Designing Multifunctional Protective PVC Electrospun Fibers with Tunable Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Electrospinning Procedure
2.3. Characterization of the Electrospun Coatings
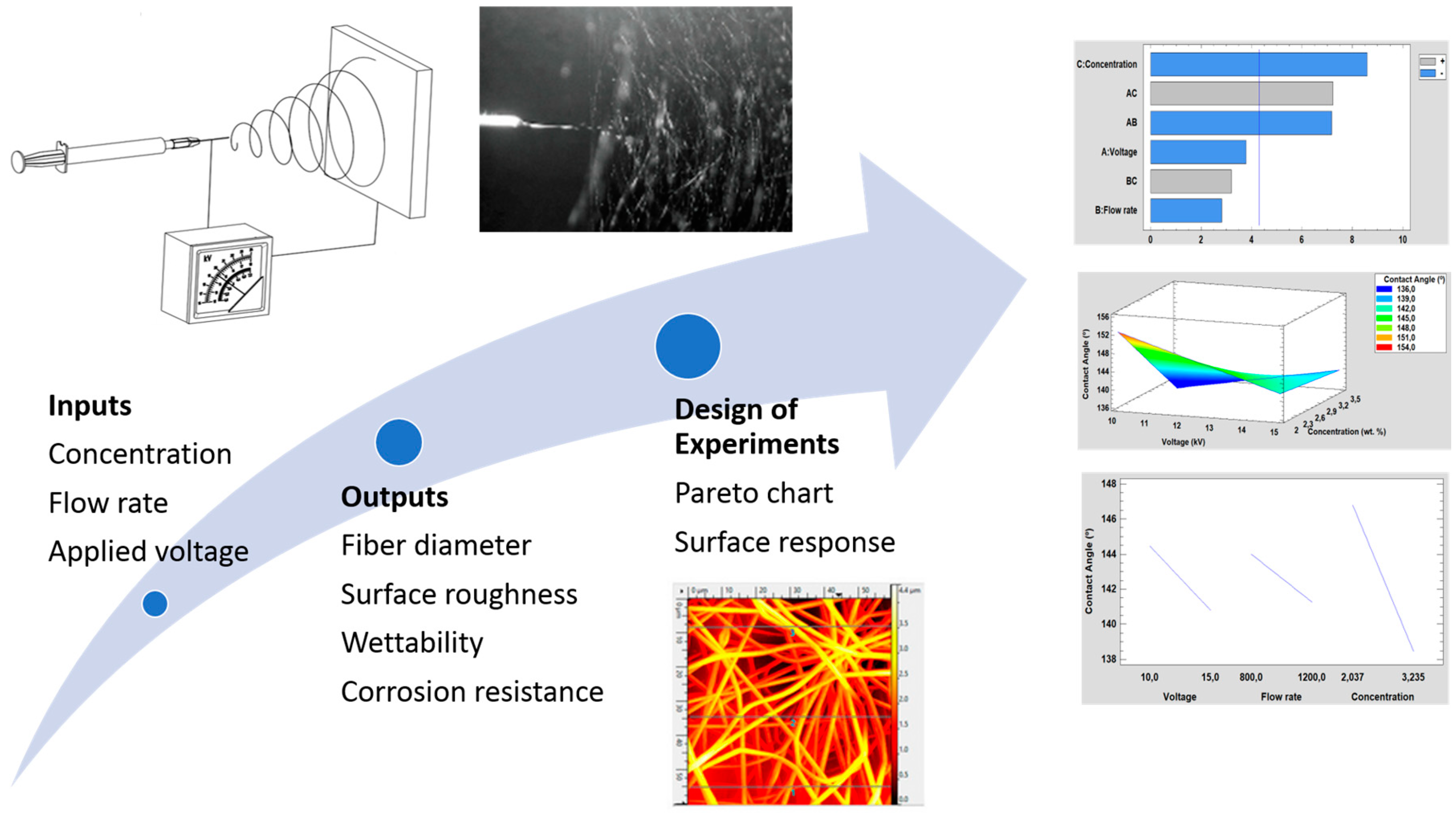
3. Results and Discussion
3.1. Surface Morphology
3.2. Wettability Properties
3.3. Optical Properties
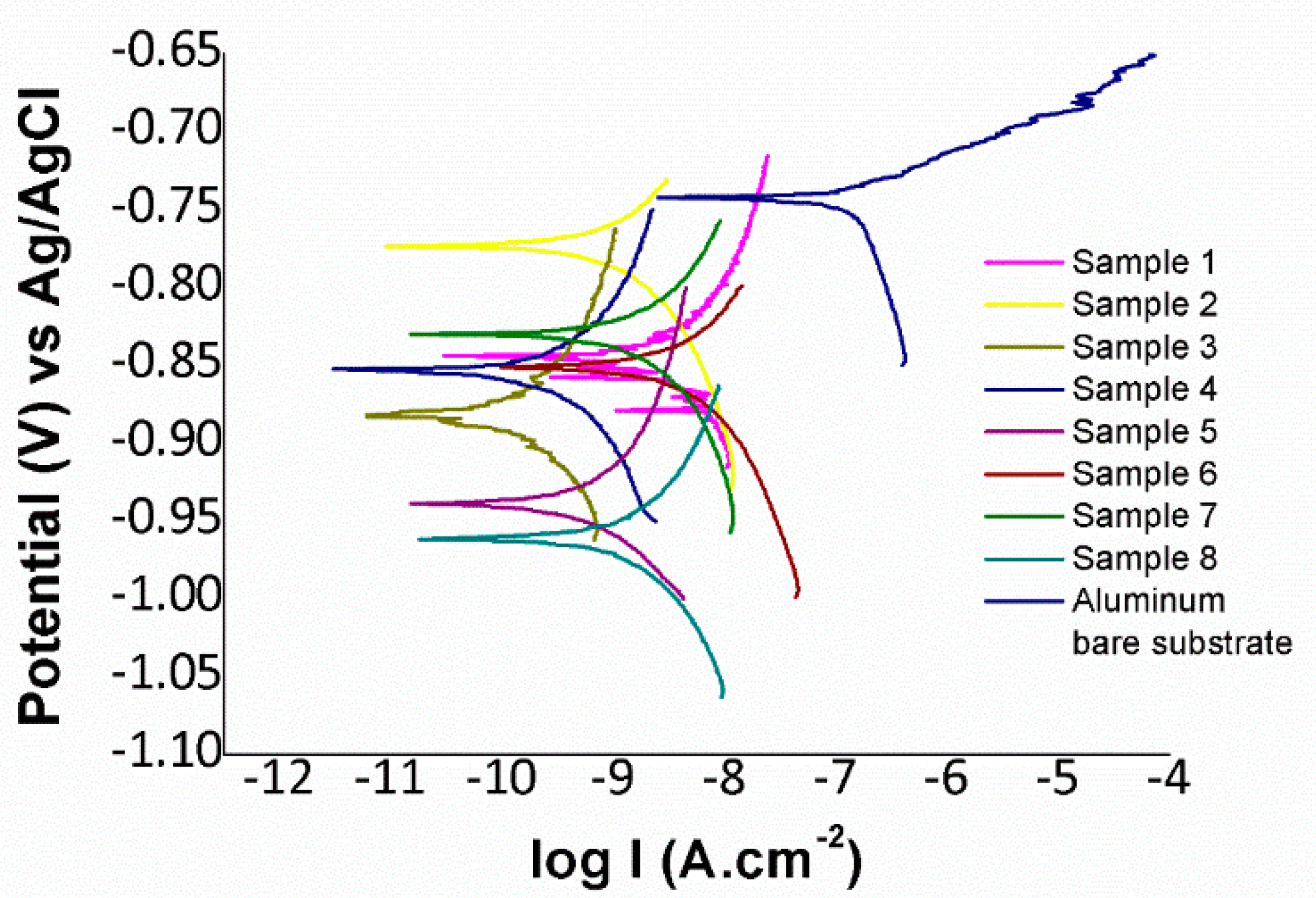
3.4. Anticorrosion Performance
3.5. Evolution of Raw Data Using Design of Experiments
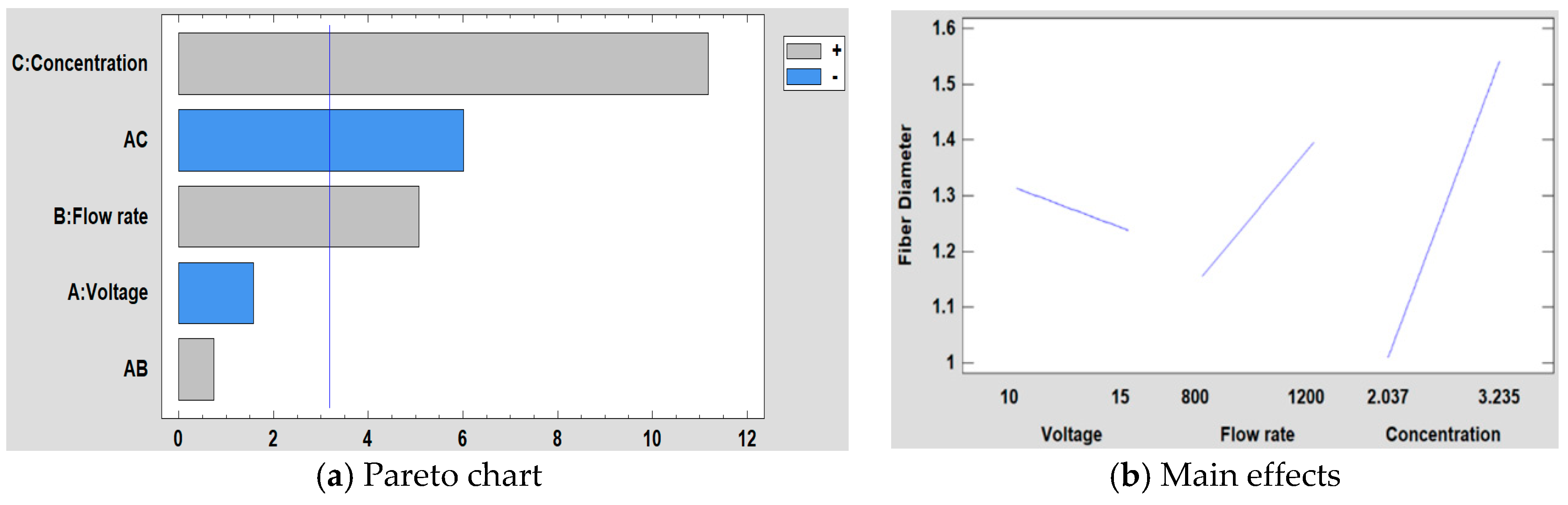
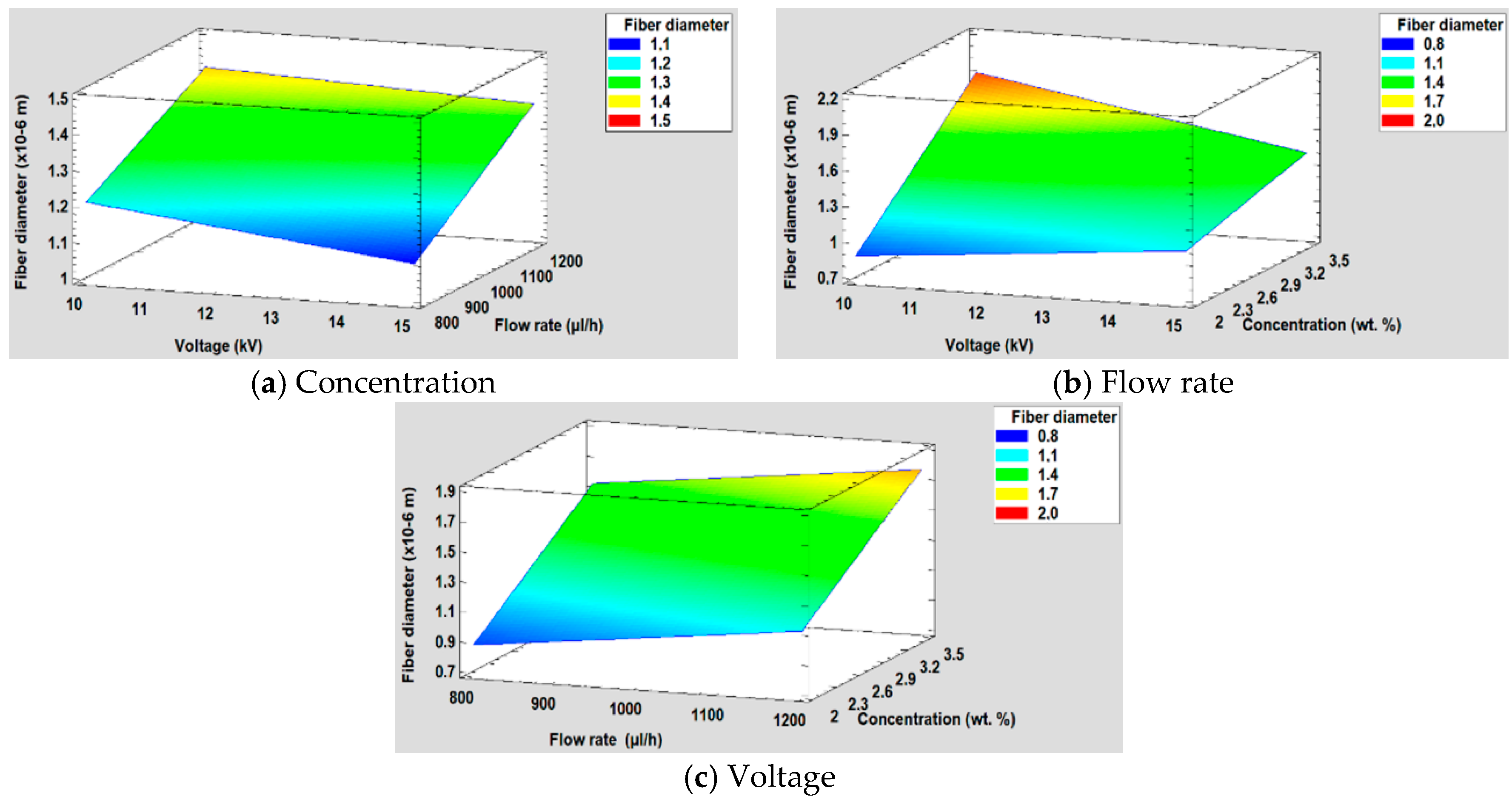
3.5.1. Effect on the Fiber Diameter
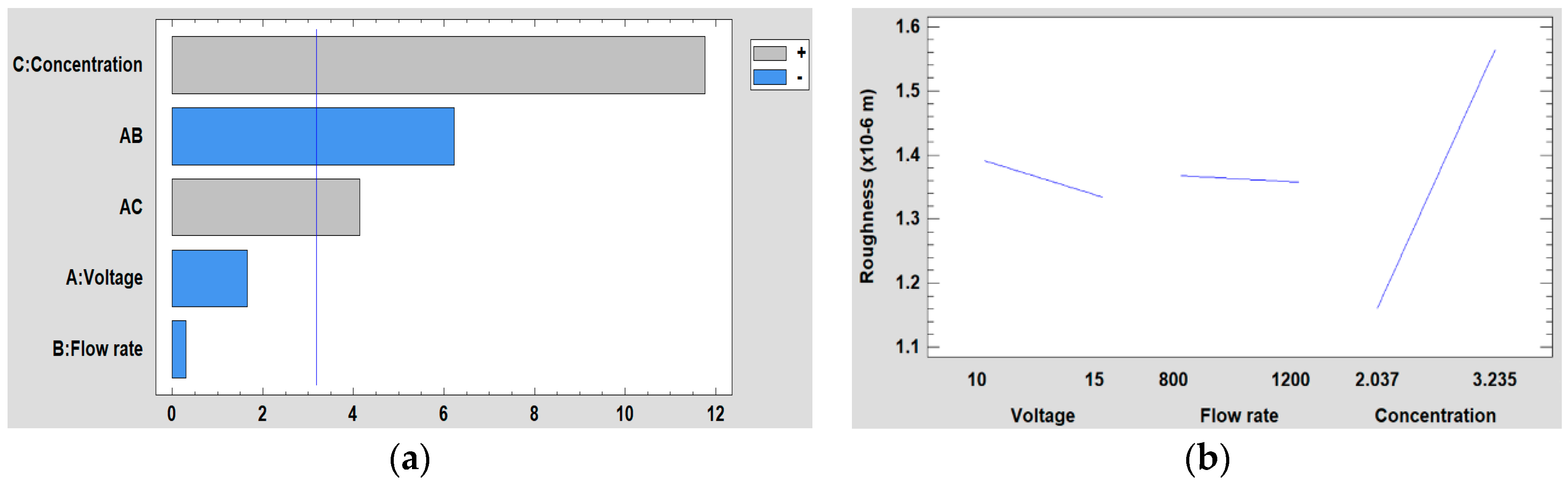
3.5.2. Effect on the Surface Roughness
3.5.3. Effect on the Water Contact Angle
3.5.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic Durable Multifunctional Self-Cleaning Nanofibrous Membrane with Outstanding Oil/Water Separation, Photodegradation of Organic Contaminants, and Antibacterial Performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, W.; Cui, J.; Wu, S.; Han, J.; Zou, Y.; Huang, C. Hydrothermal synthesized UV-resistance and transparent coating composited superoloephilic electrospun membrane for high efficiency oily wastewater treatment. J. Hazard. Mater. 2020, 383, 121152. [Google Scholar] [CrossRef]
- Ma, W.; Dinga, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Nine, J.; Tung, T.T.; Alotaibi, F.; Tran, D.N.H.; Losic, D. Facile Adhesion-Tuning of Superhydrophobic Surfaces between “Lotus” and “Petal” Effect and Their Influence on Icing and Deicing Properties. ACS Appl. Mater. Interfaces 2017, 9, 8393–8402. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Self-Cleaning Efficiency of Artificial Superhydrophobic Surfaces. Langmuir 2009, 25, 3240–3248. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically Robust Superhydrophobic Steel Surface with Anti-Icing, UV-Durability, and Corrosion Resistance Properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired Surfaces with Special Wettability. Chemin 2005, 36, 644–652. [Google Scholar] [CrossRef]
- Liu, H.; Feng, L.; Zhai, J.; Jiang, L.; Zhu, D. Reversible Wettability of a Chemical Vapor Deposition Prepared ZnO Film between Superhydrophobicity and Superhydrophilicity. Langmuir 2004, 20, 5659–5661. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic Surfaces Fabricated by Femtosecond Laser with Tunable Water Adhesion: From Lotus Leaf to Rose Petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Shiu, J.-Y.; Kuo, C.-W.; Chen, P.; Mou, C.-Y. Fabrication of Tunable Superhydrophobic Surfaces by Nanosphere Lithography. Chem. Mater. 2004, 16, 561–564. [Google Scholar] [CrossRef]
- Bravo, J.; Zhai, L.; Wu, Z.; Cohen, R.E.; Rubner, M.F. Transparent Superhydrophobic Films Based on Silica Nanoparticles. Langmuir 2007, 23, 7293–7298. [Google Scholar] [CrossRef]
- Manca, M.; Cannavale, A.; De Marco, L.; Aricò, A.; Cingolani, R.; Gigli, G. Durable Superhydrophobic and Antireflective Surfaces by Trimethylsilanized Silica Nanoparticles-Based Sol−Gel Processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Montemor, M.; Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Ran, M.; Zheng, W.; Wang, H. Fabrication of superhydrophobic surfaces for corrosion protection: A review. Mater. Sci. Technol. 2019, 35, 313–326. [Google Scholar] [CrossRef]
- Liu, H.; Szunerits, S.; Xu, W.; Boukherroub, R. Preparation of Superhydrophobic Coatings on Zinc as Effective Corrosion Barriers. ACS Appl. Mater. Interfaces 2009, 1, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Nine, J.; Cole, M.A.; Johnson, L.; Tran, D.N.H.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Grignard, B.; Vaillant, A.; De Coninck, J.; Piens, M.; Jonas, A.M.; Detrembleur, C.; Jérôme, C. Electrospinning of a Functional Perfluorinated Block Copolymer as a Powerful Route for Imparting Superhydrophobicity and Corrosion Resistance to Aluminum Substrates. Langmuir 2011, 27, 335–342. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Zhang, Z.; Yu, L. Superhydrophobic polyaniline/polystyrene micro/nanostructures as anticorrosion coatings. React. Funct. Polym. 2017, 119, 95–104. [Google Scholar] [CrossRef]
- Tarus, B.K.; Fadel, N.; Al-Oufy, A.; El Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; La, Y.M.; Lee, D.R.; Sung, N.H. Influence of a mixing solvent with tetrahydrofuran andN,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2259–2268. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Cheng, S.; Wang, K.; Mao, S.; Fan, L.-J. Preparation of high strength ultrafine polyvinyl chloride fibrous membrane and its adsorption of cationic dye. J. Polym. Res. 2009, 17, 769–777. [Google Scholar] [CrossRef]
- Lostado-Lorza, R.; Calvo, M.; Ángeles, M.; Berlanga-Labari, C.; Rivero, P.J. Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals 2018, 8, 711. [Google Scholar] [CrossRef]
- Al Haddad, Z.A.; Svinterikos, E.; Zuburtikudis, I. Designing electrospun nanocomposite poly(vinylidene fluoride) mats with tunable wettability. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 81–90. [Google Scholar] [CrossRef]
- Rivero, P.J.; Redin, D.M.; Rodríguez, R. Electrospinning: A Powerful Tool to Improve the Corrosion Resistance of Metallic Surfaces Using Nanofibrous Coatings. Metals 2020, 10, 350. [Google Scholar] [CrossRef]
- Seeram, R.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Moon, H.S.; Lyoo, W.S.; Lee, T.S.; Park, W.H. Superhydrophobicity of PHBV fibrous surface with bead-on-string structure. J. Colloid Interface Sci. 2008, 320, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Alayande, S.; Dare, E.O.; Msagati, T.A.; Akinlabi, A.K.; Aiyedun, P. Superhydrophobic and superoleophillic surface of porous beaded electrospun polystrene and polysytrene-zeolite fiber for crude oil-water separation. Phys. Chem. Earth Parts A/B/C 2016, 92, 7–13. [Google Scholar] [CrossRef]
- Cengiz, U.; Avci, M.Z.; Erbil, H.Y.; Sarac, A.S. Superhydrophobic terpolymer nanofibers containing perfluoroethyl alkyl methacrylate by electrospinning. Appl. Surf. Sci. 2012, 258, 5815–5821. [Google Scholar] [CrossRef]
- Cozza, E.S.; Monticelli, O.; Marsano, E.; Cebe, P. On the electrospinning of PVDF: Influence of the experimental conditions on the nanofiber properties. Polym. Int. 2012, 62, 41–48. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, A.D.B.; Rabolt, J.F. Micro- and Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromology 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Katti, D.S.; Robinson, K.W.; Ko, F.K.; Laurencin, C.T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. J. Biomed. Mater. Res. 2004, 70, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Bhattacharyya, S.; Bender, J.D.; Greish, Y.E.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Fabrication and Optimization of Methylphenoxy Substituted Polyphosphazene Nanofibers for Biomedical Applications. Biomacromolecules 2004, 5, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.A.; Kotaki, M.; Liu, Y.; Lu, X. Morphology, polymorphism behavior and molecular orientation of electrospun poly(vinylidene fluoride) fibers. Polymer 2007, 48, 512–521. [Google Scholar] [CrossRef]
- Chanunpanich, N.; Lee, B.; Byun, H. A study of electrospun PVDF on PET sheet. Macromol. Res. 2008, 16, 212–217. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Ki, C.-S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- LPham, Q.; Uspenskaya, M.V. Morphalogy pvc nanofiber, produced by electrospinning method. In International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management; SGEM: Sofia, Boulgaria, 2019; Volume 19, pp. 289–295. [Google Scholar]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Hekmati, A.H.; Rashidi, A.; Ghazisaeidi, R.; Drean, J.-Y. Effect of needle length, electrospinning distance, and solution concentration on morphological properties of polyamide-6 electrospun nanowebs. Text. Res. J. 2013, 83, 1452–1466. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Zhang, J. Facile method to prepare lotus-leaf-like super-hydrophobic poly(vinyl chloride) film. Appl. Surf. Sci. 2008, 254, 1593–1598. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness optimization for biomimetic superhydrophobic surfaces. Ultramicroscopy 2007, 107, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic Fabrics Produced by Electrospinning and Chemical Vapor Deposition. Macromology 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Asmatulu, R.; Ceylan, M.; Nuraje, N. Study of Superhydrophobic Electrospun Nanocomposite Fibers for Energy Systems. Langmuir 2011, 27, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Acik, G.; Cansoy, C.E.; Kamaci, M. Effect of flow rate on wetting and optical properties of electrospun poly(vinyl acetate) micro-fibers. Colloid Polym. Sci. 2018, 297, 77–83. [Google Scholar] [CrossRef]
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning superhydrophobic nanofibrous poly(vinylidene fluoride)/stearic acid coatings with excellent corrosion resistance. Thin Solid Films 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Dong, Y. Effect of the Morphology of Electrospun Polyvinylidene Fluoride Nanofiber on Corrosion Property of Q345 Steel. Int. J. Electrochem. Sci. 2017, 12, 11064–11076. [Google Scholar] [CrossRef]
- Es-Saheb, M.; Elzatahry, A.A.; Sherif, E.-S.M.; Alkaraki, A.S.; Kenawy, E.-R. A novel electrospinning application for polyvinyl chloride nanofiber coating deposition as a corrosion inhibitor for aluminum, steel, and brass in chloride solutions. Int. J. Electrochem. Sci. 2012, 7, 5962–5976. [Google Scholar]
- Iribarren, A.; Rivero, P.J.; Berlanga-Labari, C.; Larumbe, S.; Miguel, A.; Palacio, J.F.; Rodríguez, R. Multifunctional Protective PVC-ZnO Nanocomposite Coatings Deposited on Aluminum Alloys by Electrospinning. Coatings 2019, 9, 216. [Google Scholar] [CrossRef]
- Rivero, P.J.; Iribarren, A.; Larumbe, S.; Palacio, J.F.; Rodríguez, R. A Comparative Study of Multifunctional Coatings Based on Electrospun Fibers with Incorporated ZnO Nanoparticles. Coatings 2019, 9, 367. [Google Scholar] [CrossRef]
- Radwan, A.B.; Abdullah, A.M.; Mohamed, A.M.A.; Al-Maadeed, M.A. New electrospun polystyrene/Al2O3 nanocomposite superhydrophobic coatings; Synthesis, characterization, and application. Coatings 2018, 8, 65. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Qiu, H.; Xue, Y.; Yang, J. Repeated self-healing of composite coatings with core-shell fibres. Compos. Commun. 2020, 19, 220–225. [Google Scholar] [CrossRef]
- Kim, J.; Mousa, H.M.; Park, C.H.; Kim, C.S. Enhanced corrosion resistance and biocompatibility of AZ31 Mg alloy using PCL/ZnO NPs via electrospinning. Appl. Surf. Sci. 2017, 396, 249–258. [Google Scholar] [CrossRef]
- Hanas, T.; Kumar, T.S.S.; Perumal, G.; Doble, M. Tailoring degradation of AZ31 alloy by surface pre-treatment and electrospun PCL fibrous coating. Mater. Sci. Eng. C 2016, 65, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Yurrita, D.; Berlanga-Labari, C.; Palacio, J.F.; Rodríguez, R. Functionalized Electrospun Fibers for the Design of Novel Hydrophobic and Anticorrosive Surfaces. Coatings 2018, 8, 300. [Google Scholar] [CrossRef]
- Li, W.; Yu, Q.; Yao, H.; Zhu, Y.; Topham, P.D.; Yue, K.; Ren, L.; Wang, L. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019, 92, 60–70. [Google Scholar] [CrossRef]
- Lim, J.-M.; Yi, G.-R.; Moon, J.H.; Heo, C.-J.; Yang, S.-M. Superhydrophobic Films of Electrospun Fibers with Multiple-Scale Surface Morphology. Langmuir 2007, 23, 7981–7989. [Google Scholar] [CrossRef]
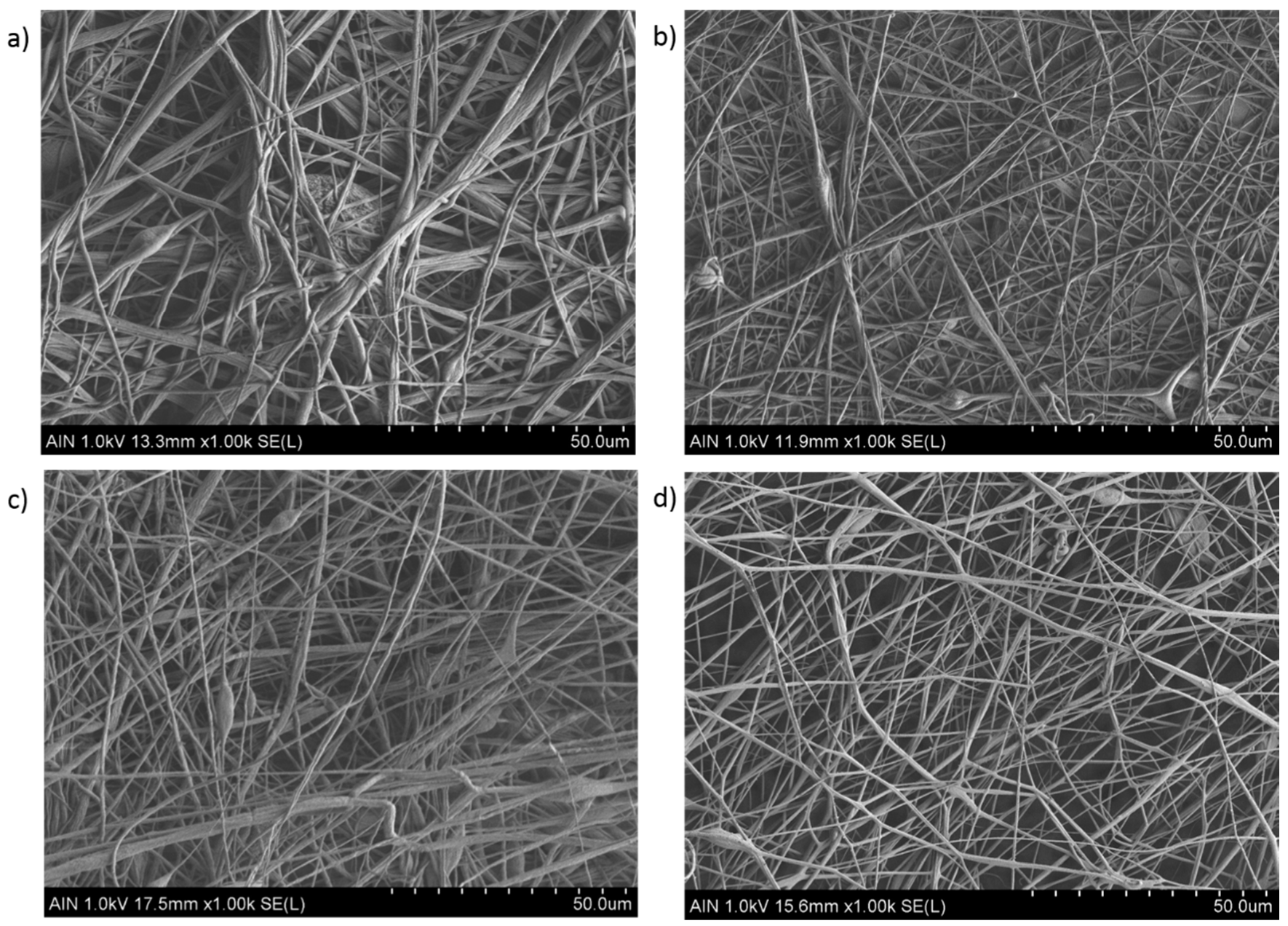


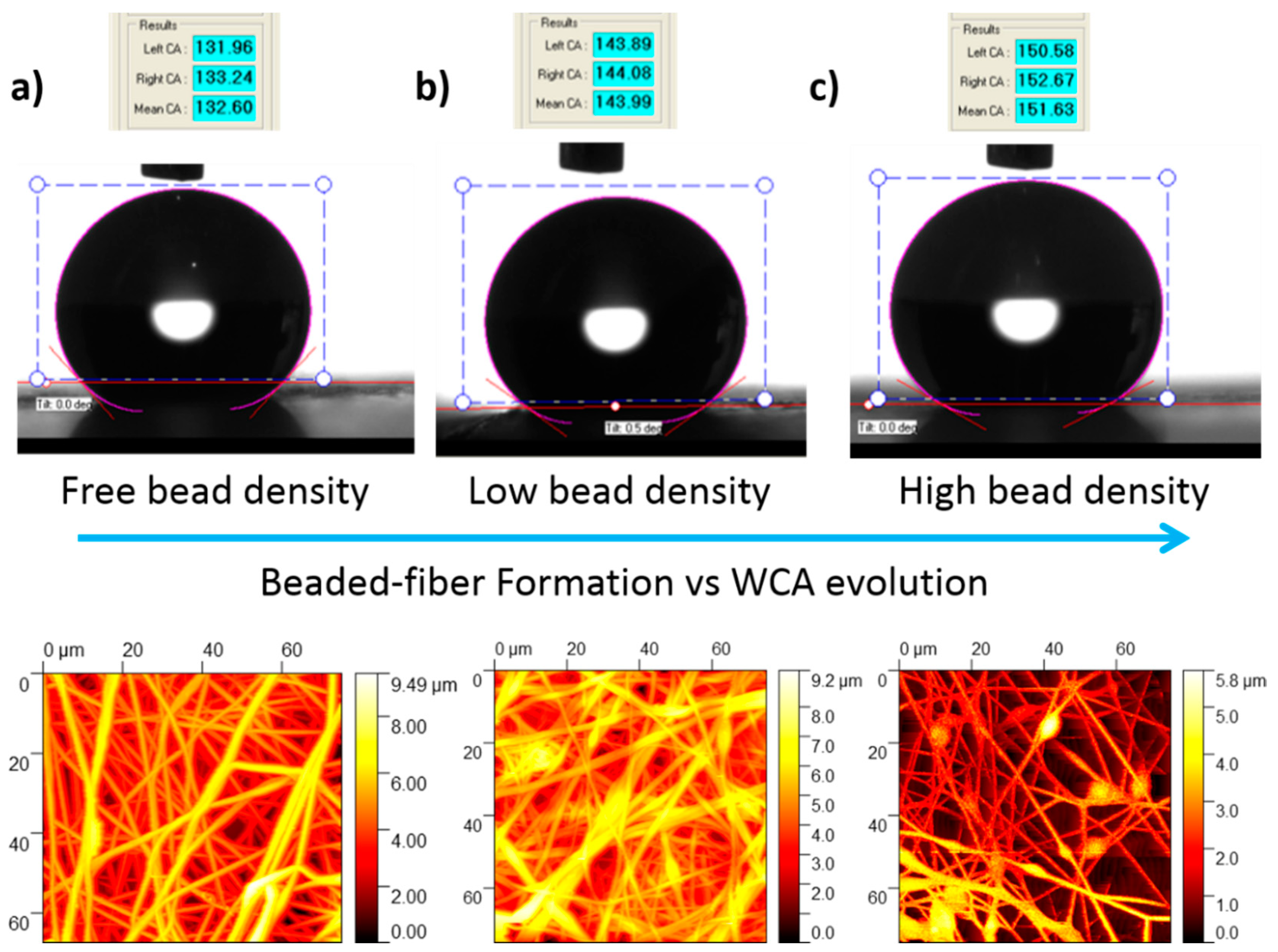
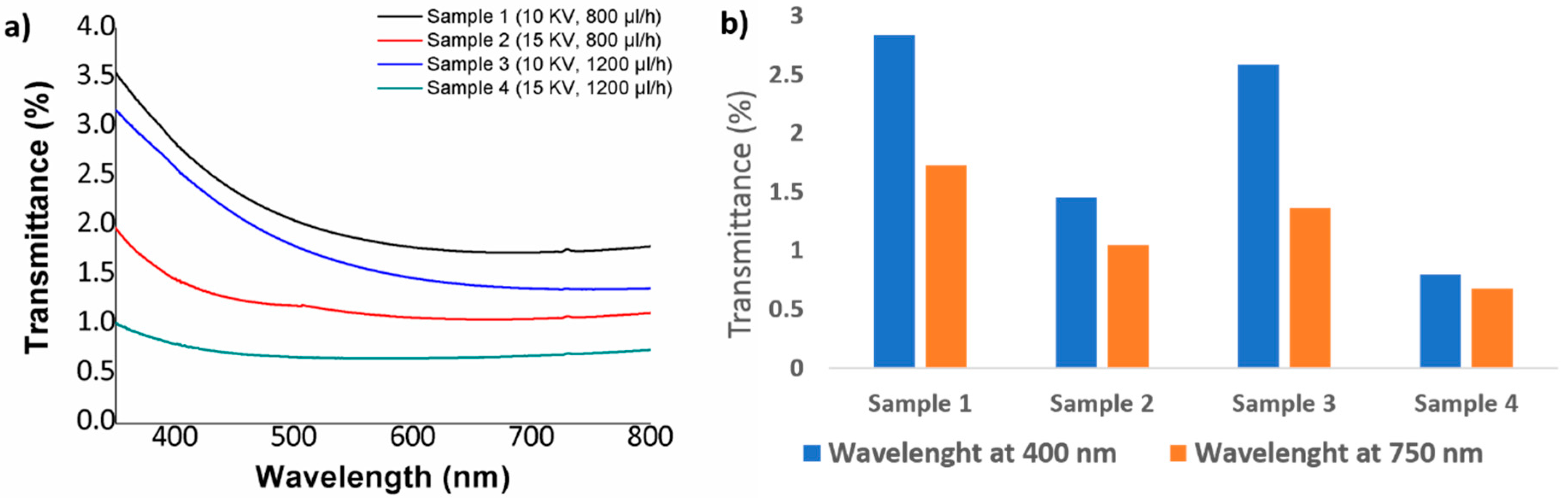

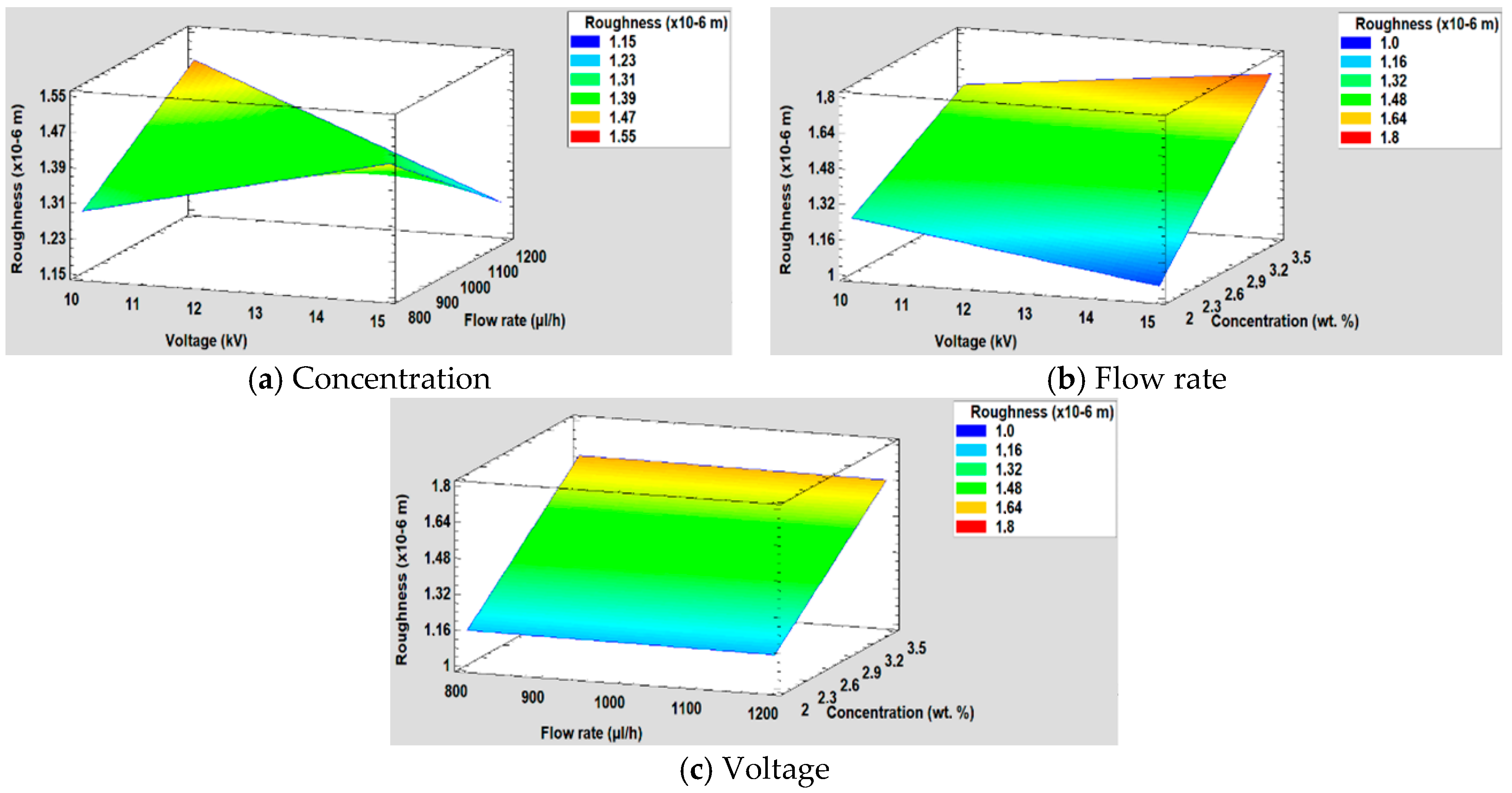
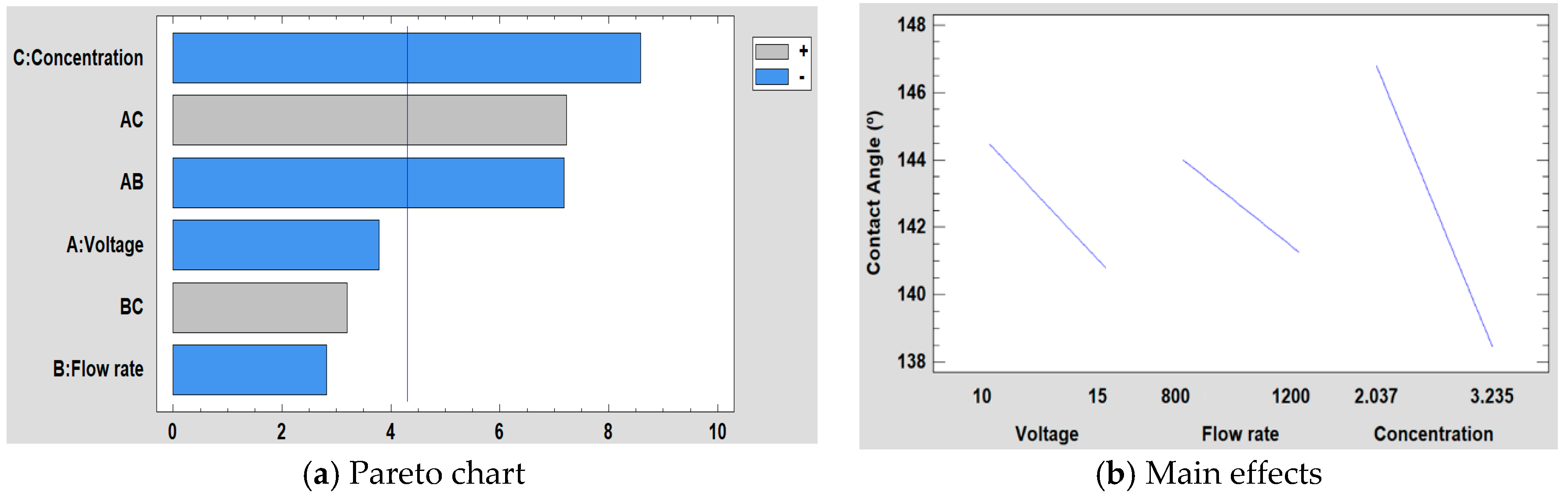


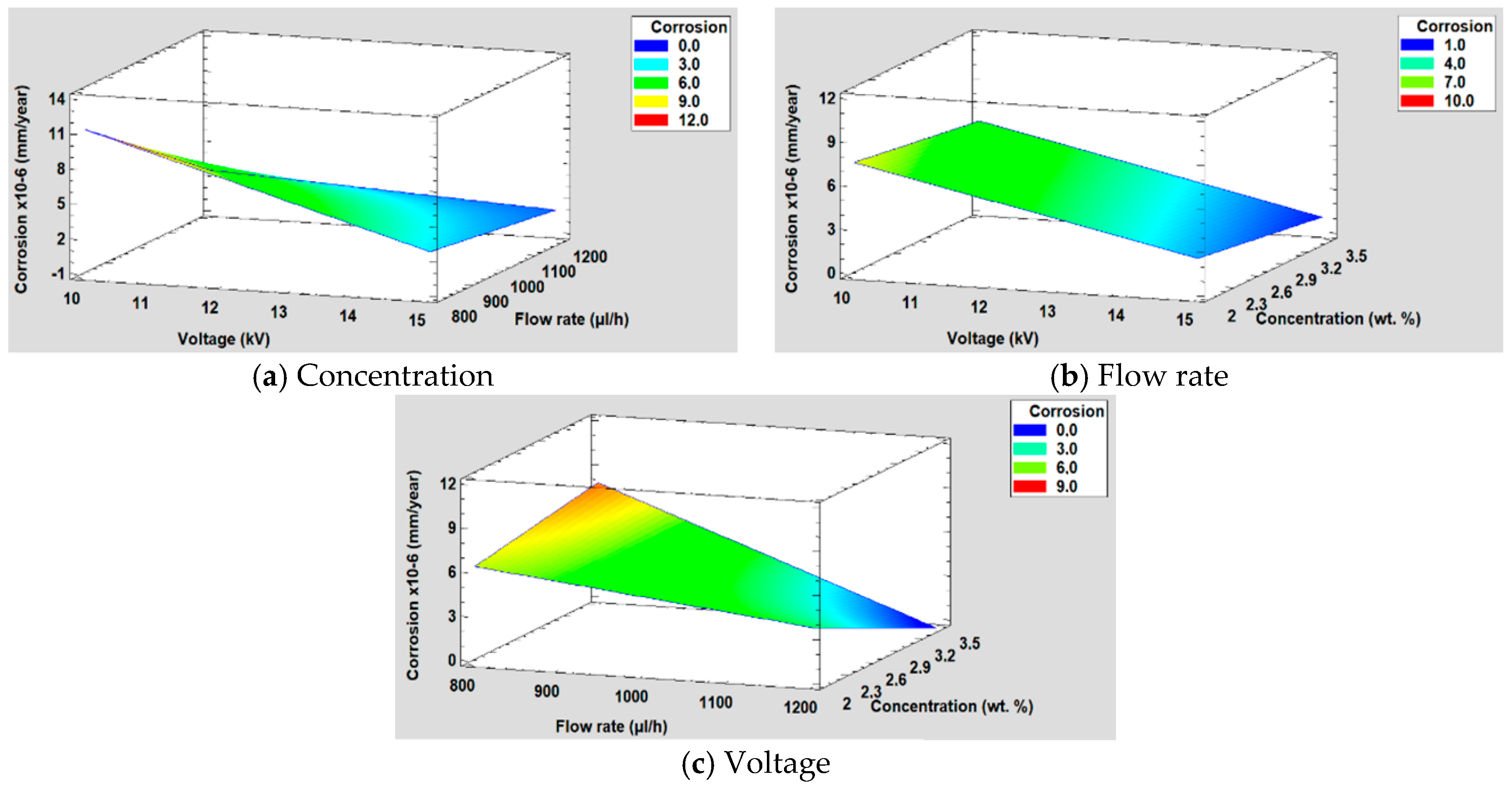
| Sample | Concentration (wt %) | Flow Rate (µL/h) | Voltage (KV) |
|---|---|---|---|
| 1 | 15 | 800 | 10 |
| 2 | 15 | 800 | 15 |
| 3 | 15 | 1200 | 10 |
| 4 | 15 | 1200 | 15 |
| 5 | 10 | 800 | 10 |
| 6 | 10 | 1200 | 10 |
| 7 | 10 | 800 | 15 |
| 8 | 10 | 1200 | 15 |
| Sample | Water Contact Angle Measurements (°) | Average Fiber Diameter (µm) | RMS Roughness Sq (µm) | Mean Roughness Sa (µm) |
|---|---|---|---|---|
| 1 | 133.1 ± 0.8 | 1.65 ± 0.43 | 1.19 | 0.95 |
| 2 | 144.1 ± 4.0 | 1.18 ± 0.16 | 1.75 | 1.43 |
| 3 | 142.1 ± 1.7 | 1.80 ± 0.61 | 1.64 | 1.39 |
| 4 | 134.8 ± 4.2 | 1.55 ± 0.15 | 1.36 | 1.09 |
| 5 | 151.1 ± 2.9 | 0.78 ± 0.16 | 1.28 | 1.06 |
| 6 | 150.6 ± 4.1 | 1.04 ± 0.15 | 1.24 | 1.08 |
| 7 | 145.8 ± 1.8 | 1.03 ± 0.19 | 1.04 | 0.93 |
| 8 | 134.7 ± 4.5 | 1.21 ± 0.18 | 0.98 | 0.87 |
| Electrospun Coating | Metallic Substrate | Parameters Used | Ref. |
|---|---|---|---|
| Poly(vinylidene fluoride)/stearic acid (PVDF/SA) | Aluminum sheets | 16 KV; 0.8 mL/h; 15 cm distance | [57] |
| Poly(vinylidene fluoride) (PVDF) | Q345 steel | 12 KV; 15 cm distance | [58] |
| Poly(vinyl chloride) (PVC) | Aluminum, copper, and brass | 70 KV; 18 cm distance | [59] |
| Poly(vinyl chloride)/zinc oxide (PVC-ZnO) | Aluminum alloy (AA6061-T6) | 8–14 KV; 0.6–1.2 mL/h; 15 cm distance | [60] |
| Poly(vinyl chloride)/zinc oxide (PVC-ZnO) and polystyrene/zinc oxide (PS/ZnO) | Aluminum alloy (AA6061-T6) | 8–14 KV; 0.6–1.2 mL/h; 15 cm distance for PVC11–17 KV; 1.5 mL/h; 15 cm distance for PS | [61] |
| Polystyrene/aluminum oxide (PS/Al2O3) | Commercial aluminum foil | 20–25 KV; 1.5–2.0 mL/h; 15 cm distance | [62] |
| Core-shell polyacrylonitrile fibers reinforced waterborne polyurethane (PU) coating | Hot dip galvanized (HDG) steel | 13.5 KV; 13.3/1.0 µL/min; 14 cm distance | [63] |
| Polycaprolactone/zinc oxide nanoparticles (PCL/ZnO) | Magnesium alloy (AZ31) | 16 KV; 1 mL/h; 15 cm distance | [64] |
| Polycaprolactone (PCL) | Magnesium alloy (AZ31) | 200 µL/h; 12 cm distance | [65] |
| Functionalized poly(acrylic acid) by chemical vapor silanization process | Aluminum alloy (AA6061-T6) | 17 KV; 0.5 mL/h; 20 cm distance | [66] |
| Perfluorinated block copolymer | Aluminum plates | 20 KV; 0.8 mL/h; 5–15 cm distance | [28] |
| Poly(vinyl chloride) (PVC) | Aluminum alloy (AA6061-T6) | Design of experiments (DoE) with 10–15 KV; 0.8–1.2 mL/h; 10–15% solution concentration; 15 cm distance | This work |
| Sample | Icorr (µA/cm2) | Ecorr (V) | Corrosion Rate (mm/year) |
|---|---|---|---|
| Aluminum (6061T6) | 0.14219 | −0.73953 | 1.4948 × 10−4 |
| 1 | 0.0038137 | −0.83989 | 4.0093 × 10−6 |
| 2 | 0.0015504 | −0.75222 | 1.6299 × 10−6 |
| 3 | 0.0001657 | −0.88928 | 1.7420 × 10−7 |
| 4 | 0.00043609 | −0.86393 | 4.5845 × 10−7 |
| 5 | 0.00075333 | −0.95068 | 7.9196 × 10−7 |
| 6 | 0.005038 | −0.83759 | 5.2963 × 10−6 |
| 7 | 0.0023467 | −0.82533 | 2.4670 × 10−6 |
| 8 | 0.0017334 | −0.96552 | 1.8223 × 10−6 |
| Factor | Low Level | High Level |
|---|---|---|
| (A) Voltage (kV) | 10 | 15 |
| (B) Flow rate (µL/h) | 800 | 1200 |
| (C) PVC concentration | 2.037 g (10 wt %) | 3.235 g (15 wt %) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, P.J.; Rosagaray, I.; Fuertes, J.P.; Palacio, J.F.; Rodríguez, R.J. Designing Multifunctional Protective PVC Electrospun Fibers with Tunable Properties. Polymers 2020, 12, 2086. https://doi.org/10.3390/polym12092086
Rivero PJ, Rosagaray I, Fuertes JP, Palacio JF, Rodríguez RJ. Designing Multifunctional Protective PVC Electrospun Fibers with Tunable Properties. Polymers. 2020; 12(9):2086. https://doi.org/10.3390/polym12092086
Chicago/Turabian StyleRivero, Pedro J., Iker Rosagaray, Juan P. Fuertes, José F. Palacio, and Rafael J. Rodríguez. 2020. "Designing Multifunctional Protective PVC Electrospun Fibers with Tunable Properties" Polymers 12, no. 9: 2086. https://doi.org/10.3390/polym12092086
APA StyleRivero, P. J., Rosagaray, I., Fuertes, J. P., Palacio, J. F., & Rodríguez, R. J. (2020). Designing Multifunctional Protective PVC Electrospun Fibers with Tunable Properties. Polymers, 12(9), 2086. https://doi.org/10.3390/polym12092086








