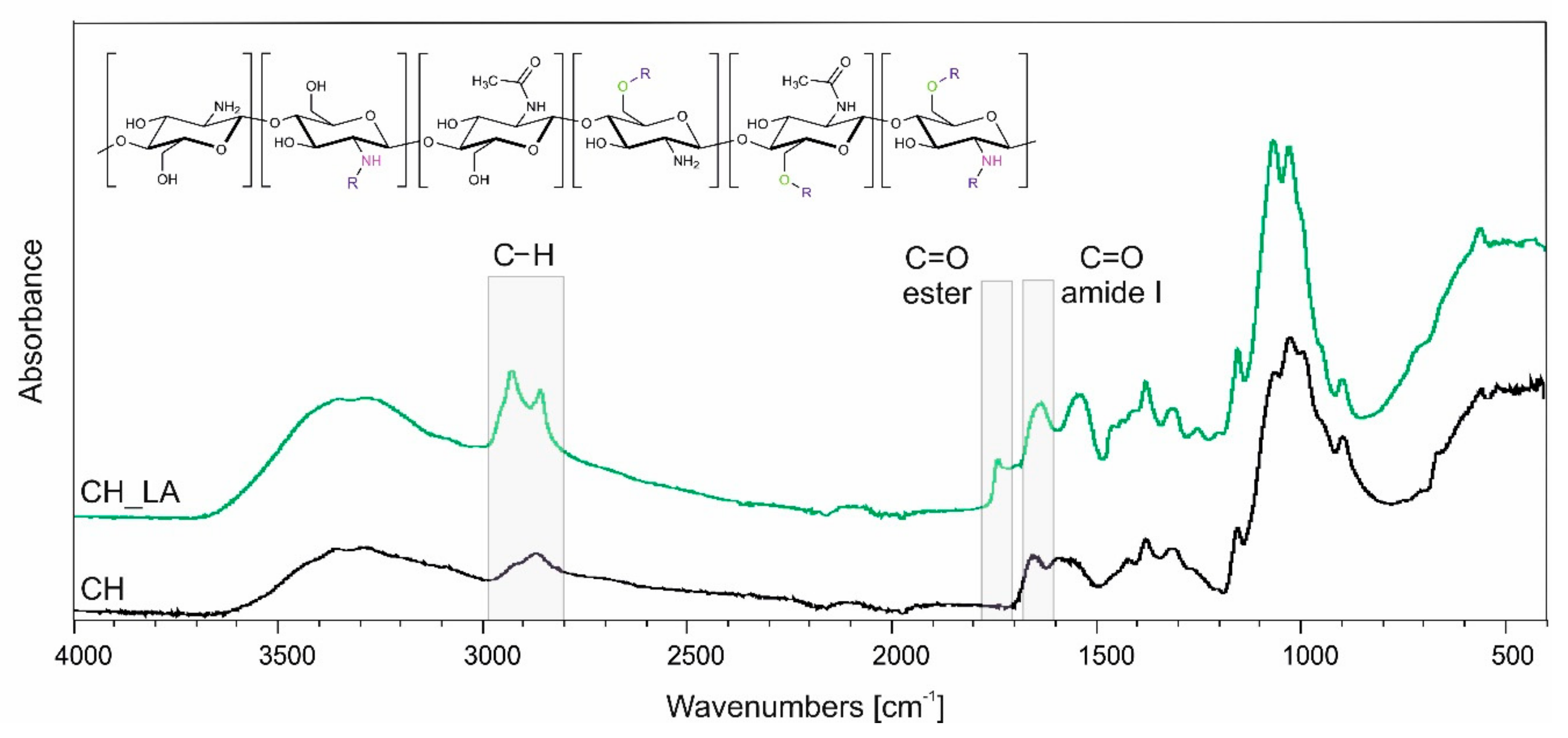
3.1. Synthesis and Chemical Structure Analysis
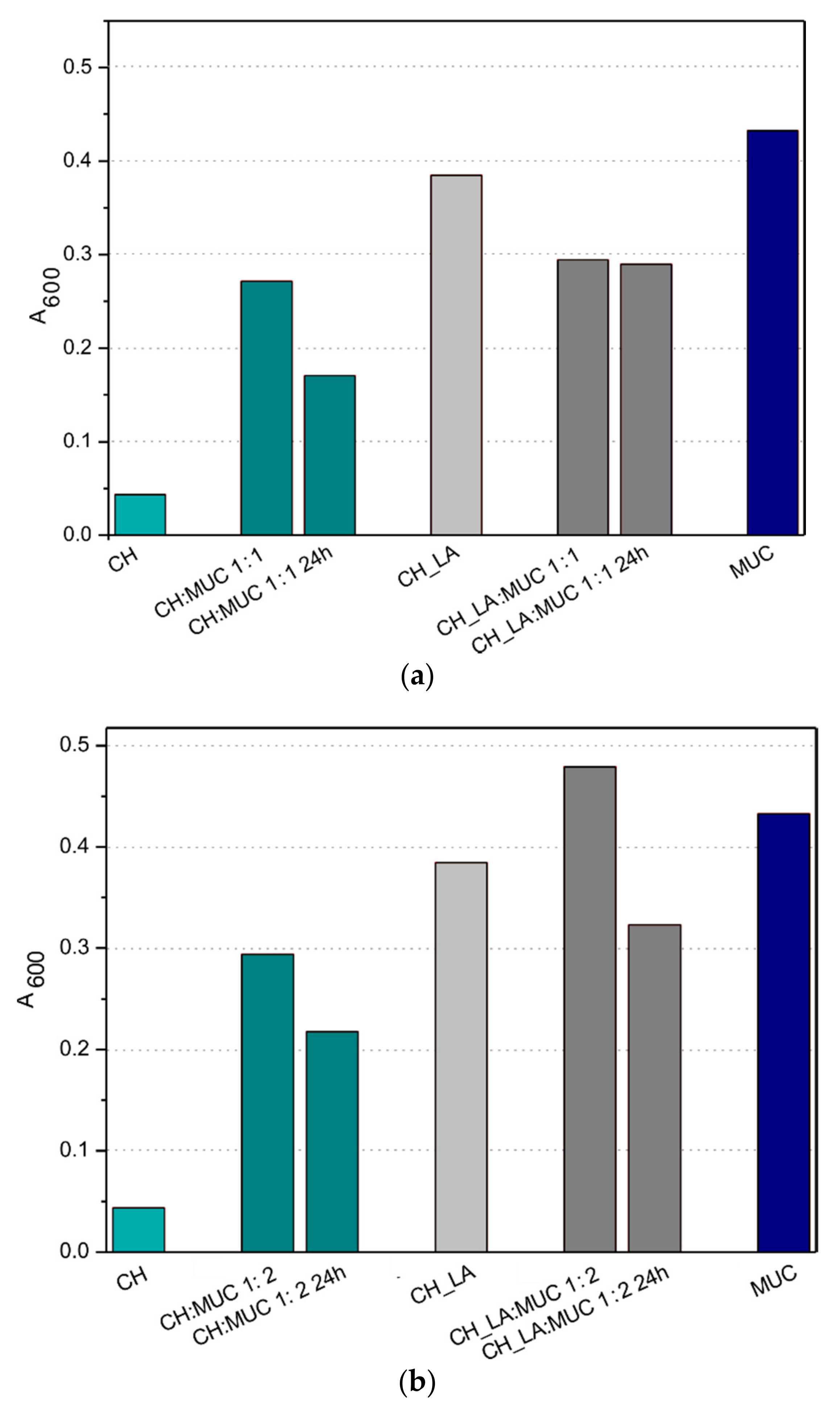
3.2. Mucin Interactions
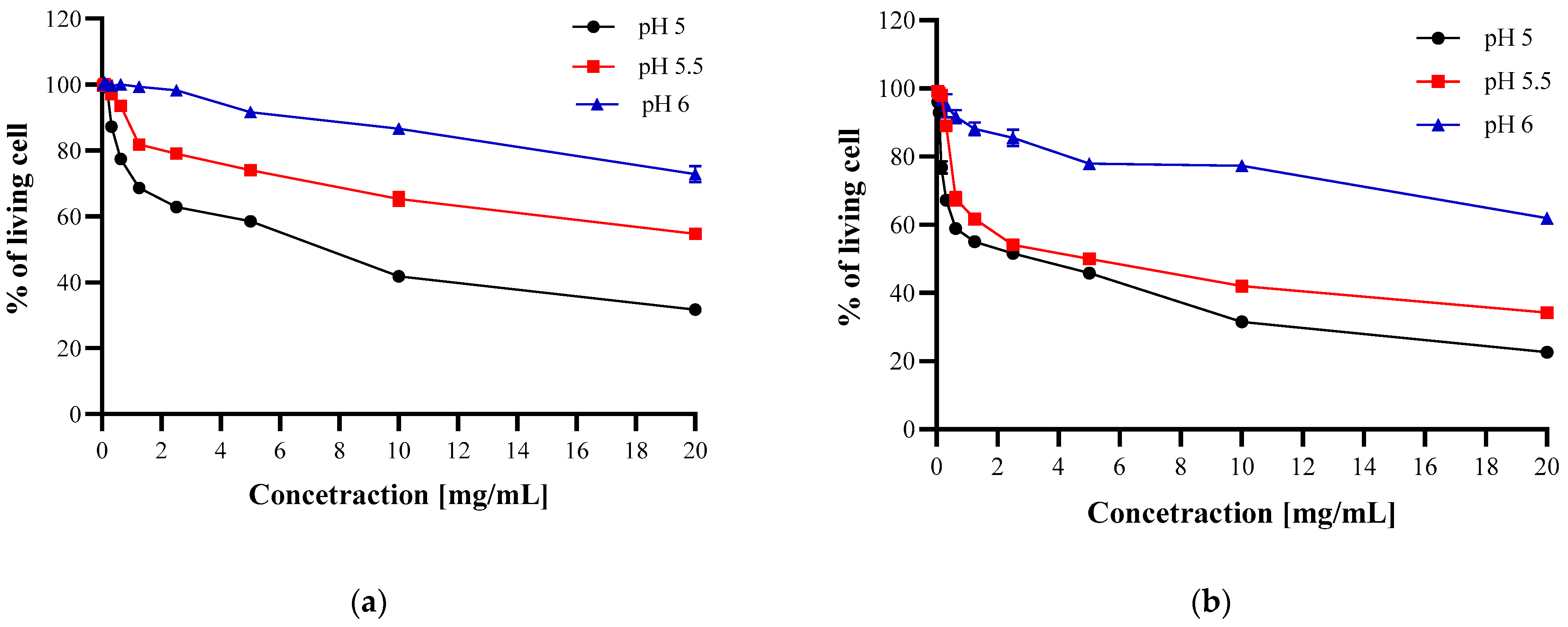
3.3. Antibacterial Activity of Chitosan
3.3.1. Diffusion Based Methods
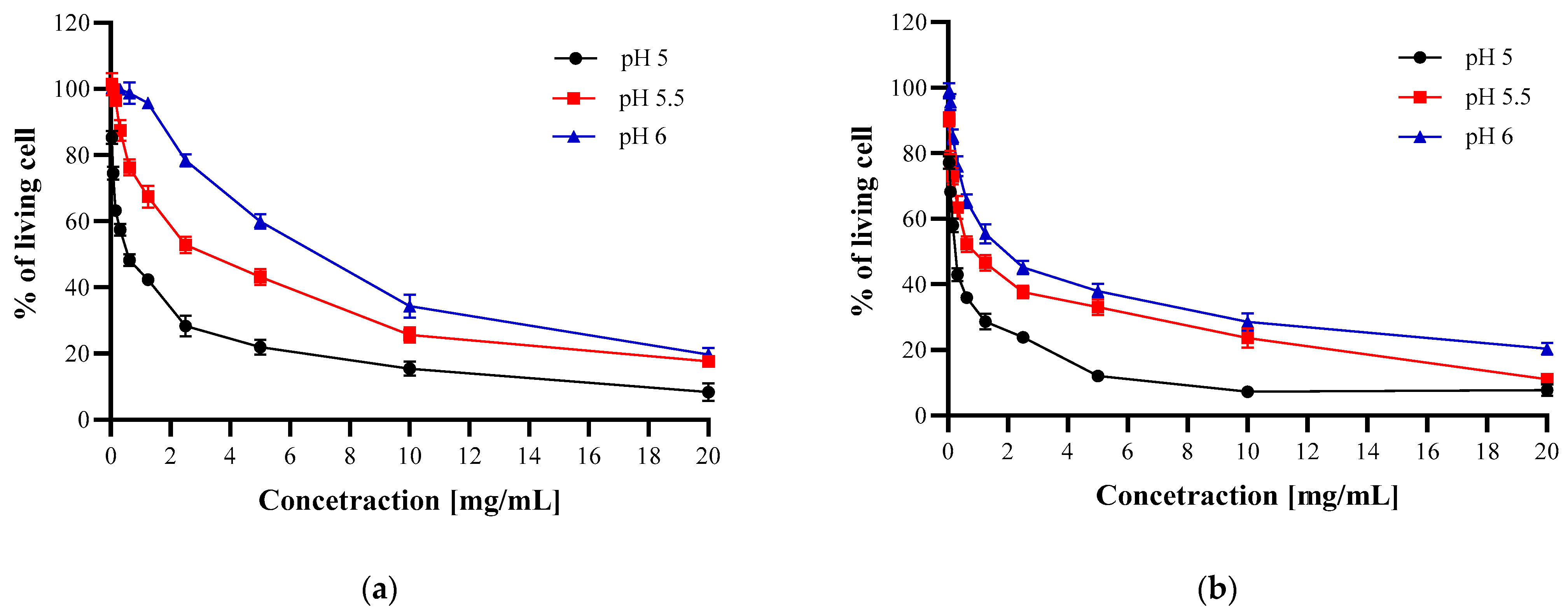
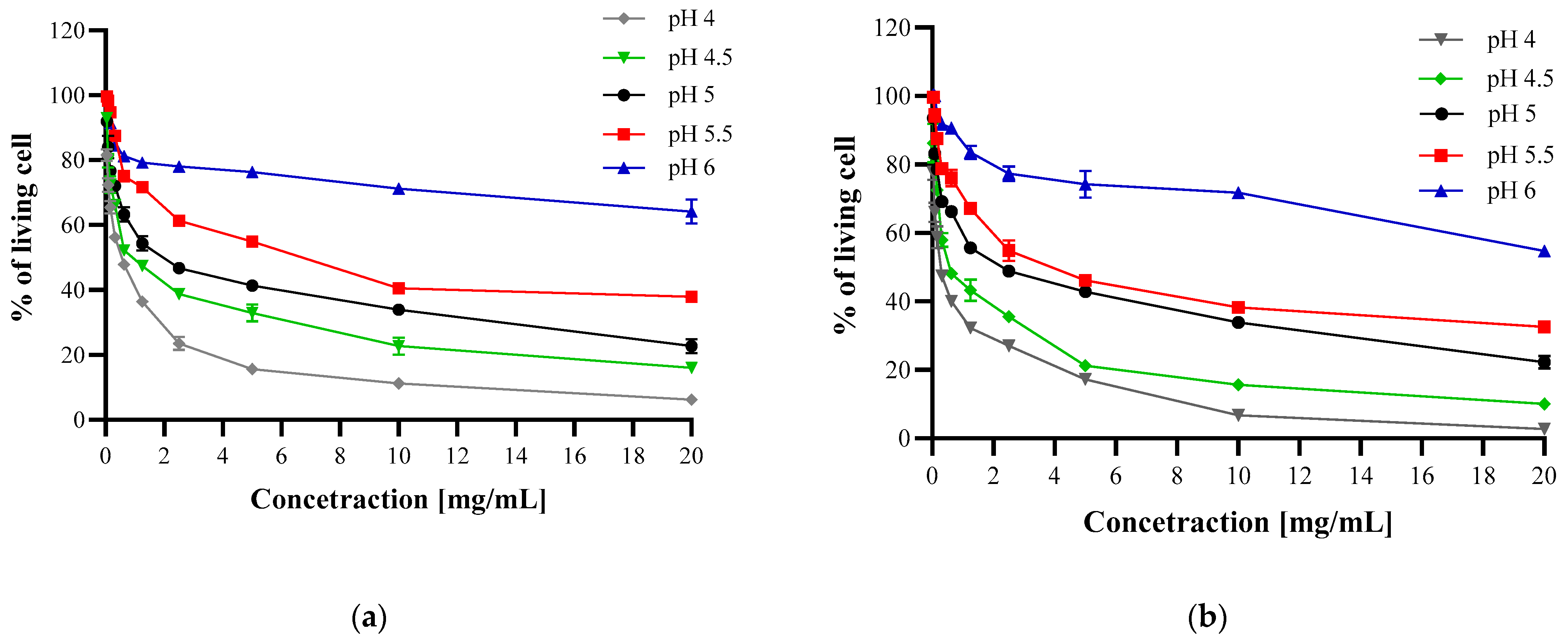
3.3.2. Microdilution Method
3.4. Dynamic Light Scattering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Aiba, S.I. Chemical Modification of Chitosan. 14:1 Synthesis of Water-Soluble Chitosan Derivatives by Simple Acetylation. Biomacromolecules 2002, 3, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, S.N. The Antiviral Activity of Chitosan (Review). Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines. Int. J. Nanomed. 2020, 15, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius Henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, Antifungal and Antiviral Activities of Chitosan from the Larvae of Housefly, Musca Domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A Potential Antifungal Effect of Chitosan against Candida Albicansis Mediated via the Inhibition of SAGA Complex Component Expression and the Subsequent Alteration of Cell Surface Integrity. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial Characteristics and Activity of Acid-Soluble Chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Mateescu, M.-A.; Ispas-Szabo, P.; Assaad, E. Chitosan and Its Derivatives as Self-Assembled Systems for Drug Delivery. In Controlled Drug Delivery; Mateescu, M.-A., Ispas-Szabo, P., Assaad, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–125. [Google Scholar]
- Kucharska, M.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 169–187. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial Action of a Novel Functionalized Chitosan-Arginine against Gram-Negative Bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef]
- Cai, J.; Dang, Q.; Liu, C.; Fan, B.; Yan, J.; Xu, Y.; Li, J. Preparation and Characterization of N-Benzoyl-O-Acetyl-Chitosan. Int. J. Biol. Macromol. 2015, 77, 52–58. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan Derivatives Obtained by Chemical Modifications for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-Solubility of Partially N-Acetylated Chitosans as a Function of PH: Effect of Chemical Composition and Depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Sashiwa, H.; Shigemasa, Y. Chemical Modification of Chitin and Chitosan 2: Preparation and Water Soluble Property of N-Acylated or N-Alkylated Partially Deacetylated Chitins. Carbohydr. Res. 1999, 39, 127–138. [Google Scholar] [CrossRef]
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically Modified Chitosan: A Bio-Based Material for Antimicrobial Active Film. Mater. Sci. Eng. C 2014, 42, 569–577. [Google Scholar] [CrossRef]
- Vo, D.T.; Lee, C.K. Antimicrobial Sponge Prepared by Hydrophobically Modified Chitosan for Bacteria Removal. Carbohydr. Polym. 2018, 187, 1–7. [Google Scholar] [CrossRef]
- Shan, P.; Shen, J.-W.; Xu, D.-H.; Shi, L.-Y.; Gao, J.; Lan, Y.-W.; Wang, Q.; Wei, X.-H. Molecular Dynamics Study on the Interaction between Doxorubicin and Hydrophobically Modified Chitosan Oligosaccharide. RSC Adv. 2014, 4, 23730. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and Characterization of Low-Toxic Amphiphilic Chitosan Derivatives and Their Application as Micelle Carrier for Antitumor Drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Dellera, E.; Rossi, S.; Ferrari, F.; Mori, M.; Caramella, C. Ionic Polymeric Micelles Based on Chitosan and Fatty Acids and Intended for Wound Healing. Comparison of Linoleic and Oleic Acid. Eur. J. Pharm. Biopharm. 2014, 87, 101–106. [Google Scholar] [CrossRef]
- Collado-González, M.; Espinosa, Y.G.; Goycoolea, F.M. Interaction between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, H.; Liu, Z.; Yao, P. Effective Enhancement of Hypoglycemic Effect of Insulin by Liver-Targeted Nanoparticles Containing Cholic Acid-Modified Chitosan Derivative. Mol. Pharm. 2016, 13, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, M.; Gao, Y.; Zhu, S.; Chen, S.; Liu, W.; Zhong, H.; Liu, J. Modified Nanoparticles with Cell-Penetrating Peptide and Amphipathic Chitosan Derivative for Enhanced Oral Colon Absorption of Insulin: Preparation and Evaluation. Drug Deliv. 2016, 23, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Park, K.; Kang, E.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Chi, D.Y.; Park, R.W.; et al. Self-Assembled Glycol Chitosan Nanoparticles for the Sustained and Prolonged Delivery of Antiangiogenic Small Peptide Drugs in Cancer Therapy. Biomaterials 2008, 29, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Piegat, A.; Goszczyńska, A.; Idzik, T.; Niemczyk, A. The Importance of Reaction Conditions on the Chemical Structure of N,O-Acylated Chitosan Derivatives. Molecules 2019, 24, 3047. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of Chitosan Hydrochloride–Mucin Interaction by Means of Viscosimetric and Turbidimetric Measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Narkar, A.R.; Cannon, E.; Yildirim-Alicea, H.; Ahn, K. Catechol-Functionalized Chitosan: Optimized Preparation Method and Its Interaction with Mucin. Langmuir 2019, 35, 16013–16023. [Google Scholar] [CrossRef]
- Ozkan, O.; Sasmazel, H.T. Antibacterial Performance of PCL-Chitosan Core-Shell Scaffolds. J. Nanosci. Nanotechnol. 2018, 18, 2415–2421. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Koc, B.; Mujtaba, M.; Ilk, S.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Yildiz, A. Diatomite as a Novel Composite Ingredient for Chitosan Film with Enhanced Physicochemical Properties. Int. J. Biol. Macromol. 2017, 105, 1401–1411. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of Diffusion and Dilution Methods to Determine the Antimicrobial Activity of Water-Soluble Chitosan Derivatives. J. Appl. Microbiol. 2013, 114, 956–963. [Google Scholar] [CrossRef]
- Balti, R.; Mansour, M.B.; Sayari, N.; Yacoubi, L.; Rabaoui, L.; Brodu, N.; Massé, A. Development and Characterization of Bioactive Edible Films from Spider Crab (Maja Crispata) Chitosan Incorporated with Spirulina Extract. Int. J. Biol. Macromol. 2017, 105, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Champer, J.; Patel, J.; Fernando, N.; Salehi, E.; Wong, V.; Kim, J. Chitosan against Cutaneous Pathogens. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Presser, K.A.; Ross, T.; Ratkowsky, D.A. Modelling the Growth Limits (Growth/No Growth Interface) of Escherichia Coli as a Function of Temperature, PH, Lactic Acid Concentration, and Water Activity. Appl. Environ. Microbiol. 1998, 64, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Athmann, C.; Zeng, N.; Kang, T.; Marcus, E.A.; Scott, D.R.; Rektorschek, M.; Buhmann, A.; Melchers, K.; Sachs, G. Local PH Elevation Mediated by the Intrabacterial Urease of Helicobacter Pylori Cocultured with Gastric Cells. J. Clin. Investig. 2000, 106, 339–347. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia Coli in the Environment: Fundamental and Public Health Aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V. Chitosan and Its Hydrophobic Derivatives: Preparation and Aggregation in Dilute Aqueous Solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.G.; Lin, J.G. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial Action of Chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic Force Microscopy Study of the Antibacterial Effects of Chitosans on Escherichia Coli and Staphylococcus Aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific Manipulations of a Rigid Polysaccharide: Syntheses of Novel Chitosan Derivatives with Excellent Solubility in Common Organic Solvents by Regioselective Chemical Modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]

| CFU × 106/mL | ||||
|---|---|---|---|---|
| Concertation [mg/mL] | E. coli | S. aureus | H. pylori | |
| CH | 10 | 70 ± 2 | 42 ± 2 | 61 ± 3 |
| 5 | 67 ± 3 | 39 ± 3 | 60 ± 3 | |
| 2 | 69 ± 4 | 40 ± 6 | 59 ± 6 | |
| 1 | 71 ± 3 | 42 ± 4 | 66 ± 5 | |
| CH_LA | 10 | 68 ± 3 | 50 ± 3 | 61 ± 3 |
| 5 | 71 ± 6 | 47 ± 2 | 65 ± 5 | |
| 2 | 73 ± 5 | 49 ± 1 | 60 ± 6 | |
| 1 | 69 ± 4 | 51 ± 3 | 63 ± 5 | |
| Control | 70 ± 2 | 41 ± 2 | 62 ± 6 | |
| pH of Growth Medium | Bacteria * | ||
|---|---|---|---|
| S. aureus | E. coli | H. pyroli | |
| 7.0 | 0.61 ± 0.05 | 2.26 ± 0.08 | 1.19 ± 0.08 |
| 6.5 | 0.70 ± 0.04 | 2.34 ± 0.04 | 1.26 ± 0.04 |
| 6.0 | 0.71 ± 0.07 | 2.46 ± 0.06 | 1.36 ± 0.06 |
| 5.5 | 0.73 ± 0.08 | 2.34 ± 0.04 | 1.15 ± 0.04 |
| 5.0 | 0.67 ± 0.10 | 2.41 ± 0.09 | 1.18 ± 0.06 |
| 5.5 | 0.03 ± 0.06 | 2.73 ± 0.06 | 0.37 ± 0.09 |
| 4.0 | 0.03 ± 0.07 | 2.22 ± 0.06 | 0.37 ± 0.06 |
| pH | Z-Average Rh [nm] | PdI | Peak 1 Mean Size [nm] | Peak 1 Area [%] | Peak 2 Mean Size [nm] | Peak 2 Area [%] | Diffusion Coefficient [µm2/s] | |
|---|---|---|---|---|---|---|---|---|
| CH | 4 | 343 ± 32 | 0.39 ± 0.05 | 199 ± 10 | 83 ± 1.0 | 30 ± 3 | 17 ± 1 | 1.9 ± 0.2 |
| 5 | 302 ± 118 | 0.40 ± 0.06 | 164 ± 42 | 87 ± 4.8 | 27 ± 14 | 13 ± 5 | 2.4 ± 0.8 | |
| 7 | 248 ± 49 | 0.38 ± 0.04 | 180 ± 33 | 87 ± 2.8 | 35 ± 11 | 13 ± 3 | 2.7 ± 0.5 | |
| CH_LA | 4 | 4990 ± 1760 | 0.49 ± 0.20 | 3835 ± 1380 | 100 | - | - | 0.13 ± 0.05 |
| 5 | 6220 ± 795 | 1 | N/A | N/A | N/A | N/A | 0.10 ± 0.02 | |
| 7 | 4644 ± 970 | 1 | N/A | N/A | N/A | N/A | 0.15 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piegat, A.; Żywicka, A.; Niemczyk, A.; Goszczyńska, A. Antibacterial Activity of N,O-Acylated Chitosan Derivative. Polymers 2021, 13, 107. https://doi.org/10.3390/polym13010107
Piegat A, Żywicka A, Niemczyk A, Goszczyńska A. Antibacterial Activity of N,O-Acylated Chitosan Derivative. Polymers. 2021; 13(1):107. https://doi.org/10.3390/polym13010107
Chicago/Turabian StylePiegat, Agnieszka, Anna Żywicka, Agata Niemczyk, and Agata Goszczyńska. 2021. "Antibacterial Activity of N,O-Acylated Chitosan Derivative" Polymers 13, no. 1: 107. https://doi.org/10.3390/polym13010107
APA StylePiegat, A., Żywicka, A., Niemczyk, A., & Goszczyńska, A. (2021). Antibacterial Activity of N,O-Acylated Chitosan Derivative. Polymers, 13(1), 107. https://doi.org/10.3390/polym13010107






