Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. ZnO Nanoparticle Fabrication
2.4. Nanofiber Fabrication
3. Results
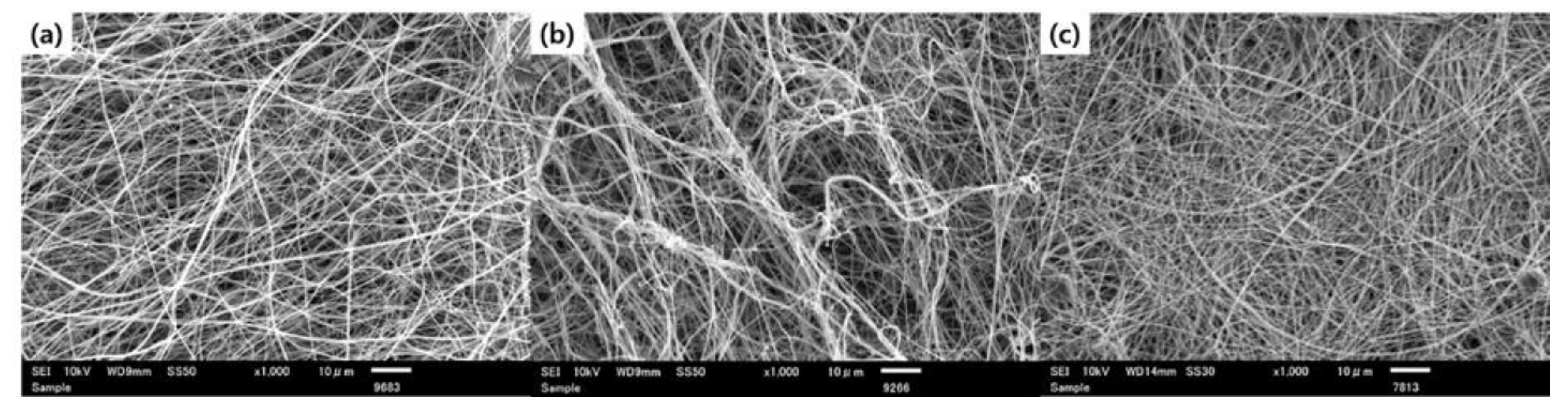
3.1. The Effect of Post-Electrospinning Treatment of Nanofibers on Composite Structure Formation
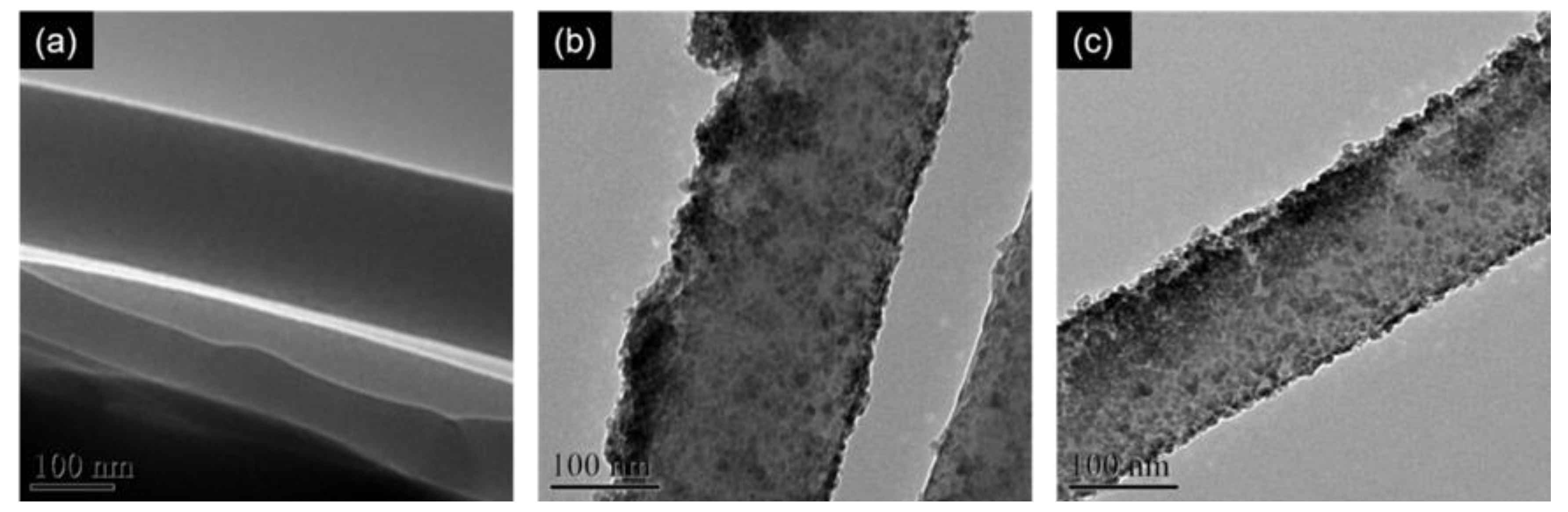
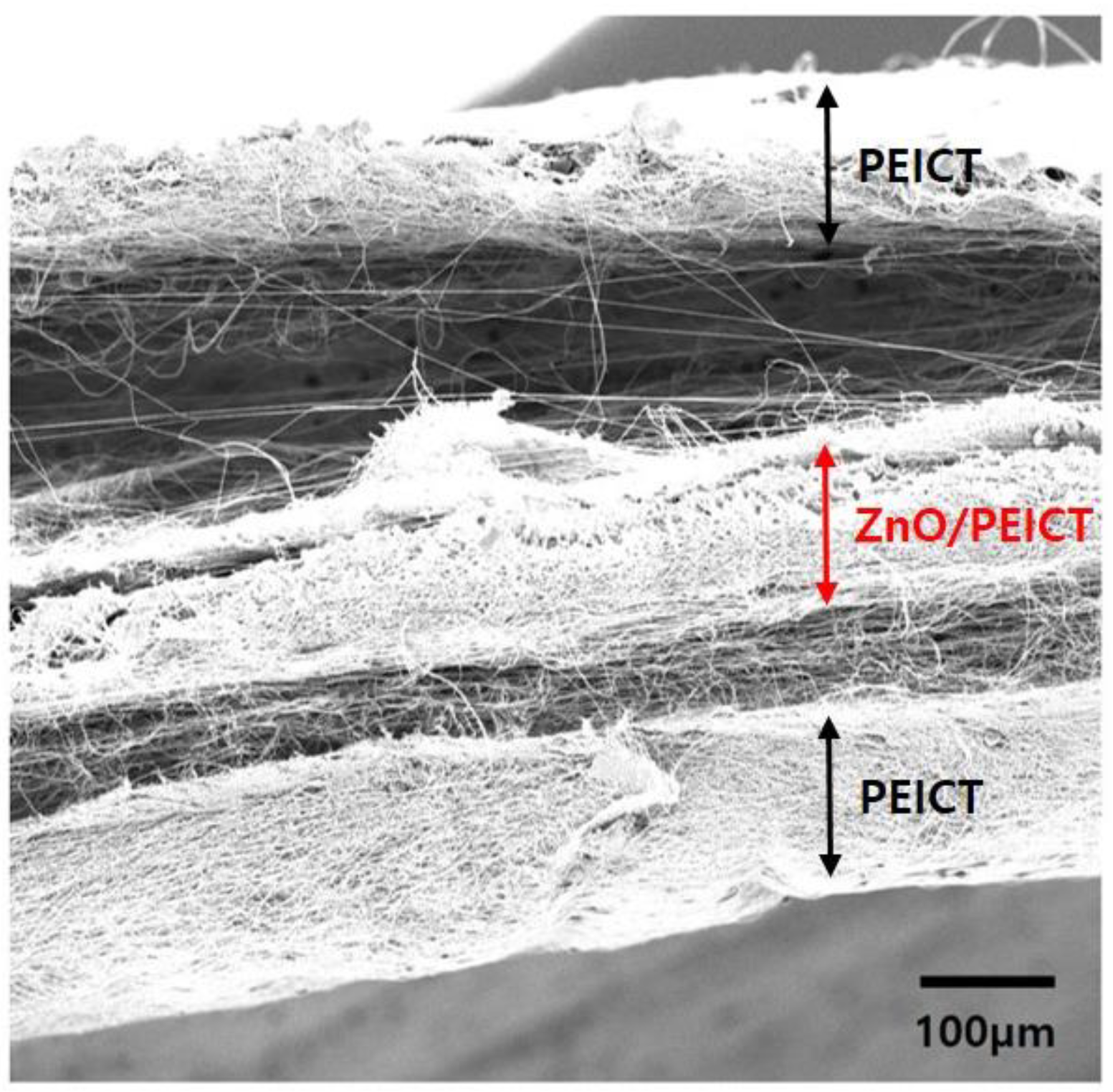
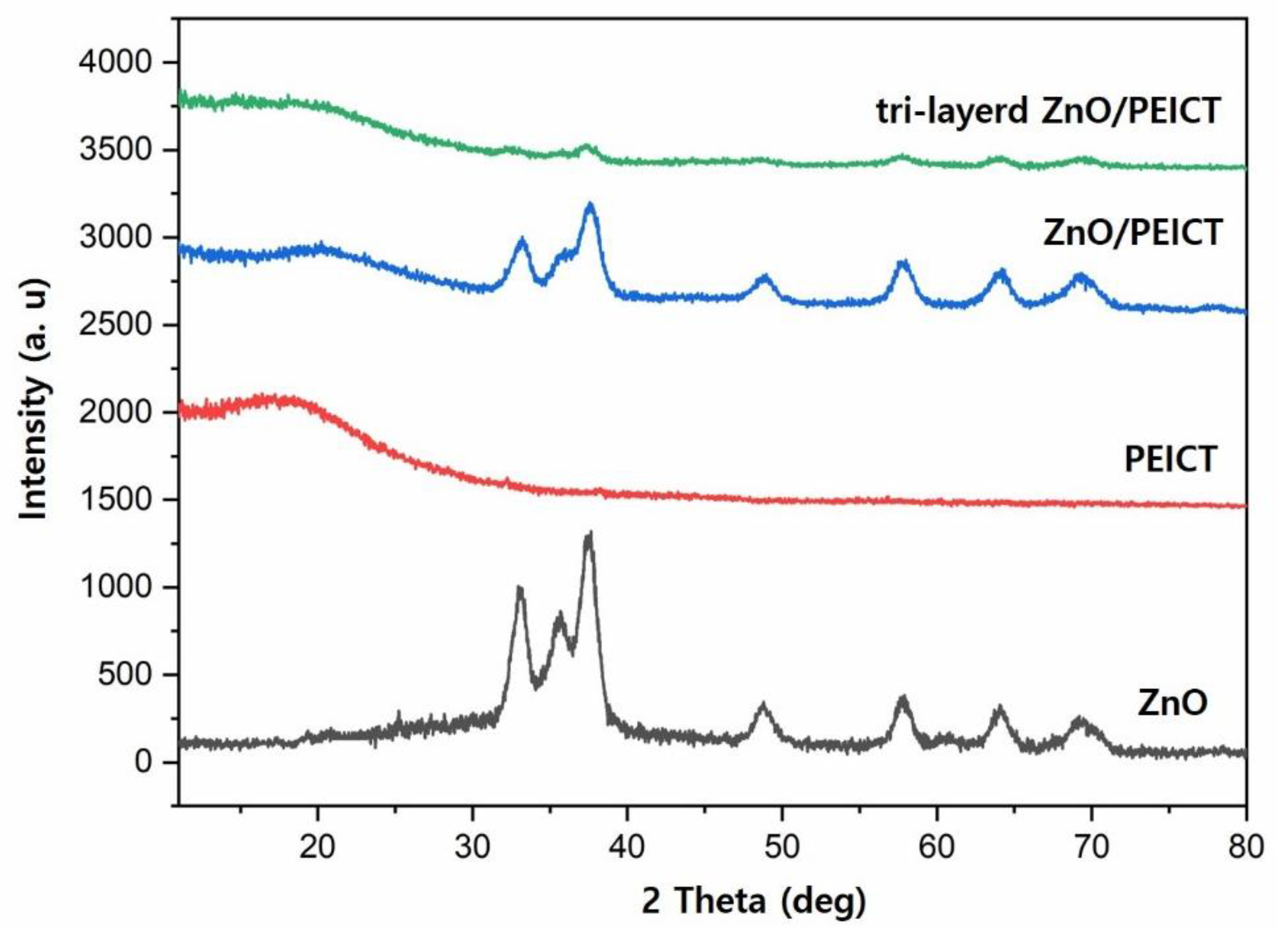
3.2. Envelopment of ZnO Nanoparticles on Nanofibers by the Formation of Layer-by-Layer Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Kim, I.S. Nanofibers: Emerging Progress on Fabrication Using Mechanical Force and Recent Applications. Polym. Rev. 2018, 58, 688–716. [Google Scholar] [CrossRef]
- Lee, H.; Xu, G.; Kharaghani, D.; Nishino, M.; Song, K.H.; Lee, J.S.; Kim, I.S. Electrospun tri-layered zein/PVP-GO/zein nanofiber mats for providing biphasic drug release profiles. Int. J. Pharm. 2017, 531, 101–107. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.; Xie, J.; Shen, L.; Tao, J.; Zhu, J. Facile Strategy to Generate Aligned Polymer Nanofibers: Effects on Cell Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 1566–1574. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Li, W.; Gao, J.; Xue, H.; Li, R.K.Y.; Mai, Y.W. TiO2 nanoparticle decorated carbon nanofibers for removal of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 205–211. [Google Scholar] [CrossRef]
- Tzitzios, V.; Georgakilas, V.; Oikonomou, E.; Karakassides, M.; Petridis, D. Synthesis and characterization of carbon nanotube/metal nanoparticle composites well dispersed in organic media. Carbon 2006, 44, 848–853. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.Q. Cellulose acetate/multi-wall carbon nanotube/Ag nanofiber composite for antibacterial applications. Mater. Sci. Eng. C 2020, 110, 110679. [Google Scholar] [CrossRef]
- Kharaghani, D.; Lee, H.; Ishikawa, T.; Nagaishi, T.; Kim, S.H.; Kim, I.S. Comparison of fabrication methods for the effective loading of Ag onto PVA nanofibers. Text. Res. J. 2019, 89, 625–634. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Sohn, D.; Kim, S.H.; Oh, S.G.; Im, S.S.; Kim, I.S. Electrospun tungsten trioxide nanofibers decorated with palladium oxide nanoparticles exhibiting enhanced photocatalytic activity. RSC Adv. 2017, 7, 6108–6113. [Google Scholar] [CrossRef]
- Lee, H.; Watanabe, K.; Kim, M.; Gopiraman, M.; Song, K.H.; Lee, J.S.; Kim, I.S. Handspinning Enabled Highly Concentrated Carbon Nanotubes with Controlled Orientation in Nanofibers. Sci. Rep. 2016, 6, 37590. [Google Scholar] [CrossRef]
- He, H.; Chen, R.; Zhang, L.; Williams, T.; Fang, X.; Shen, W. Fabrication of single-crystalline gold nanowires on cellulose nanofibers. J. Colloid Interface Sci. 2020, 562, 333–341. [Google Scholar] [CrossRef]
- Im, J.S.; Kim, M.I.; Lee, Y.S. Preparation of PAN-based electrospun nanofiber webs containing TiO2 for photocatalytic degradation. Mater. Lett. 2008, 62, 3652–3655. [Google Scholar] [CrossRef]
- Lee, H.; Koo, J.M.; Sohn, D.; Kim, I.S.; Im, S.S. High thermal stability and high tensile strength terpolyester nanofibers containing biobased monomer: Fabrication and characterization. RSC Adv. 2016, 6, 40383–40388. [Google Scholar] [CrossRef]
- Moazzami, K.; Murphy, T.E.; Phillips, J.D.; Cheung, M.C.K.; Cartwright, A.N. Sub-bandgap photoconductivity in ZnO epilayers and extraction of trap density spectra. Semicond. Sci. Technol. 2006, 21, 717–723. [Google Scholar] [CrossRef]
- Yildiz, A.; Uzun, S.; Serin, N.; Serin, T. Influence of grain boundaries on the figure of merit of undoped and Al, In, Sn doped ZnO thin films for photovoltaic applications. Scr. Mater. 2016, 113, 23–26. [Google Scholar] [CrossRef]
- Khan, M.Q.; Lee, H.; Koo, J.M.; Khatri, Z.; Sui, J.; Im, S.S.; Zhu, C.; Kim, I.S. Self-cleaning effect of electrospun poly (1,4-cyclohexanedimethylene isosorbide terephthalate) nanofibers embedded with zinc oxide nanoparticles. Text. Res. J. 2018, 88, 2493–2498. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Li, Z.; Yang, R.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L. Cellular Level Biocompatibility and Biosafety of ZnO Nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef]
- Li, Z.; Tang, H.; Yuan, W.; Song, W.; Niu, Y.; Yan, L.; Yu, M.; Dai, M.; Feng, S.; Wang, M.; et al. Ag nanoparticle–ZnO nanowire hybrid nanostructures as enhanced and robust antimicrobial textiles via a green chemical approach. Nanotechnology 2014, 25, 145702. [Google Scholar] [CrossRef]
- Gu, G.Q.; Han, C.B.; Tian, J.J.; Lu, C.X.; He, C.; Jiang, T.; Li, Z.; Wang, Z.L. Antibacterial Composite Film-Based Triboelectric Nanogenerator for Harvesting Walking Energy. ACS Appl. Mater. Interfaces 2017, 9, 11882–11888. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Liang, J.; Huang, Y.; Tang, C. ZnO Nanofiber and Nanoparticle Synthesized Through Electrospinning and Their Photocatalytic Activity Under Visible Light. J. Am. Ceram. Soc. 2008, 91, 1287–1291. [Google Scholar] [CrossRef]
- Krebs, F.C.; Thomann, Y.; Thomann, R.; Andreasen, J.W. A simple nanostructured polymer/ZnO hybrid solar cell—Preparation and operation in air. Nanotechnology 2008, 19, 424013. [Google Scholar] [CrossRef]
- Tan, L.; Yu, Z.; Tan, X.; Fang, M.; Wang, X.; Wang, J.; Xing, J.; Ai, Y.; Wang, X. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinetics, spectroscopic analyses and MD simulations. Environ. Pollut. 2019, 246, 999–1007. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Jo, Y.K.; Lee, H.; Oh, S.G.; Hwang, D.S.; Khatri, Z.; Cha, H.J.; Kim, I.S. Antibacterial efficacy of poly(vinyl alcohol) composite nanofibers embedded with silver-anchored silica nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Sridhar, R.; Sundarrajan, S.; Venugopal, J.R.; Ravichandran, R.; Ramakrishna, S. Electrospun inorganic and polymer composite nanofibers for biomedical applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 365–385. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced Polymerization and Properties of Biobased High Tg polyester of Isosorbide and 1,4-Cyclohexanedicarboxylic Acid through in Situ Acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Jayalakshmi, G.; Saravanan, K.; Pradhan, J.; Magudapathy, P.; Panigrahi, B.K. Facile synthesis and enhanced luminescence behavior of ZnO: Reduced graphene oxide(rGO) hybrid nanostructures. J. Lumin. 2018, 203, 1–6. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, D.-N.; Choi, H.Y.; Oh, S.-G.; Kim, M.; Lee, H. Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure. Polymers 2020, 12, 1859. https://doi.org/10.3390/polym12091859
Phan D-N, Choi HY, Oh S-G, Kim M, Lee H. Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure. Polymers. 2020; 12(9):1859. https://doi.org/10.3390/polym12091859
Chicago/Turabian StylePhan, Duy-Nam, Hyeong Yeol Choi, Seong-Geun Oh, Myungwoong Kim, and Hoik Lee. 2020. "Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure" Polymers 12, no. 9: 1859. https://doi.org/10.3390/polym12091859
APA StylePhan, D.-N., Choi, H. Y., Oh, S.-G., Kim, M., & Lee, H. (2020). Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure. Polymers, 12(9), 1859. https://doi.org/10.3390/polym12091859






