Mechanical Integrity Degradation and Control of All-Solid-State Lithium Battery with Physical Aging Poly (Vinyl Alcohol)-Based Electrolyte
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Test Methods
- (1)
- For the purpose of determining the tensile properties of SPE films in the period of physical aging, quasi-static uniaxial tension was conducted at the strain rate of 5 × 10−3 s−1.
- (2)
- The stress relaxation was carried out at 0.4% strain, which could ensure that the mechanical response of the sample was within a linear range. The stress required to keep a constant strain was recorded with a sampling rate of 10 Hz.
3. Model Formulation
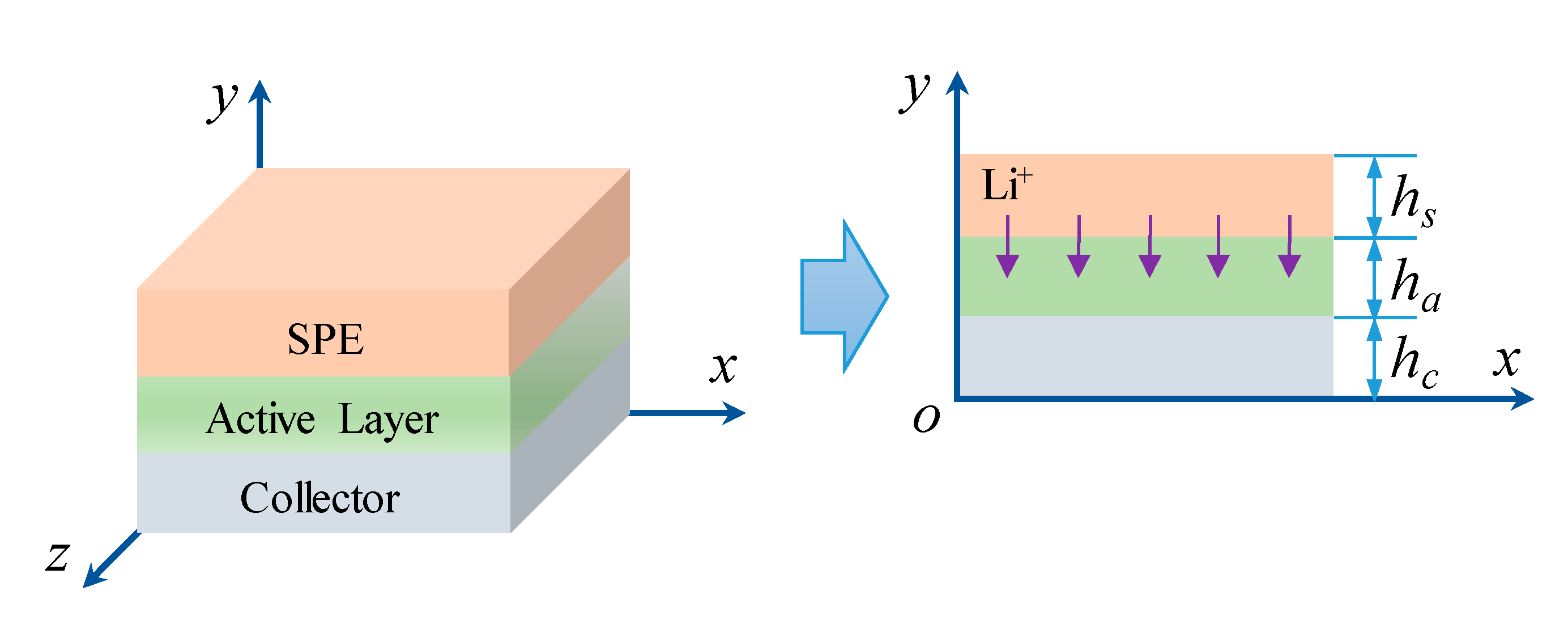
3.1. Migration of Lithium
3.2. Internal Stress
3.3. Solving Conditions
4. Results and Discussions
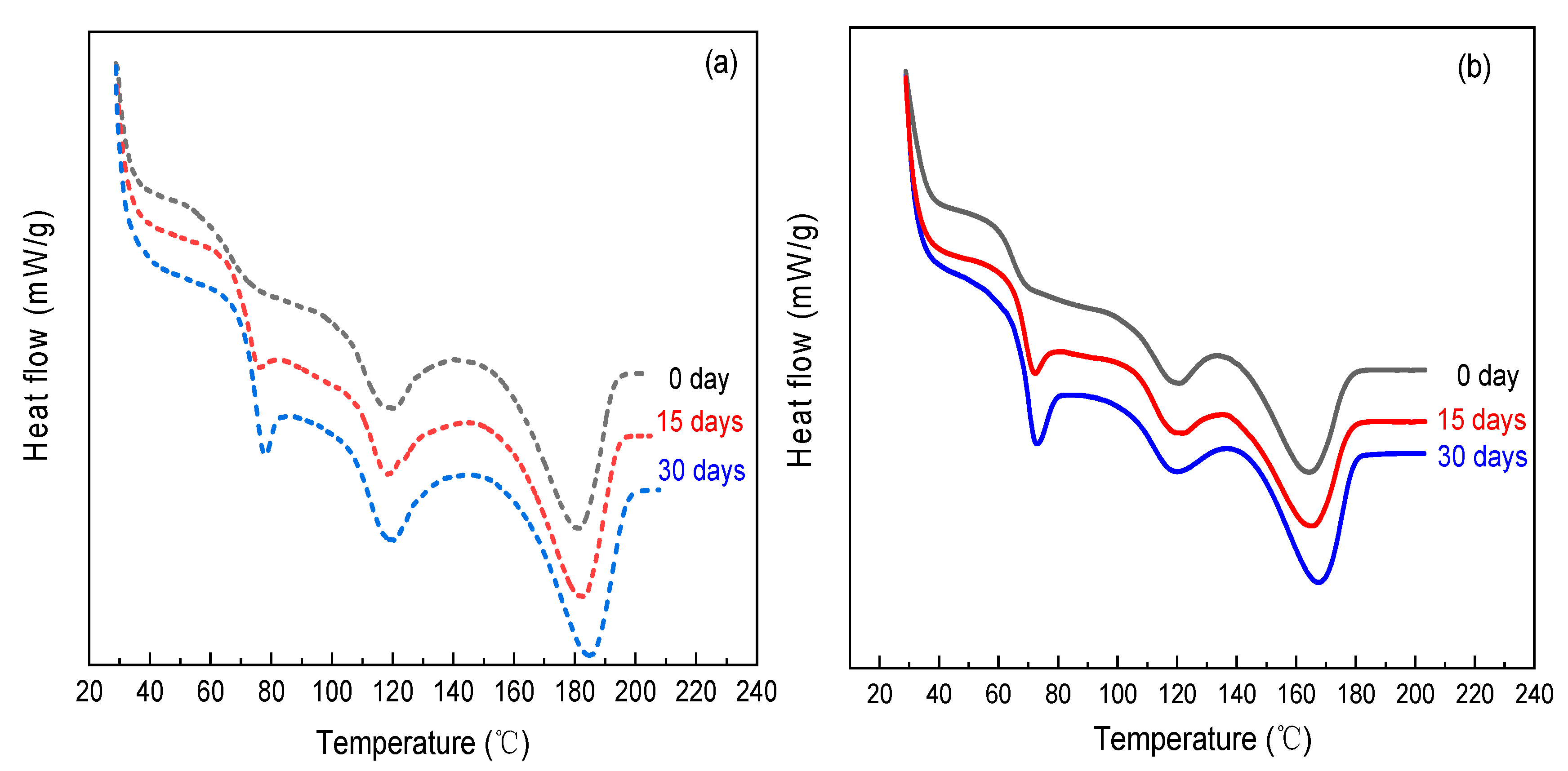
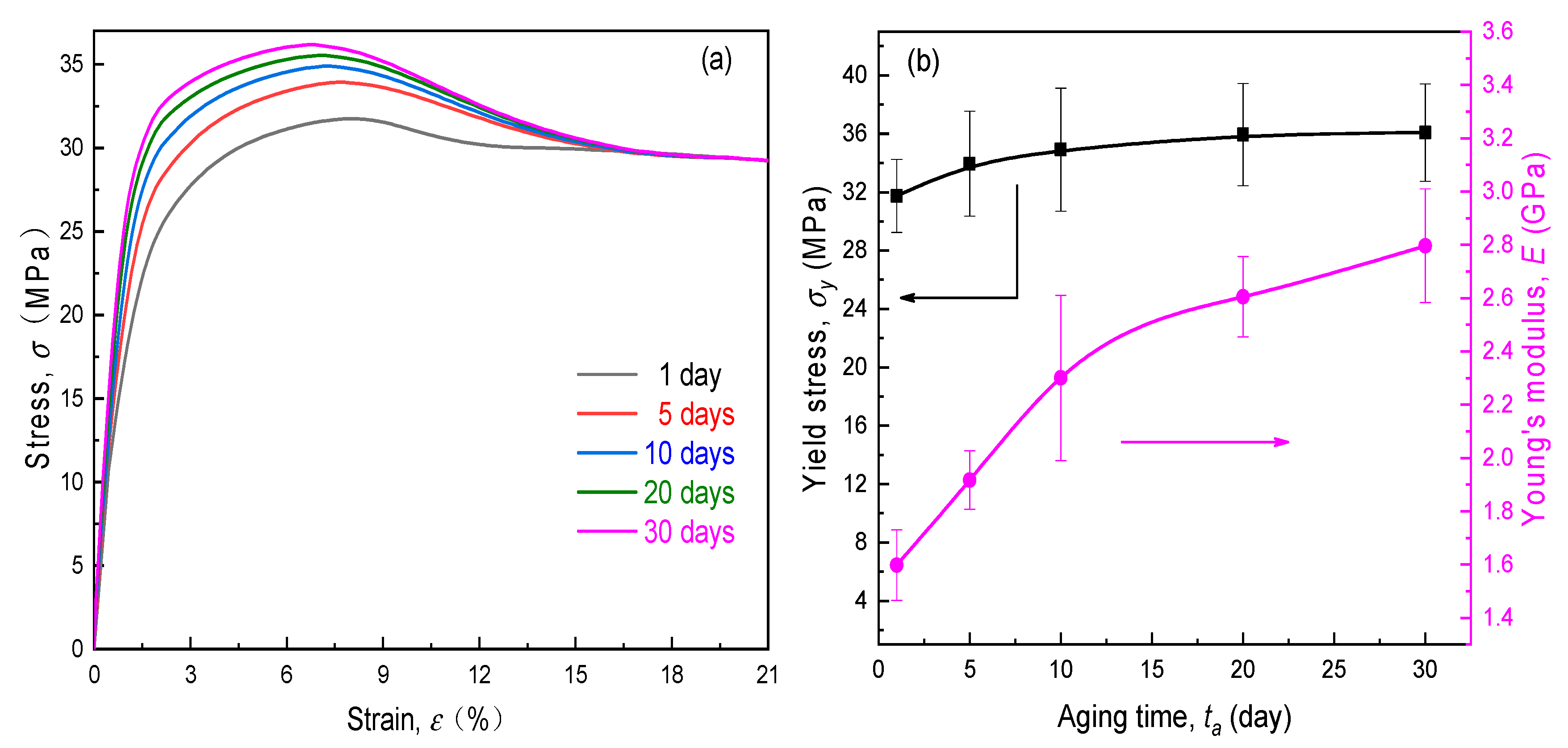
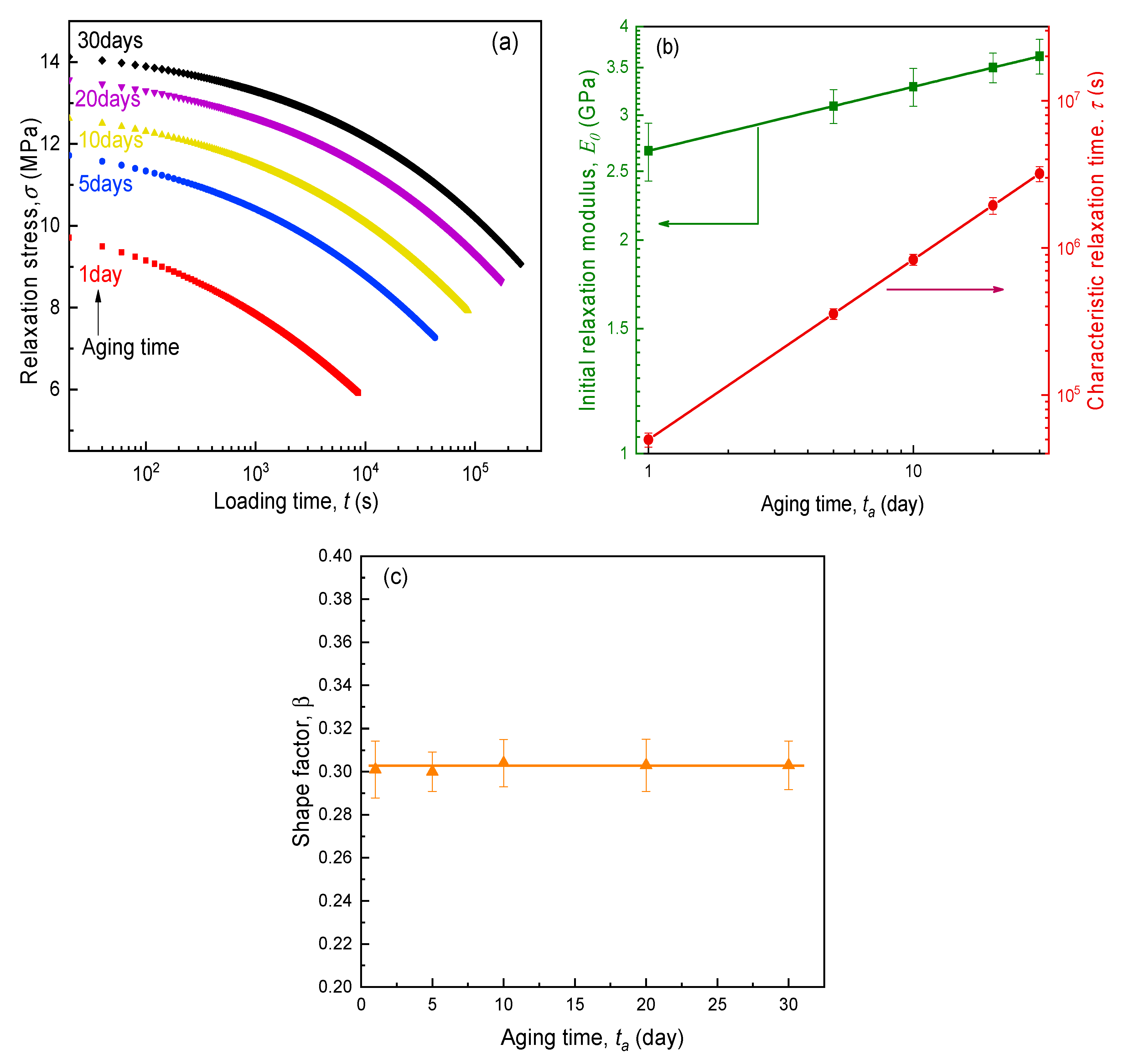
4.1. Mechanical Performance of SPEs during Physical Aging
4.2. Impact of Physical Aging on SPE Stress during Electrode Lithiation
4.3. Impact of Relative Thickness of SPE Film
4.4. Impact of Electrochemical Loading Rate
5. Conclusions
- (1)
- The PVA-based SPE films exhibited a pronounced physical aging trend under ambient storage conditions, in which both stiffness and yield strength enhanced with the elapsed time. However, the tensile rupture stress nearly did not rely on the aging time, due to the mechanical rejuvenation in the process of plastic deformation.
- (2)
- The KWW time-decay function could describe the evolution of elastic modulus for aging SPEs during the stress relaxation period. Furthermore, it is found that the physical aging contributed to the increase in initial modulus and characteristic relaxation time, while the shape factor remained constant for the specimen at different aging stages. Accordingly, the ideal momentary relaxation master curve could be obtained using the classical Struik shift method in terms of the time–aging time superposition.
- (3)
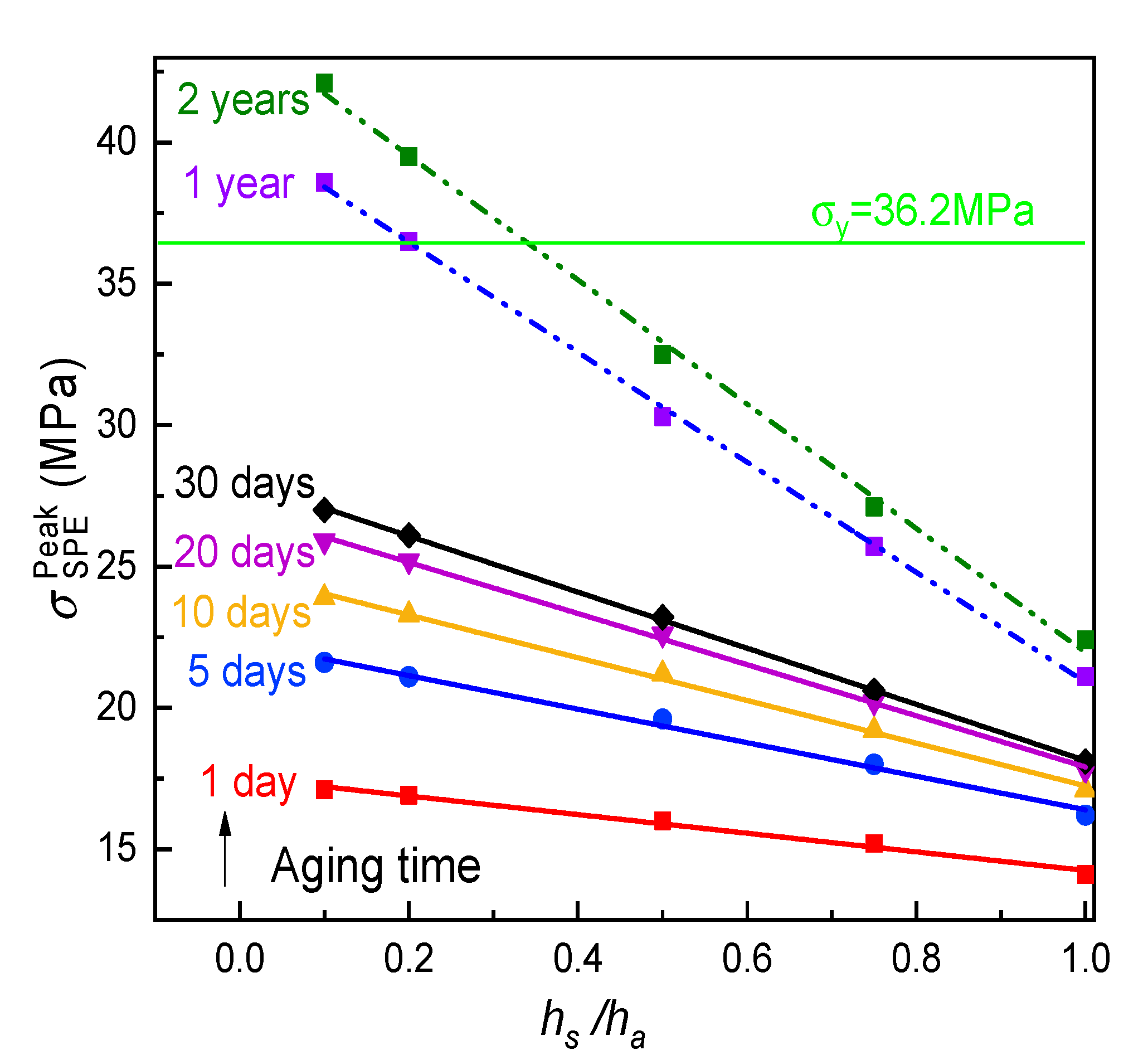
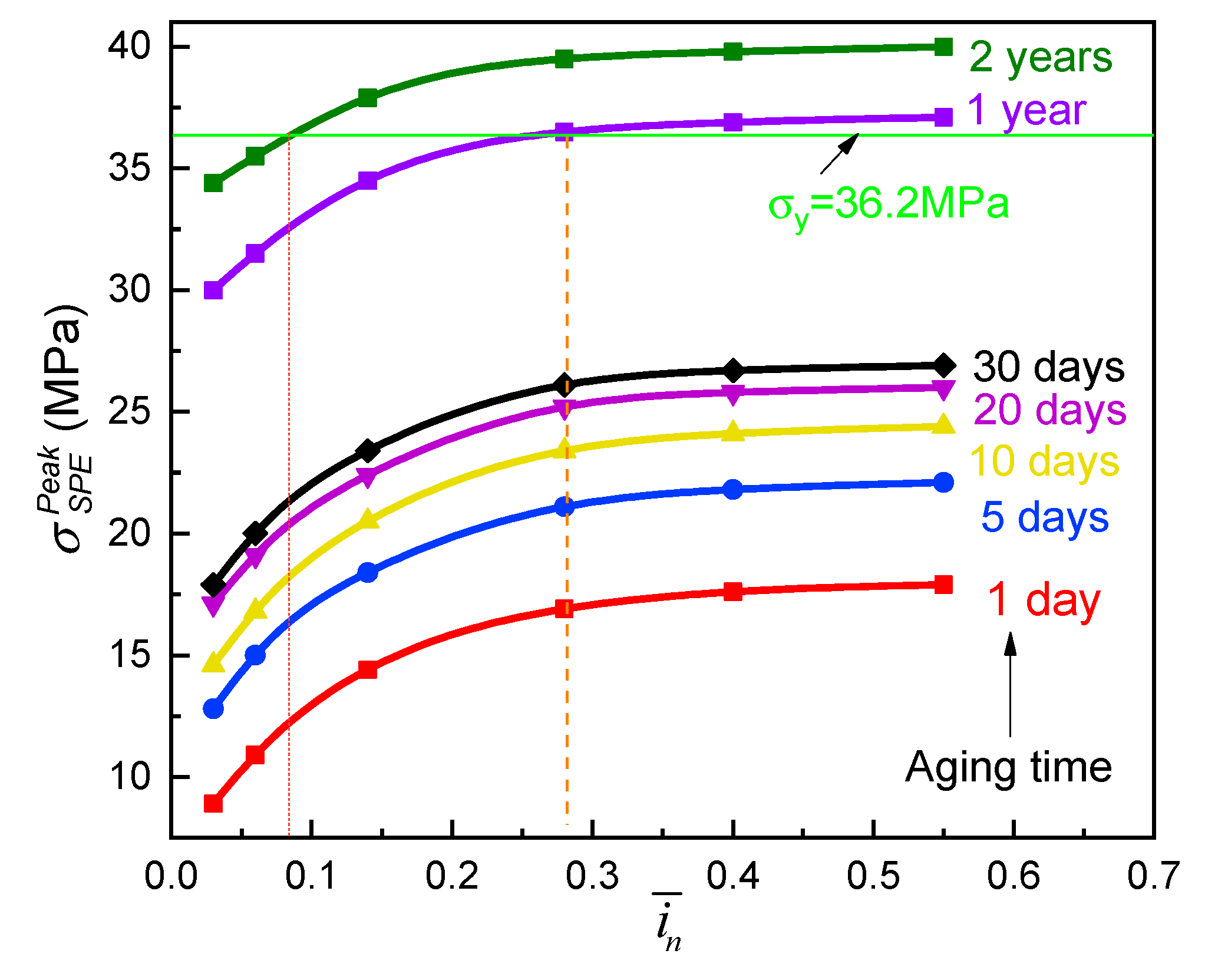
- The peak tensile stress in the SPE film occurred at the electrolyte/cathode interface for a full discharged ASSB, it would significantly enlarge with the aging on account of the stiffening of the electrolyte composite easily resulting in the mechanical failure of the cell system. However this negative effect may be restrained by increasing the relative thickness of the solid electrolyte to the composite electrode. In addition a lower rate discharge is a benefit of the durability of the SPE during physical aging.
- (4)
- In order to meet the requirement of a two-year lifetime needed for potential commercialization, the relative thickness of the electrolyte to the electrode should be larger than 0.355 for the ASSB of the Li/PVA-40% LiClO4/LiCoO2 in the viewpoint of mechanics.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schnell, J.; Gunther, T.; Knoche, T.; Vieider, C.; Kohler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Liu, K.; Ding, F.; Liu, X.J. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Yue, L.P.; Ma, J.; Zhang, J.J.; Zhao, J.W.; Dong, S.M.; Liu, Z.H.; Cui, G.L.; Chen, L.Q. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Xue, Z.G.; He, D.; Xie, X.L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, X.; Zhang, G.; Ma, A.J.; Chen, W.X.; Shao, L.; Shen, C.; Xie, K.Y. High-Performance Solid Composite Polymer Electrolyte for all Solid-State Lithium Battery through Facile Microstructure Regulation. Front. Chem. 2019, 7, 388. [Google Scholar] [CrossRef]
- Money, B.K.; Hariharan, K.; Swenson, J. Relation between structural and conductivity relaxation in PEO and PEO based electrolytes. Solid State Ion. 2014, 262, 785–789. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P.; Magistris, A. PEO-based composite polymer electrolytes. Solid State Ion. 1998, 110, 1–14. [Google Scholar] [CrossRef]
- Jia, W.S.; Li, Z.L.; Wu, Z.R.; Wang, L.P.; Wu, B.; Wang, Y.H.; Cao, Y.; Li, J.Z. Graphene oxide as a filler to improve the performance of PAN-LiClO4 flexible solid polymer electrolyte. Solid State Ion. 2018, 315, 7–13. [Google Scholar] [CrossRef]
- Chen-Yang, Y.W.; Chen, H.C.; Lin, F.J.; Chen, C.C. Polyacrylonitrile electrolytes: 1. A novel high-conductivity composite polymer electrolyte based on PAN, LiClO4 and alpha-Al2O3. Solid State Ion. 2002, 150, 327–335. [Google Scholar] [CrossRef]
- Huang, B.Y.; Wang, Z.X.; Chen, L.Q.; Xue, R.J.; Wang, F.S. The mechanism of lithium ion transport in polyacrylonitrile-based polymer electrolytes. Solid State Ion. 1996, 91, 279–284. [Google Scholar] [CrossRef]
- Voigt, N.; van Wullen, L. The mechanism of ionic transport in PAN-based solid polymer electrolytes. Solid State Ion. 2012, 208, 8–16. [Google Scholar] [CrossRef]
- Kotobuki, M.; Lu, L.; Savilov, S.V.; Aldoshin, S.M. Poly(vinylidene fluoride)-Based Al Ion Conductive Solid Polymer Electrolyte for Al Battery. J. Electrochem. Soc. 2017, 164, A3868–A3875. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Chen, M.; Jin, W.; Zhu, Q.Y.; Chen, W.; Mho, S.I. Characterization of (PVDF + LiFePO4) solid polymer electrolyte. J. Appl. Electrochem. 2007, 37, 637–642. [Google Scholar] [CrossRef]
- Stolarska, M.; Niedzicki, L.; Borkowska, R.; Zalewska, A.; Wieczorek, W. Structure, transport properties and interfacial stability of PVdF/HFP electrolytes containing modified inorganic filler. Electrochim. Acta 2007, 53, 1512–1517. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Albuquerque, T.; Gattu, B.; Kumta, P.N. Novel Composite Polymer Electrolytes of PVdF-HFP Derived by Electrospinning with Enhanced Li-Ion Conductivities for Rechargeable Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2018, 1, 483–494. [Google Scholar] [CrossRef]
- Rajendran, S.; Uma, T. Effect of ceramic oxide on PMMA based polymer electrolyte systems. Mater. Lett. 2000, 45, 191–196. [Google Scholar] [CrossRef]
- Kurapati, S.; Gunturi, S.S.; Nadella, K.J.; Erothu, H. Novel solid polymer electrolyte based on PMMA: CH3COOLi effect of salt concentration on optical and conductivity studies. Polym. Bull. 2019, 76, 5463–5481. [Google Scholar] [CrossRef]
- Shamrao, P.V.; Vithya, K.; Premalatha, M.; Sundaresan, B. AC Impedance Study of PMMA-LiNO3 Electrolyte. Macromol. Symp. 2019, 387, 1800187. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, M.; Subadevi, R. Effect of salt concentration in poly(vinyl alcohol)-based solid polymer electrolytes. J. Power Sources 2003, 124, 225–230. [Google Scholar] [CrossRef]
- Malathi, J.; Kumaravadivel, M.; Brahmanandhan, G.M.; Hema, M.; Baskaran, R.; Selvasekarapandian, S. Structural, thermal and electrical properties of PVA-LiCF3SO3 polymer electrolyte. J. Non-Cryst. Solids 2010, 356, 2277–2281. [Google Scholar] [CrossRef]
- Noor, I.S.; Majid, S.R.; Arof, A.K. Poly(vinyl alcohol)-LiBOB complexes for lithium-air cells. Electrochim. Acta 2013, 102, 149–160. [Google Scholar] [CrossRef]
- Sundaramahalingam, K.; Muthuvinayagam, M.; Nallamuthu, N.; Vanitha, D.; Vahini, M. Investigations on lithium acetate-doped PVA/PVP solid polymer blend electrolytes. Polym. Bull. 2019, 76, 5577–5602. [Google Scholar] [CrossRef]
- Li, J.; Zhu, K.J.; Yao, Z.R.; Qian, G.M.; Zhang, J.; Yan, K.; Wang, J. A promising composite solid electrolyte incorporating LLZO into PEO/PVDF matrix for all-solid-state lithium-ion batteries. Ionics 2019, 26, 1101–1108. [Google Scholar] [CrossRef]
- Patla, S.K.; Ray, R.; Asokan, K.; Karmakar, S. Investigation of ionic conduction in PEO-PVDF based blend polymer electrolytes. J. Appl. Phys. 2018, 123, 125102. [Google Scholar] [CrossRef]
- Jinisha, B.; Femy, A.F.; Ashima, M.S.; Jayalekshmi, S. Polyethylene oxide (PEO)/polyvinyl alcohol (PVA) complexed with lithium perchlorate (LiClO4) as a prospective material for making solid polymer electrolyte films. Mater. Today-Proc. 2018, 5, 21189–21194. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, Z.H.; Xia, Q.L.; Xiao, Q.Z.; Lei, G.T.; Zhou, X.D. Electrochemical study of P(VDF-HFP)/PMMA blended polymer electrolyte with high-temperature stability for polymer lithium secondary batteries. Ionics 2012, 18, 275–281. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, S.T.; Chien, W.C. All solid-state electric double-layer capacitors based on alkaline polyvinyl alcohol polymer electrolytes. J. Power Sources 2005, 152, 303–310. [Google Scholar] [CrossRef]
- Every, H.A.; Zhou, F.; Forsyth, M.; MacFarlane, D.R. Lithium ion mobility in poly(vinyl alcohol) based polymer electrolytes as determined by Li-7 NMR spectroscopy. Electrochim. Acta 1998, 43, 1465–1469. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, M.; Subadevi, R. Li-ion conduction of plasticized PVA solid polymer electrolytes complexed with various lithium salts. Solid State Ion. 2004, 167, 335–339. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.Y.; Zhu, P.; Zhang, X.W. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier Scientific Publishing Company: New York, NY, USA, 1978. [Google Scholar] [CrossRef]
- Hu, H.J.; Fan, X.M.; He, Y.L. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Koka, S.; Rodrigues, S.J.; Nookala, M. Physical aging effects on conductivity in polymer electrolytes. Solid State Ion. 2003, 156, 163–170. [Google Scholar] [CrossRef]
- Lasinska, A.K.; Marzantowicz, M.; Dygas, J.R.; Krok, F.; Florjanczyk, Z.; Tomaszewska, A.; Zygadlo-Monikowska, E.; Zukowska, Z.; Lafont, U. Study of ageing effects in polymer-in-salt electrolytes based on poly (acrylonitrile-co-butyl acrylate) and lithium salts. Electrochim. Acta 2015, 169, 61–72. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Dhatarwal, P.; Choudhary, S. Study of time-ageing effect on the ionic conduction and structural dynamics in solid polymer electrolytes by dielectric relaxation spectroscopy. Solid State Ion. 2018, 324, 247–259. [Google Scholar] [CrossRef]
- Grazioli, D.; Zadin, V.; Brandell, D.; Simone, A. Electrochemical-mechanical modeling of solid polymer electrolytes: Stress development and non-uniform electric current density in trench geometry microbatteries. Electrochim. Acta 2019, 296, 1142–1162. [Google Scholar] [CrossRef]
- Ganser, M.; Hildebrand, F.E.; Kamlah, M.; McMeeking, R.M. A finite strain electro-chemo-mechanical theory for ion transport with application to binary solid electrolytes. J. Mech. Phys. Solids 2019, 125, 681–713. [Google Scholar] [CrossRef]
- Yu, H.C.; Taha, D.; Thompson, T.; Taylor, N.J.; Drews, A.; Sakamoto, J.; Thornton, K. Deformation and stresses in solid-state composite battery cathodes. J. Power Sources 2019, 440, 227116. [Google Scholar] [CrossRef]
- Wang, P.; Qu, W.J.; Song, W.L.; Chen, H.S.; Chen, R.J.; Fang, D.N. Electro-Chemo-Mechanical Issues at the Interfaces in Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2019, 29, 1900950. [Google Scholar] [CrossRef]
- He, Y.M.; Lu, C.Y.; Liu, S.; Zheng, W.J.; Luo, J.Y. Interfacial Incompatibility and Internal Stresses in All-Solid-State Lithium Ion Batteries. Adv. Energy Mater. 2019, 9, 1901810. [Google Scholar] [CrossRef]
- Bucci, G.; Swamy, T.; Chiang, Y.M.; Carter, W.C. Modeling of internal mechanical failure of all-solidstate batteries during electrochemical cycling, and implications for battery design. J. Mater. Chem. A 2017, 5, 19422–19430. [Google Scholar] [CrossRef]
- Tippens, J.; Miers, J.C.; Afshar, A.; Lewis, J.A.; Cortes, F.J.Q.; Qiao, H.P.; Marchese, T.S.; Di Leo, C.V.; Saldana, C.; McDowell, M.T. Visualizing Chemomechanical Degradation of a Solid-State Battery Electrolyte. ACS Energy Lett. 2019, 4, 1475–1483. [Google Scholar] [CrossRef]
- Klinsmann, M.; Hildebrand, F.E.; Ganser, M.; McMeeking, R.M. Dendritic cracking in solid electrolytes driven by lithium insertion. J. Power Sources 2019, 442, 227226. [Google Scholar] [CrossRef]
- Rouse, J.J.; Mohamed, F.; van der Walle, C.F. Physical ageing and thermal analysis of PLGA microspheres encapsulating protein or DNA. Int. J. Pharm. 2007, 339, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.G.; Dudney, N.J.; Rochow, M.; Thole, V.; Hackney, S.A. On the mechanisms of stress relaxation and intensification at the lithium/solid-state electrolyte interface. J. Mater. Res. 2019, 34, 3593–3616. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, H.J.; Zhang, K.F.; Li, S.; Chen, J.H. Mechanical insights into the stability of heterogeneous solid electrolyte interphase on an electrode particle. J. Mater. Sci. 2017, 52, 2836–2848. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Zheng, B.L. Effects of concentration-dependent elastic modulus on Li-ions diffusion and diffusion-induced stresses in spherical composition-gradient electrodes. J. Appl. Phys. 2015, 118, 105102. [Google Scholar] [CrossRef]
- Danilov, D.; Niessen, R.A.H.; Notten, P.H.L. Modeling All-Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A215–A222. [Google Scholar] [CrossRef]
- Jones, E.M.C.; Silberstein, M.N.; White, S.R.; Sottos, N.R. In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling. Exp. Mech. 2014, 54, 971–985. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, H.J.; Song, Y.C.; Guo, Z.S.; Liu, C.; Zhang, J.Q. Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power Sources 2014, 248, 517–523. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta. 2000, 45, 1481–1490. [Google Scholar] [CrossRef]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Effect of nano-silica filler in polymer electrolyte on Li dendrite formation in Li/poly(ethylene oxide)-Li(CF3SO2)2 N/Li. J. Power Sources 2010, 195, 6847–6853. [Google Scholar] [CrossRef]
- Li, Q.; Sun, H.Y.; Takeda, Y.; Imanishi, N.; Yang, J.; Yamamoto, O. Interface properties between a lithium metal electrode and a poly(ethylene oxide) based composite polymer electrolyte. J. Power Sources 2001, 94, 201–205. [Google Scholar] [CrossRef]
- Masoud, E.M.; El-Bellihi, A.A.; Bayoumy, W.A.; Mousa, M.A. Effect of LiAlO2 nanoparticle filler concentration on the electrical properties of PEO-LiClO4 composite. Mater. Res. Bull. 2013, 48, 1148–1154. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Dautzenberg, G.; Mastragostino, M.; Ronci, F.; Scrosati, B.; Soavi, F.; Zanelli, A.; Alessandrini, F.; Prosini, P.P. Composite polymer electrolytes with improved lithium metal electrode interfacial properties—I. Electrochemical properties of dry PEO-LiX systems. J. Electrochem. Soc. 1998, 145, 4126–4132. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]

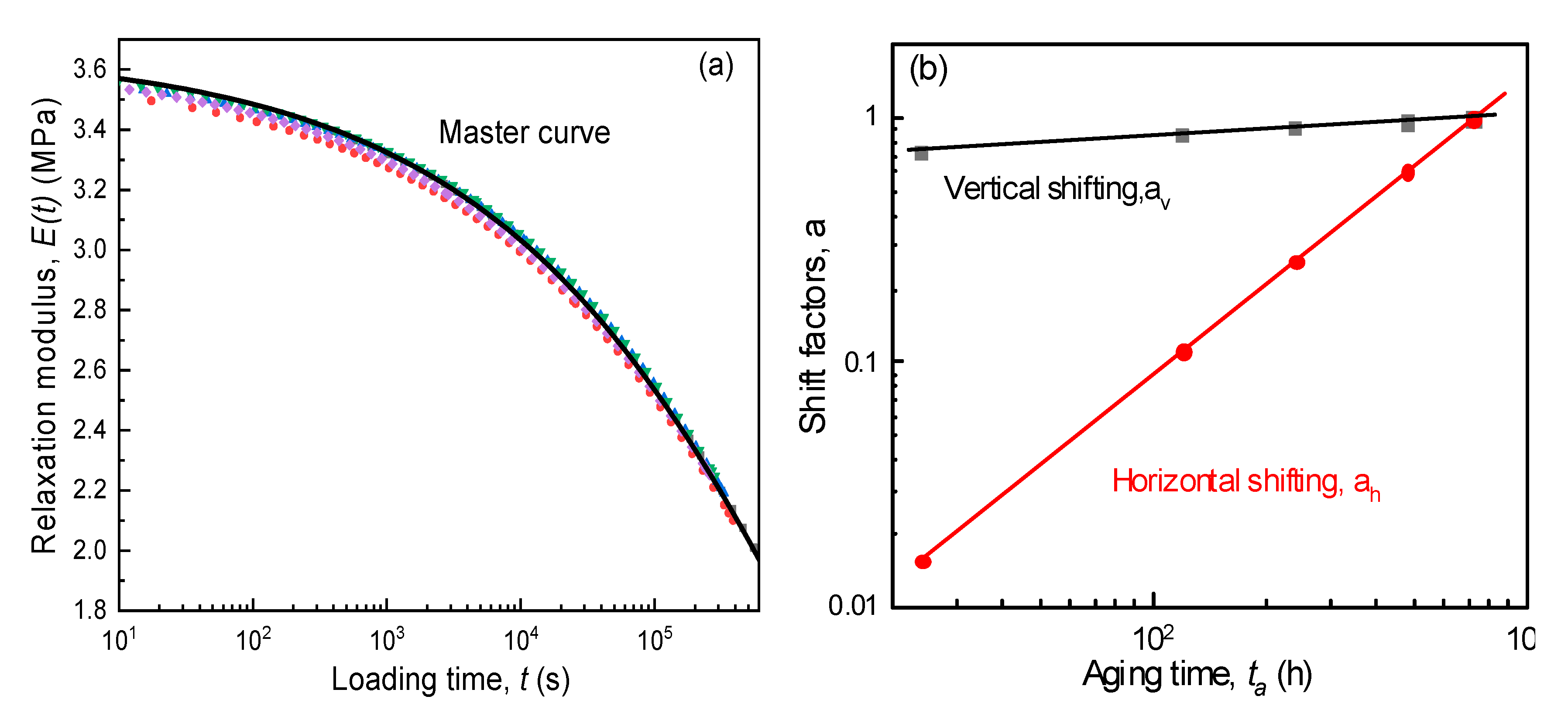



| SPE (PVA-40% LiClO4) | Cathode (LiCoO2:PVdF:CB(Carbon Black) = 85:10:5) | Current Collector (Al) | |
|---|---|---|---|
| Elastic modulus, E (GPa) | Indicated in Figure 4 | 1.97 * | 70 |
| Poisson ratio, μ | 0.3 | 0.31 * | 0.3 |
| Partial molar volume of solute Ω (m3·mol−1) | / | 4.17 × 10–6 [50] | / |
| Diffusion coefficient, D (m2·s−1) | / | 1.76 × 10–15 [51] | / |
| Stoichiometric maximum concentration, cmax (mol m−3) | / | 2.33 × 104 [51] | / |
| Thickness, h (μm) | 5 | 10 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, S.; Zhou, S.; Hu, H. Mechanical Integrity Degradation and Control of All-Solid-State Lithium Battery with Physical Aging Poly (Vinyl Alcohol)-Based Electrolyte. Polymers 2020, 12, 1886. https://doi.org/10.3390/polym12091886
He Y, Li S, Zhou S, Hu H. Mechanical Integrity Degradation and Control of All-Solid-State Lithium Battery with Physical Aging Poly (Vinyl Alcohol)-Based Electrolyte. Polymers. 2020; 12(9):1886. https://doi.org/10.3390/polym12091886
Chicago/Turabian StyleHe, Yaolong, Shufeng Li, Sihao Zhou, and Hongjiu Hu. 2020. "Mechanical Integrity Degradation and Control of All-Solid-State Lithium Battery with Physical Aging Poly (Vinyl Alcohol)-Based Electrolyte" Polymers 12, no. 9: 1886. https://doi.org/10.3390/polym12091886
APA StyleHe, Y., Li, S., Zhou, S., & Hu, H. (2020). Mechanical Integrity Degradation and Control of All-Solid-State Lithium Battery with Physical Aging Poly (Vinyl Alcohol)-Based Electrolyte. Polymers, 12(9), 1886. https://doi.org/10.3390/polym12091886






