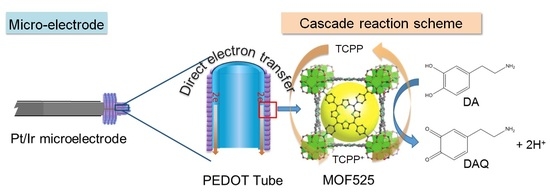
Synthesis of MOF525/PEDOT Composites as Microelectrodes for Electrochemical Sensing of Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of MOF525
2.2. Electropolymerization of 3,4-Ethylenedioxythiophene (EDOT)-Based Monomers
2.3. Preparation of MOF525/PEDOT Composites Modified Microelectrode
2.4. Material Characterizations
2.5. Electrochemical Determination of Dopamine
3. Results and Discussion
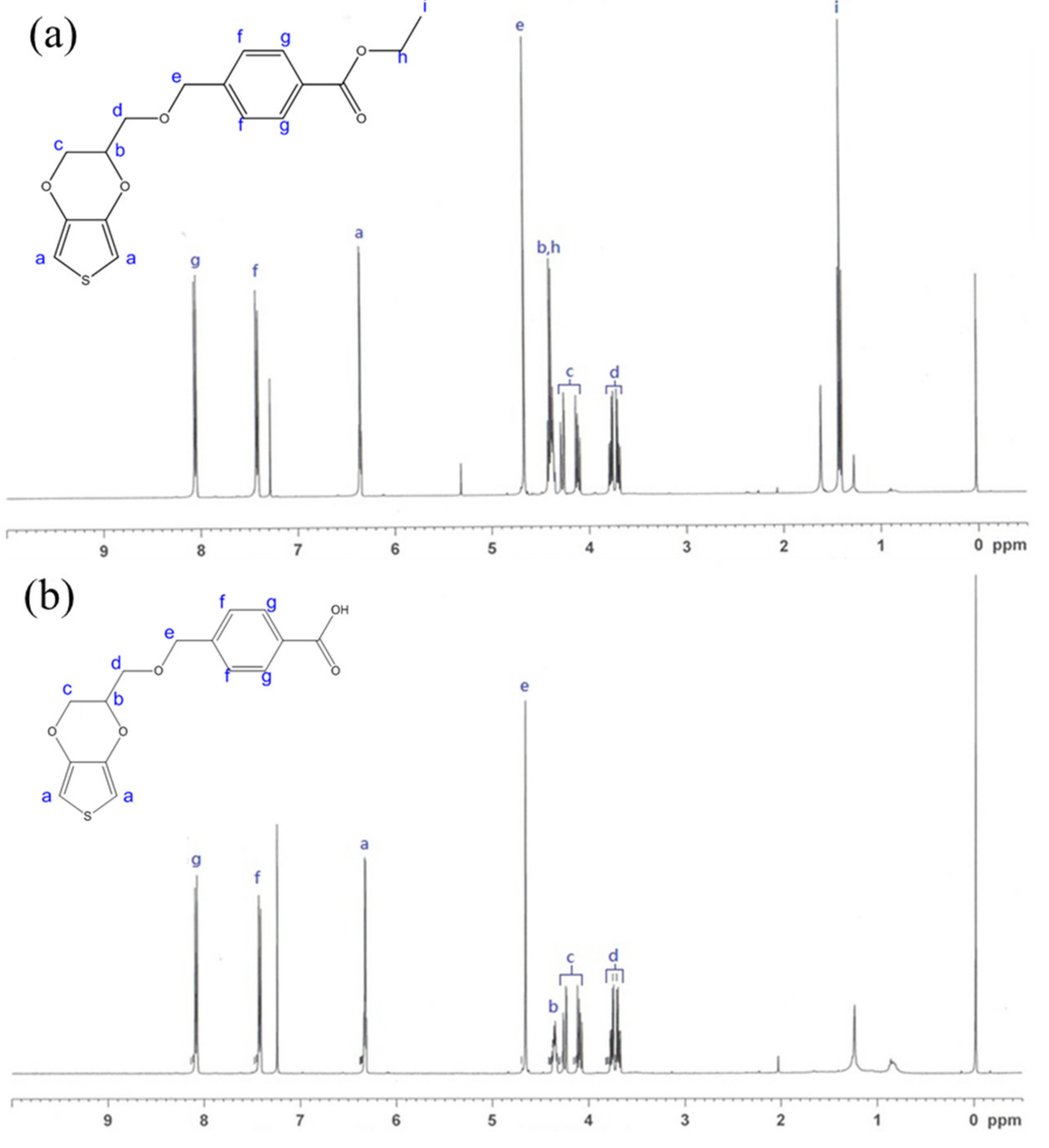
3.1. NMR Analysis of EDOT Monomers
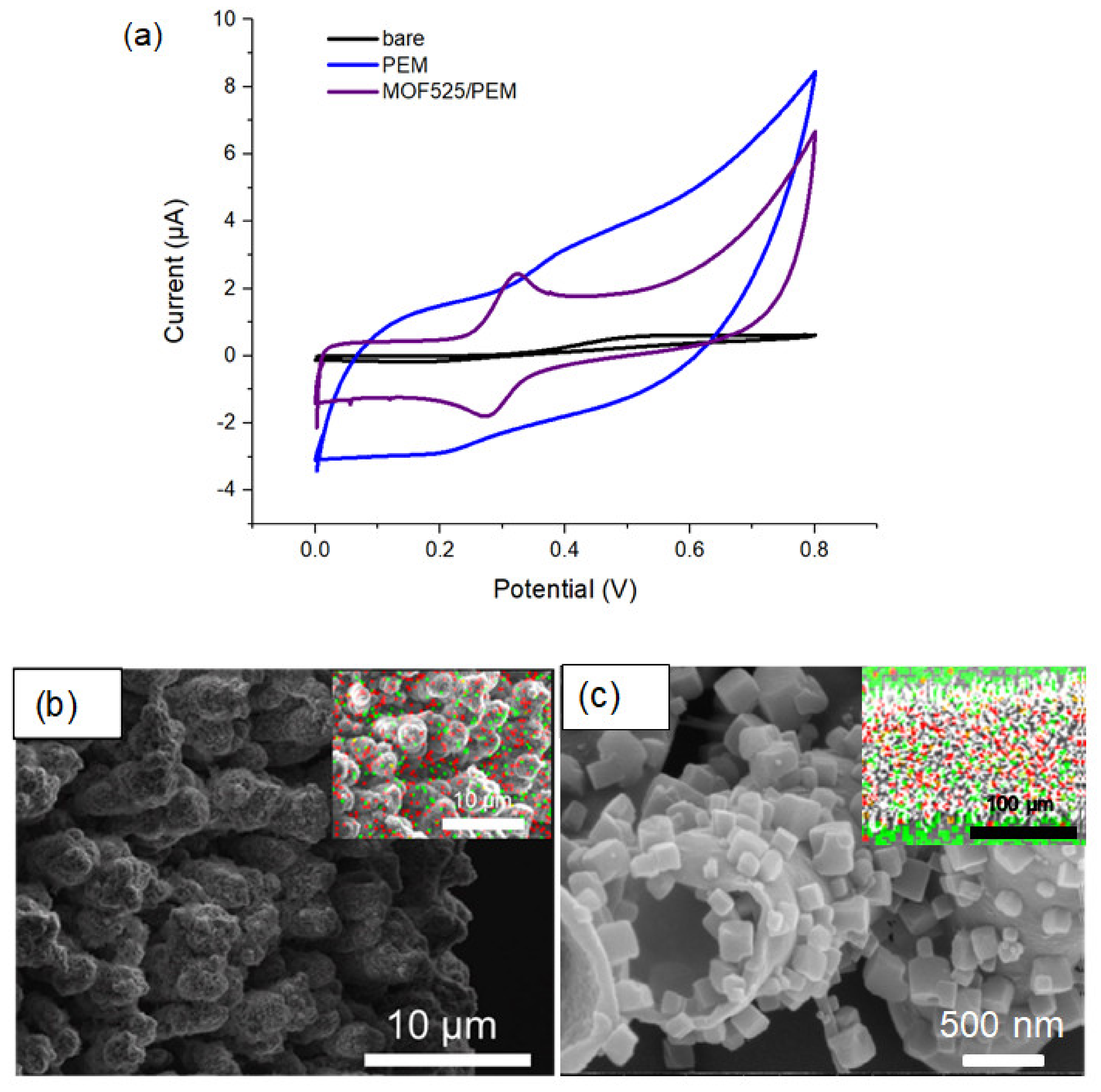
3.2. Characterization of MOF525
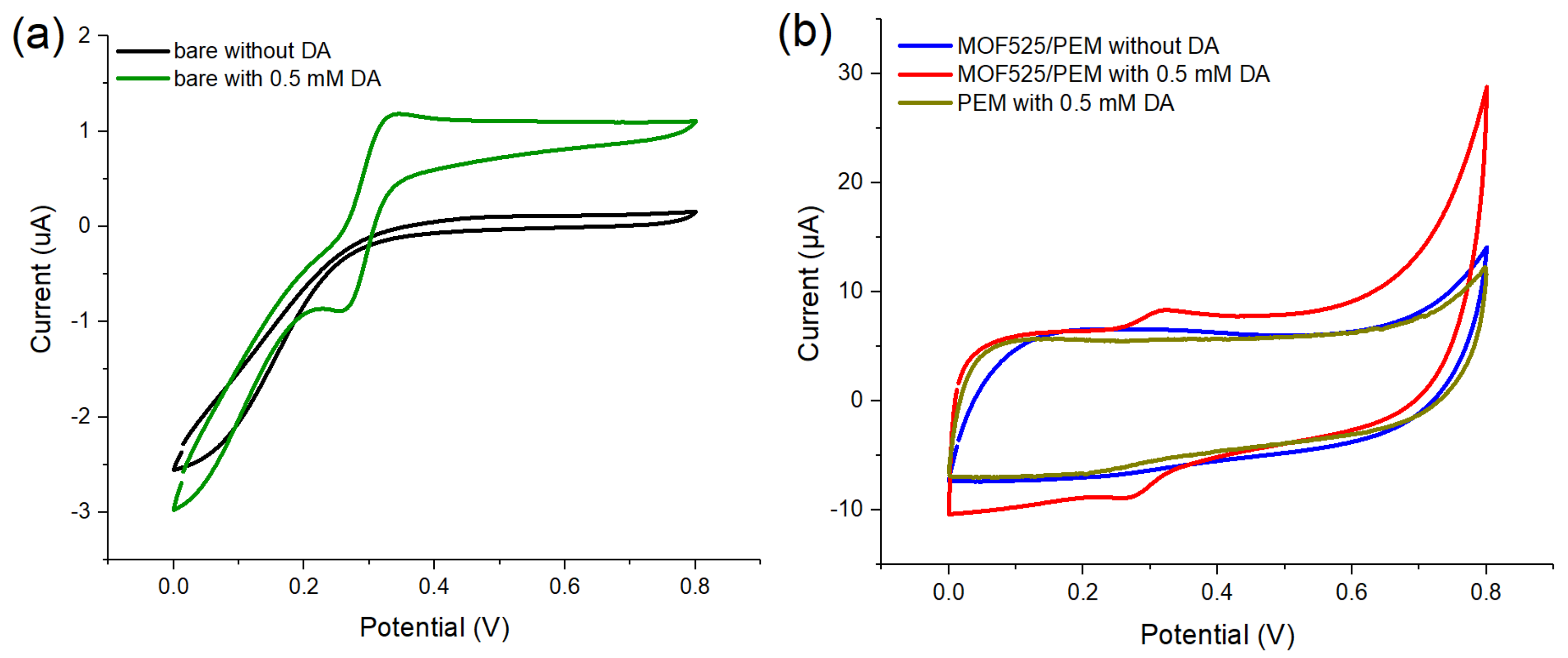
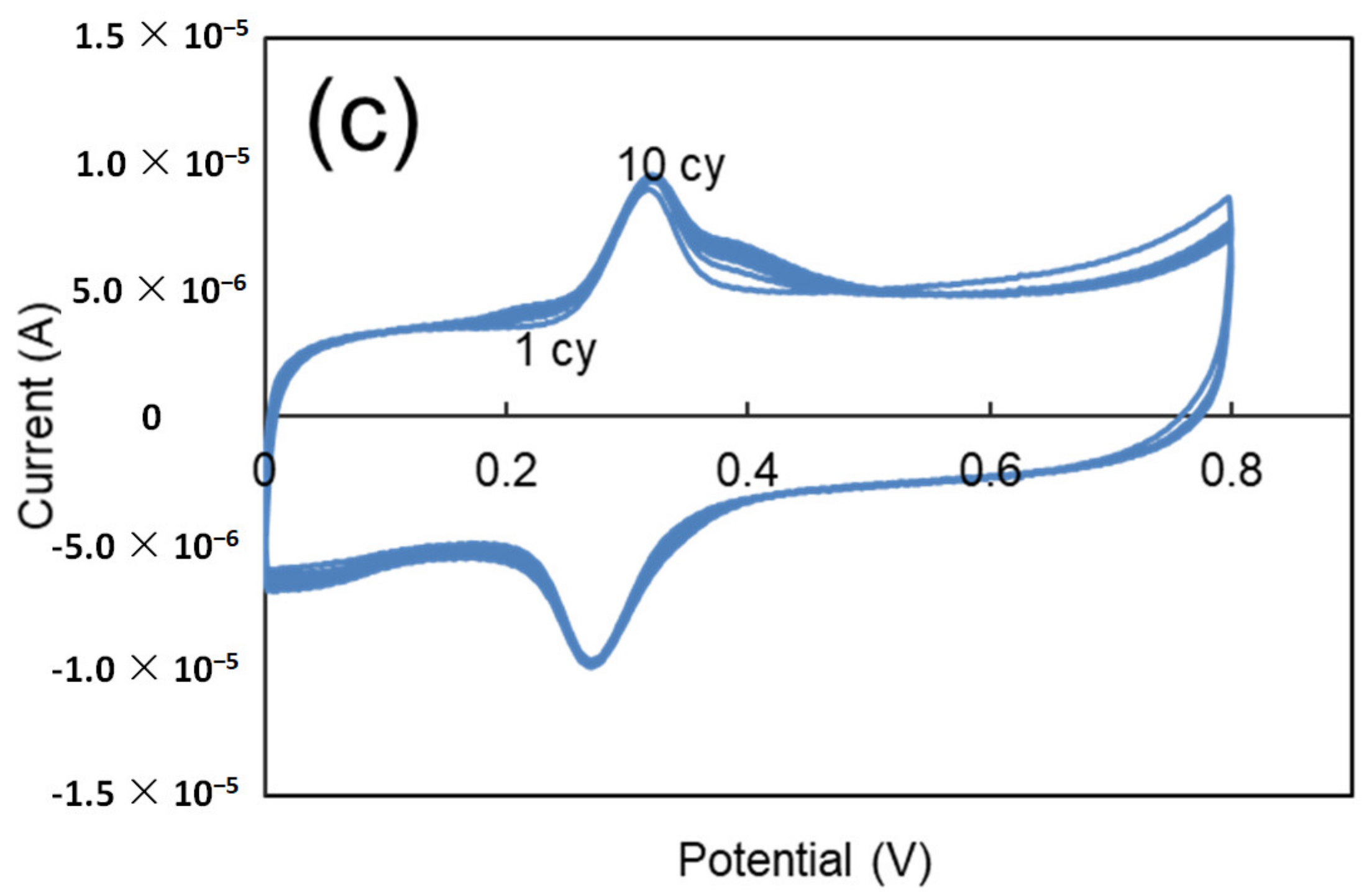
3.3. MOF525/PEM Composites Modified Microelectrode
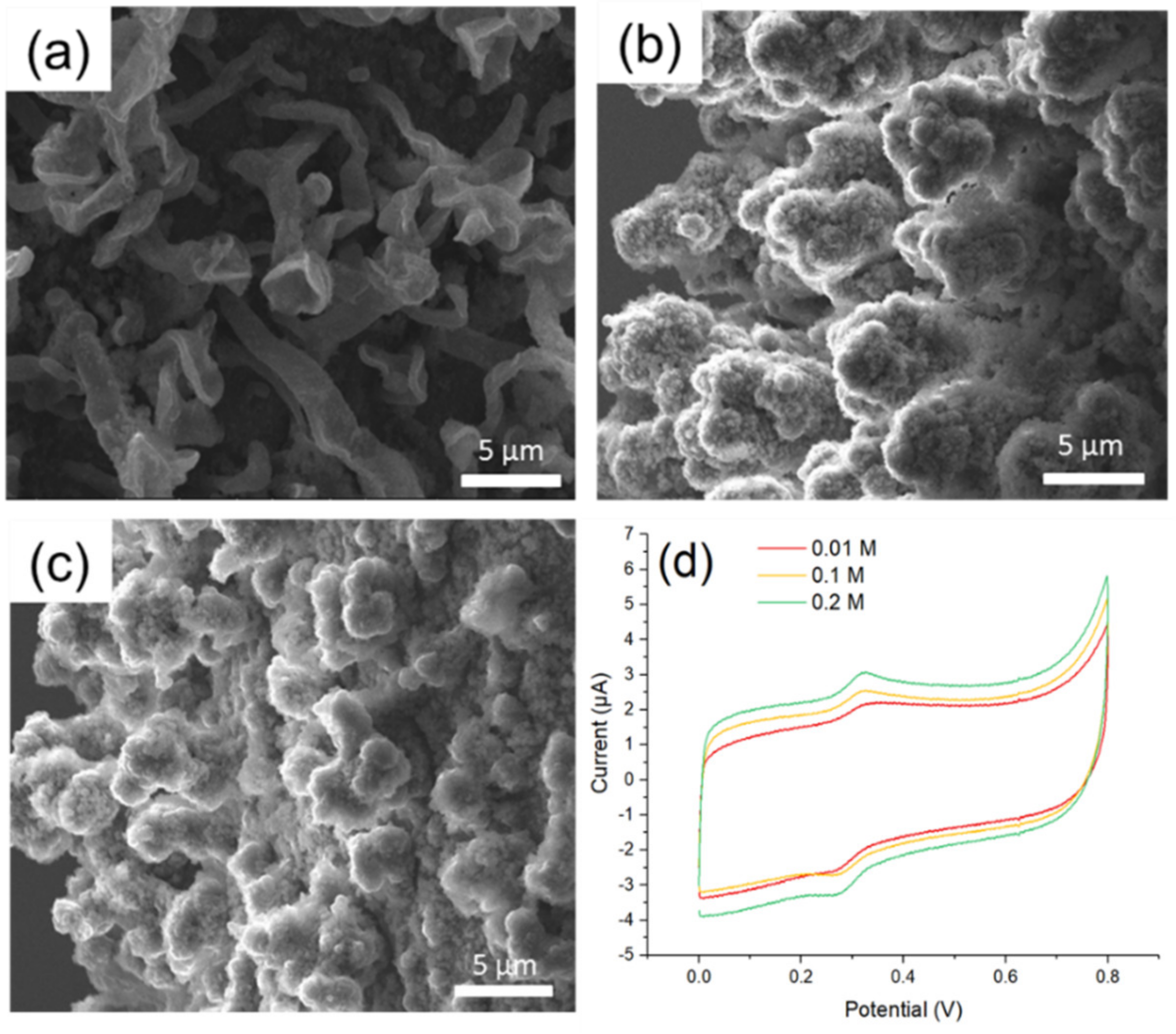
3.4. MOF525/PEpC Composites Modified Microelectrode
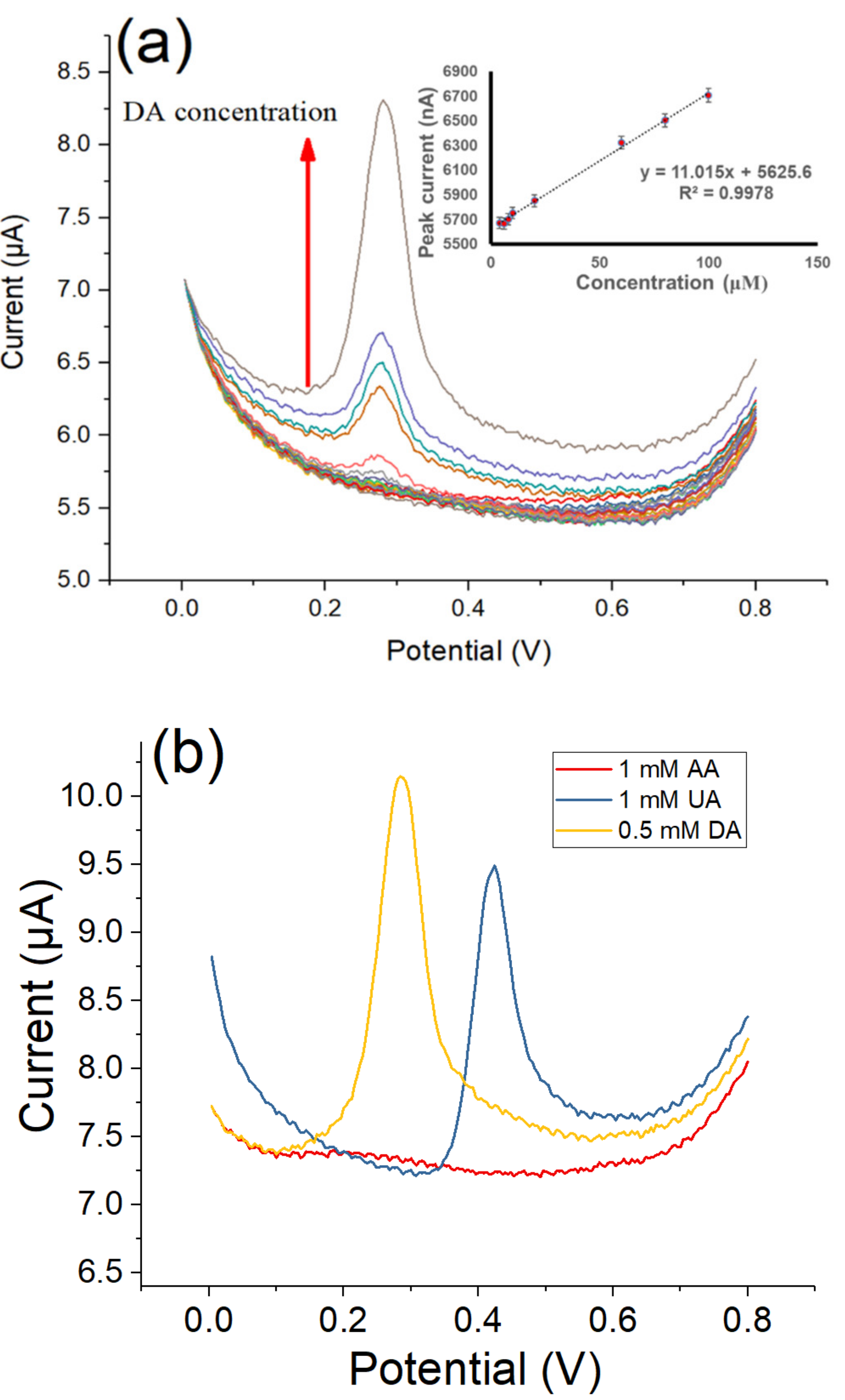
3.5. Detection of Dopamine by Differential Pulse Voltammetry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Jones, S.; Giros, B.; Caron, M.G. The dopamine transporter: A crucial component regulating dopamine transmission. Mov. Disord. Soc. 1997, 12, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Vantol, H.H.; Bunzow, J.R.; Guan, H.C.; Sunahara, R.K.; Seeman, P.; Niznik, H.B.; Civelli, O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 1991, 350, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Van Tol, H.H. Dopamine receptor pharmacology. Trends Pharmacol. Sci. 1994, 15, 264–270. [Google Scholar] [CrossRef]
- Biederman, J.; Faraone, S.V. Attention-deficit hyperactivity disorder. Lancet 2005, 366, 237–248. [Google Scholar] [CrossRef]
- Fahn, S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov. Disord. Soc. 2008, 23, S497–S508. [Google Scholar] [CrossRef]
- Su, R.; Lin, J.M.; Qu, F.; Chen, Z.; Gao, Y.; Yamada, M. Capillary electrophoresis microchip coupled with on-line chemiluminescence detection. Anal. Chim. Acta 2004, 508, 11–15. [Google Scholar] [CrossRef]
- Carrera, V.; Sabater, E.; Vilanova, E.; Sogorb, M.A. A simple and rapid HPLC–MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: Application to the secretion of bovine chromaffin cell cultures. J. Chromatogr. B 2007, 847, 88–94. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, Y.; Shi, M.; Liu, Y.M. Quantification of biogenic amines by microchip electrophoresis with chemiluminescence detection. J. Chromatogr. A 2009, 1216, 5155–5159. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Shi, H.; Ma, C.; Cong, B.; Kang, W. Determination of three major catecholamines in human urine by capillary zone electrophoresis with chemiluminescence detection. Anal. Biochem. 2012, 427, 10–17. [Google Scholar] [CrossRef]
- Pandikumar, A.; How, G.T.S.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, K.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, S.A.; et al. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RSC Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Application of laccase and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzym. Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Tembe, S.; Kubal, B.S.; Karve, M.; Souza, S.F. Glutaradelhyde activated eggshell membrane for immobilization of tyrosinase from Amorphophallus companulatus: Application in construction oe electrochemical biosensor for dopamine. Anal. Chim. Acta. 2008, 612, 212–217. [Google Scholar] [CrossRef]
- Njagi, J.; Ispas, C.; Andreescu, S. Mixed ceria-based metal oxides biosensor for operation in oxygen restrictive environments. Anal. Chem. 2008, 80, 7266–7274. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, Y.; Xu, H.; Tan, Y.; Wang, S. Detection of dopamine based tyrosinase-Fe3O4 nanoparticles-chitosan nanocomposite biosensor. Am. J. Biomed. Sci. 2010, 2, 209–216. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, C.; Ye, J. Graphene-modified carbon fiber microelectrode for the detection of dopamine in mice hippocampus tissue. Electroanalysis 2011, 23, 907–914. [Google Scholar] [CrossRef]
- Bala, K.; Sharma, D.; Gupta, N. Carbon-nanotube-based materials for electrochemical sensing of the neurotransmitter dopamine. ChemElectroChem 2019, 6, 274–288. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in vivo electrochemical detection of resting dopamine using Poly (3, 4-ethylenedioxythiophene)/Carbon Nanotube functionalized microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zeng, X.; Liu, X.; Kong, B.; Wei, W.; Luo, S. Selective and sensitive detection of dopamine in the presence of ascorbic acid by molecular sieve/ionic liquids composite electrode. Electrochim. Acta 2011, 23, 2730–2734. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, D.; Zhang, S.; Luan, F.; Chen, L. Reduced graphene oxide functionalized with a CoS2/ionic liquid composite and decorated with gold nanoparticles for voltammetric sensing of dopamine. Microchim. Acta 2018, 185, 166. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, Y.; Tok, M.; Bilici, E.; Mikoliunaite, L.; Yazicigil, Z.; Ramanaviciene, A.; Ramanavicius, A. Copper nanoparticle modified carbon electrode for determination of dopamine. Electrochim. Acta 2012, 76, 201–207. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.K.; Aruna, S.; Kumar, M.; Shashanka, R.; Jayadevappa, H. Preparation of NiO/ZnO hybrid nanoparticles for electrochemical sensing of dopamine and uric acid. Chem. Sensors 2012, 2, 1–7. [Google Scholar]
- Wang, H.H.; Chen, X.J.; Li, W.T.; Zhou, W.H.; Guo, X.C.; Kang, W.Y.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Tian, Q.W.; et al. ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 2018, 176, 573–581. [Google Scholar] [CrossRef]
- Huang, T.Y.; Kung, C.W.; Liao, Y.T.; Kao, S.Y.; Cheng, M.; Chang, T.H.; Henzie, J.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; et al. Enhanced charge collection in MOF-525–PEDOT nanotube composites enable highly sensitive biosensing. Adv. Sci. 2017, 4, 1700261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Duan, D.; Liu, S.; Zhang, Y.; Leng, L.; Li, X.; Chen, N.; Zhang, Y. Metal-organic framework-based molecularly imprinted polymer as a high sensitive and selective hybrid for the determination of dopamine in injections and human serum samples. Biosens. Bioelectron. 2018, 118, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de novo approach: Size-selective sheltering of catalase in metal–organic framework microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef]
- Kung, C.W.; Han, P.C.; Chuang, C.H.; Wu, K.C.W. Electronically conductive metal–organic framework-based materials. APL Mater. 2019, 7, 110902. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.; Shieh, F.K.; Wu, K.C.W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, S.; Xue, H.; Pang, H. Metal–organic framework composites and their electrochemical applications. J. Mater. Chem. A 2019, 7, 7301–7327. [Google Scholar] [CrossRef]
- Tang, A.; Bungay, P.M.; Gonzales, R.A. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J. Neurosci. Methods 2003, 126, 1–11. [Google Scholar] [CrossRef]
- Borland, L.M.; Shi, G.; Yang, H.; Michael, A.C. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J. Neurosci. Methods 2005, 146, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Suzuki, A.; Ivandini, T.A.; Yoshimi, K.; Fujishima, A.; Oyama, G.; Nakazato, T.; Hattori, N.; Kitazawa, S.; Einaga, Y. Fabrication, characterization, and application of boron-doped diamond microelectrodes for in vivo dopamine detection. Anal. Chem. 2007, 79, 8608–8615. [Google Scholar] [CrossRef]
- Kung, C.W.; Chang, T.H.; Chou, L.Y.; Hupp, J.T.; Farha, O.K.; Ho, K.C. Post metalation of solvothermally grown electroactive porphyrin metal–organic framework thin films. Chem. Commun. 2015, 51, 2414–2417. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Kunimatsu, K.; Senzaki, T.; Samjeské, G.; Tsushima, M.; Osawa, M. Hydrogen adsorption and hydrogen evolution reaction on a polycrystalline Pt electrode studied by surface-enhanced infrared absorption spectroscopy. Electrochim. Acta 2007, 52, 5715–5724. [Google Scholar] [CrossRef]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Jang, L.S.; Hsu, C.Y.; Chen, C.H. Effect of electrode geometry on performance of EWOD device driven by battery-based system. Biomed. Microdevices 2009, 11, 1029. [Google Scholar] [CrossRef]
- Siewert, E.; Schein, J.; Forster, G. Determination of enthalpy, temperature, surface tension and geometry of the material transfer in PGMAW for the system argon–iron. J. Phys. D Appl. Phys. 2013, 46, 224008. [Google Scholar] [CrossRef]
- Luo, S.C.; Sekine, J.; Zhu, B.; Zhao, H.; Nakao, A.; Yu, H.H. Polydioxythiophene nanodots, nonowires, nano-networks, and tubular structures: The effect of functional groups and temperature in template-free electropolymerization. ACS Nano 2012, 6, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Li, C.T.; Ho, K.C. A template-free synthesis of the hierarchical hydroxymethyl PEDOT tube-coral array and its application in dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 384–394. [Google Scholar] [CrossRef]
- Luo, S.C.; Thomas, J.L.; Guo, H.Z.; Liao, W.T.; Lee, M.H.; Lin, H.Y. Electrosynthesis of Nanostructured, Imprinted Poly (hydroxymethyl 3, 4-ethylenedioxythiophene) for the Ultrasensitive Electrochemical Detection of Urinary Progesterone. ChemistrySelect 2017, 2, 7935–7939. [Google Scholar] [CrossRef]
- Castagnola, V.; Bayon, C.; Descamps, E.; Bergaud, C. Morphology and conductivity of PEDOT layers produced by different electrochemical routes. Synth. Met. 2014, 189, 7–16. [Google Scholar] [CrossRef]
- Seki, Y.; Takahashi, M.; Takashiri, M. Enhanced thermoelectric properties of electropolymerized poly (3, 4-ethylenedioxythiophene) thin films by optimizing electrolyte temperature and thermal annealing temperature. Org. Electron. 2018, 55, 112–116. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Zhang, Y.; Chen, X.; Chi, Z.; Yang, J.; Ou, J.; Zhang, M.Q.; Li, D.; Wang, D.; et al. The steric effect of aromatic pendant groups and electrical bistability in π-stacked polymers for memory devices. Phys. Chem. Chem. Phys. 2012, 14, 4640–4650. [Google Scholar] [CrossRef]
- Mortier, C.; Darmanin, T.; Guittard, F. 3, 4-Ethylenedioxypyrrole (EDOP) monomers with aromatic substituents for parahydrophobic surfaces by electropolymerization. Macromolecules 2015, 48, 5188–5195. [Google Scholar] [CrossRef]
- Song, B.; Wang, Z.; Chen, S.; Zhang, X.; Fu, Y.; Smet, M.; Dehaen, W. The introduction of π–π stacking moieties for fabricating stable micellar structure: Formation and dynamics of disklike micelles. Angew. Chem. Int. Ed. 2005, 44, 4731–4735. [Google Scholar] [CrossRef]
- Meng, L.; Turner, A.P.; Mak, W.C. Modulating electrode kinetics for discrimination of dopamine by a PEDOT: COOH interface doped with negatively charged tricarboxylate. ACS Appl. Mater. Interfaces 2019, 11, 34497–34506. [Google Scholar] [CrossRef] [PubMed]
- Eskelsen, J.R.; Wang, Y.; Qui, Y.; Ray, M.; Handlin, M.; Hipps, K.W.; Mazur, U. Protonation state of core nitrogens in the meso-tetra (4-carboxyphenyl) porphyrin impacts the chemical and physical properties of nanostructures formed in acid solutions. J. Porphyr. Phthalocyanines 2012, 16, 1233–1243. [Google Scholar] [CrossRef]




| Conditions | S/B Ratio | |
|---|---|---|
| EDOT-MeOH:EDOT a | 3:1 | 1.32 |
| 2:1 | 1.22 | |
| 1:1 | 1.21 | |
| 0.33:1 | 1.17 | |
| 0.2:1 | 1.11 | |
| TBAP concentration (M) | 0.01 | 1.19 |
| 0.1 | 1.17 | |
| 0.2 | 1.15 | |
| TBAP concentration (M) b | 0.005 | 1.70 |
| 0.01 | 1.26 | |
| 0.02 | 1.26 | |
| Polymerization Time (s) | S/B Ratio |
|---|---|
| 60 | 1.68 |
| 80 | 1.74 |
| 100 | 2.02 |
| 120 | 1.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.S.; Han, P.-C.; Kuok, W.-K.; Lu, J.-Y.; Gu, Y.; Ahamad, T.; Alshehri, S.M.; Ayalew, H.; Yu, H.-h.; Wu, K.C.-W. Synthesis of MOF525/PEDOT Composites as Microelectrodes for Electrochemical Sensing of Dopamine. Polymers 2020, 12, 1976. https://doi.org/10.3390/polym12091976
Chen SS, Han P-C, Kuok W-K, Lu J-Y, Gu Y, Ahamad T, Alshehri SM, Ayalew H, Yu H-h, Wu KC-W. Synthesis of MOF525/PEDOT Composites as Microelectrodes for Electrochemical Sensing of Dopamine. Polymers. 2020; 12(9):1976. https://doi.org/10.3390/polym12091976
Chicago/Turabian StyleChen, Season S., Po-Chun Han, Wai-Kei Kuok, Jian-Yu Lu, Yesong Gu, Tansir Ahamad, Saad M. Alshehri, Hailemichael Ayalew, Hsiao-hua Yu, and Kevin C.-W. Wu. 2020. "Synthesis of MOF525/PEDOT Composites as Microelectrodes for Electrochemical Sensing of Dopamine" Polymers 12, no. 9: 1976. https://doi.org/10.3390/polym12091976
APA StyleChen, S. S., Han, P.-C., Kuok, W.-K., Lu, J.-Y., Gu, Y., Ahamad, T., Alshehri, S. M., Ayalew, H., Yu, H.-h., & Wu, K. C.-W. (2020). Synthesis of MOF525/PEDOT Composites as Microelectrodes for Electrochemical Sensing of Dopamine. Polymers, 12(9), 1976. https://doi.org/10.3390/polym12091976