The Effect of Natural Additives on the Composting Properties of Aliphatic Polyesters
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
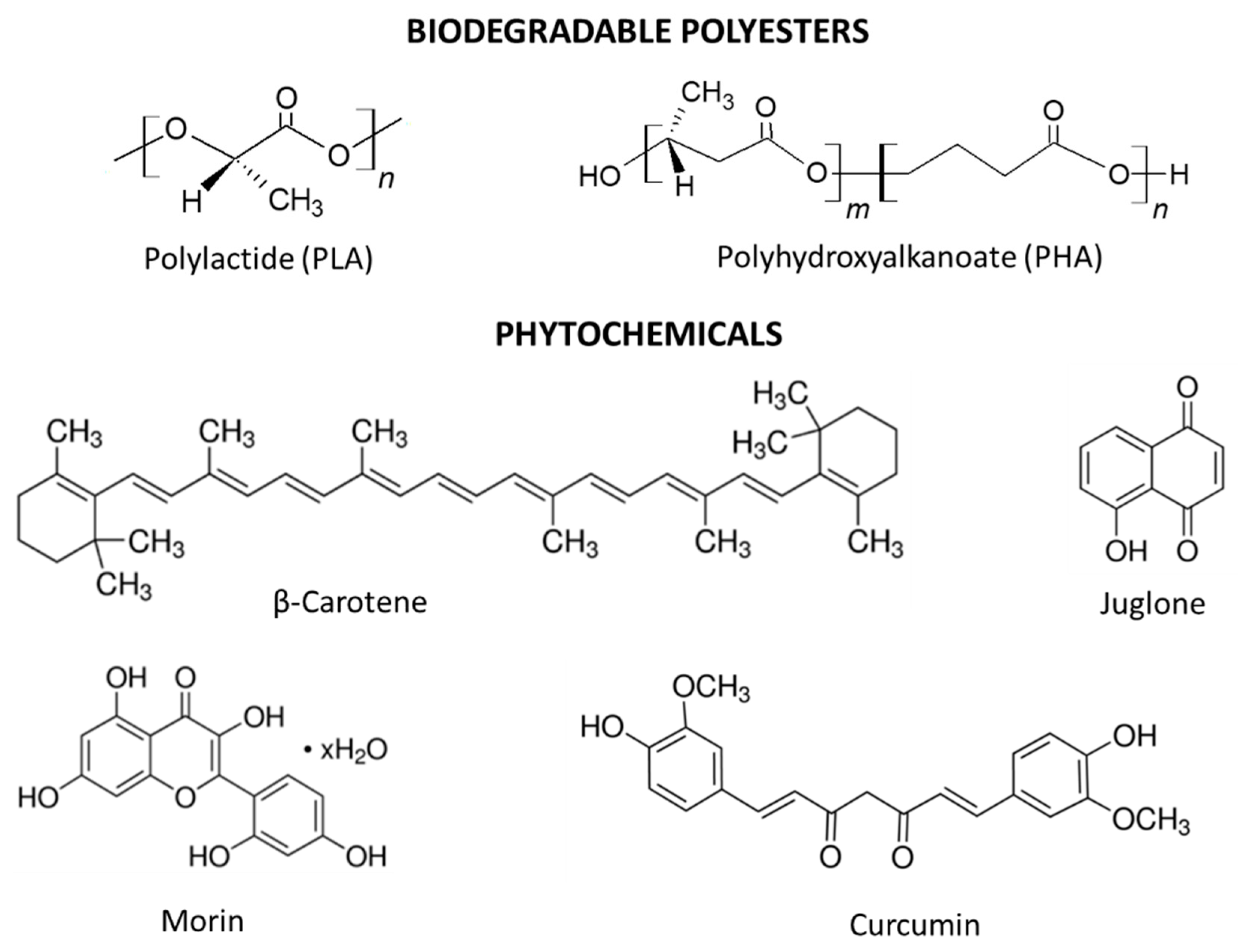
2.2. Method of Preparation of PLA and PHA with Phytochemicals
2.3. Method for Composting Polyester Samples
2.4. Measurement Methods
2.4.1. Surface Free Energy
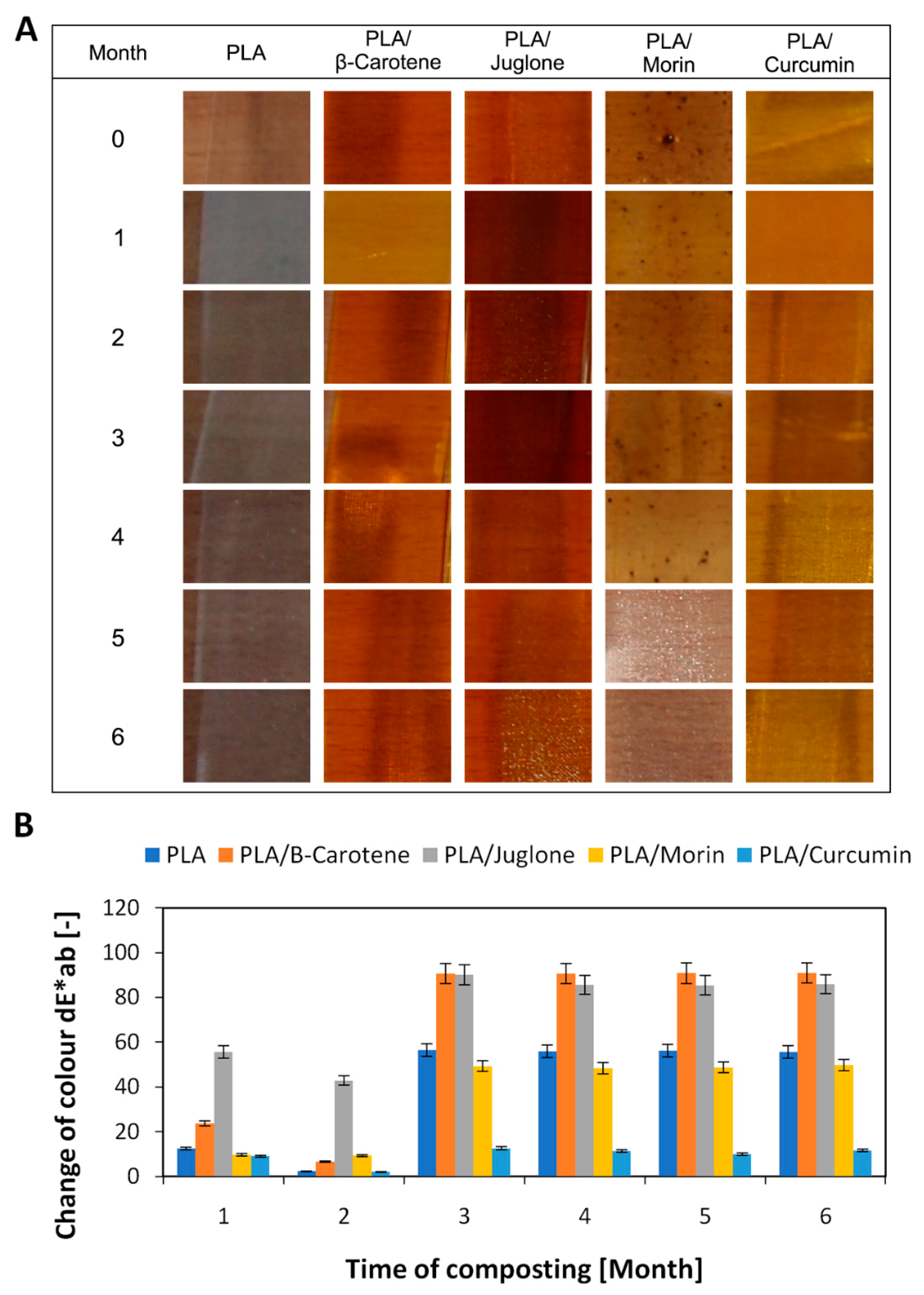
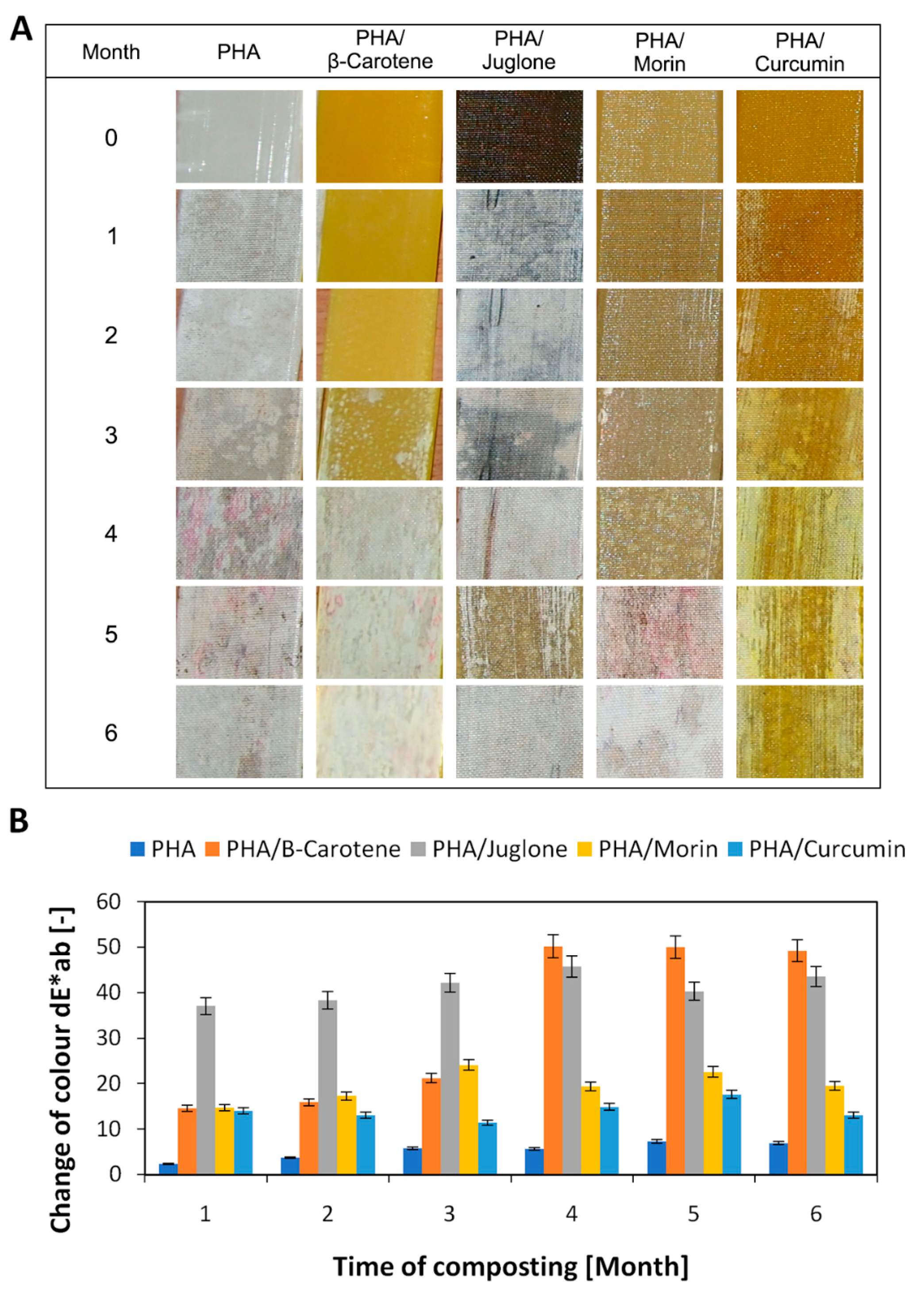
2.4.2. Change of Color
2.4.3. Mechanical Properties
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
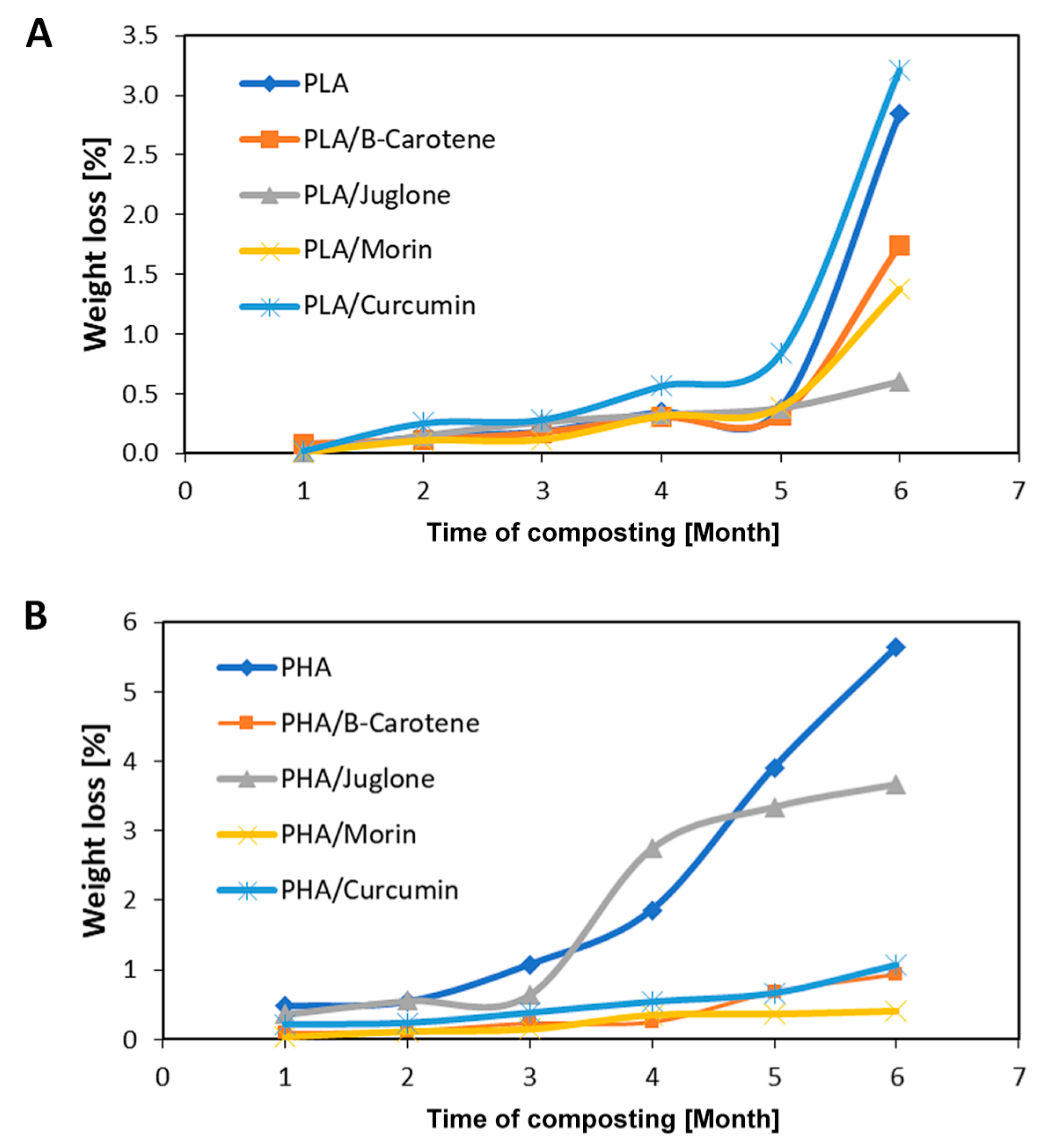
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef]
- Musioł, M.; Jurczyk, S.; Sobota, M.; Klim, M.; Sikorska, W.; Zięba, M.; Janeczek, H.; Rydz, J.; Kurcok, P.; Johnston, B.; et al. (Bio)Degradable Polymeric Materials for Sustainable Future—Part 3: Degradation Studies of the PHA/Wood Flour-Based Composites and Preliminary Tests of Antimicrobial Activity. Materials 2020, 13, 2200. [Google Scholar] [CrossRef] [PubMed]
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of poly(lactic acid)/starch/coir biocomposites under controlled composting conditions. Polym. Degrad. Stabil. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- Kalea, G.; Aurasa, R.; Singha, S.P.; Narayanb, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Nurul Fazita, M.R.; Jayaraman, K.; Bhattacharyya, D.; Hossain, M.S.; Mohamad Haafiz, M.K.; Abdul Khalil, H.P.S. Disposal Options of Bamboo Fabric-Reinforced Poly(Lactic) Acid Composites for Sustainable Packaging: Biodegradability and Recyclability. Polymers 2015, 7, 1476–1496. [Google Scholar] [CrossRef]
- Jurczyk, S.; Musioł, M.; Sobota, M.; Klim, M.; Hercog, A.; Kurcok, P.; Janeczek, H.; Rydz, J. (Bio)degradable Polymeric Materials for Sustainable Future—Part 2: Degradation Studies of P(3HB-co-4HB)/Cork Composites in Different Environments. Polymers 2019, 11, 547. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tatraaljai, D.; Foldes, E.; Pukanszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stabil. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Hussain, T.; Tausif, M.; Ashraf, M. A review of progress in the dyeing of eco-friendly aliphatic polyester based polylactic acid fabrics. J. Clean. Prod. 2015, 108, 476–483. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, J.S. Anthocyanin—A Natural Dye for Smart Food Packaging Systems. Korean J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Tichoniuk, M.; Radomska, N.; Cierpiszewski, R. The Application of Natural Dyes in Food Freshness Indicators Designed for Intelligent Packaging. Stud. Oeconomica Posnaniensia 2017, 5, 19–34. [Google Scholar] [CrossRef]
- Doudin, K.; Al-Malaika, S.; Sheena, H.H.; Tverezovskiy, V.; Fowler, P. New genre of antioxidants from renewable natural resources: Synthesis and characterisation of rosemary plant-derived antioxidants and their performance in polyolefins. Polym. Degrad. Stabil. 2016, 130, 126–134. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Schrade, C. Modifying biodegradable plastics with additives based on condensed tannin esters. J. Appl. Polym. Sci. 2015, 132, 41626. [Google Scholar] [CrossRef]
- Gordobil, O.; Egües, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stabil. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Nwakaudu, A.A.; Nwakaudu, M.S.; Owuamanam, C.I.; Iheaturu, N.C. The Use of Natural Antioxidant Active Polymer Packaging Films for Food Preservation. Appl. Signals Rep. 2015, 2, 38–50. [Google Scholar]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part. A 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Lagaron, J.M. Improvement of UV stability and mechanical properties of biopolyesters through the addition of β-carotene. Polym. Degrad. Stabil. 2010, 95, 2162–2168. [Google Scholar] [CrossRef]
- Lionetto, F.; López-Muñoz, R.; Espinoza-González, C.; Mis-Fernández, R.; Rodríguez-Fernández, O.; Maezzoli, A. A Study on Exfoliation of Expanded Graphite Stacks in CandelillaWax. Materials 2019, 12, 2530. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Degli Esposti, M.; Fabbri, P. Poly(hydroxyalkanoate)s-Based Hydrophobic Coatings for the Protection of Stone in Cultural Heritage. Materials 2018, 11, 165. [Google Scholar] [CrossRef]
- Keceli, T.M.; Erginkaya, Z.; Turkkan, E.; Kaya, U. Antioxidant and Antibacterial Effects of Carotenoids Extracted from Rhodotorula glutinis Strains. Asian J. Chem. 2013, 25, 42–46. [Google Scholar] [CrossRef]
- Manimala, M.R.A.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Nat. Appl. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Hayashi, M.; Naknukool, S.; Hayakawa, S.; Ogawa, M.; Nimatulah, A.-B.A. Enhancement of antimicrobial activity of a lactoperoxidase system by carrot extract and b-carotene. Food Chem. 2012, 130, 541–546. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from henna leaves and its dyeing on cotton by exhaust method. J. Clean Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Tan, D.T.C.; Osman, H.; Mohamad, S.; Kamaruddin, A.H. Synthesis and antibacterial activity of juglone derivatives. J. Chem. Chem. Eng. 2012, 6, 8489. [Google Scholar]
- Montenegro, R.C.; Araujo, A.J.; Molina, M.T.; Filho, J.D.B.M.; Rocha, D.D.; Lopéz-Montero, E.; Goulart, M.O.F.; Bento, E.S.; Alves, A.P.N.N.; Pessoa, C.; et al. Cytotoxic activity of naphthoquinones with special emphasis on juglone and its 5-methyl derivative. Chem. Biol. Interact. 2010, 184, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 23262331. [Google Scholar] [CrossRef]
- Bonjar, G.S.; Aghighi, S.; Nik, A.K. Antibacterial and antifungal survey in plants used in indigenous herbal medicine of south east regions of Iran. J. Biol. Sci. 2004, 4, 405412. [Google Scholar]
- Kopacz, M.; Woznicka, E.; Gruszecka, J. Antibacterial Activity of Morin and Its Complexes with La(III), Gd(III) and Lu(III) Ions. ACTA Pol. Pharm. 2005, 62, 65–67. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ou, J.-L.; Mizushina, Y.; Wang, S.-Y.; Chuang, D.-Y.; Nadar, M.; Hsu, W.-L. Structure–activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013, 280, 5829–5840. [Google Scholar] [CrossRef]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest, Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M. The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA). Polymers 2018, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Latos, M.; Masek, A.; Zaborski, M. The potential of juglone as natural dye and indicator for biodegradable polyesters. Proc. Inst. Mech. Eng. L J. Mater. Des. Appl. 2019, 233, 276–285. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life. 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Diakowska, K.; Zaborski, M. Application of β-carotene, a natural flavonoid dye, to polymeric materials as a natural antioxidant and determination of its characteristics using cyclic voltammetry and FTIR spectroscopy. Int. J. Electrochem. Sci. 2015, 10, 3372–3386. [Google Scholar]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 11097. [Google Scholar] [CrossRef]
- Moraczewski, K.; Malinowski, R.; Sikorska, W.; Karasiewicz, T.; Stepczynska, M.; Jagodzinski, B.; Rytlewski, P. Composting of Polylactide Containing Natural Anti-Aging Compounds of Plant Origin. Polymers 2019, 11, 1582. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Biodegradable Polyester Materials Containing Gallates. Polymers 2020, 12, 677. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Atkinson, H. A simplified theory of crystallisation induced by polymer chain scissions for biodegradable polyesters. Polym. Degrad. Stabil. 2012, 97, 1616–1620. [Google Scholar] [CrossRef]

| Liquid | Contact Angle during 6 Months of Composting [°] | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| PLA | |||||||
| Water | 61.3 | 77.1 | 70.8 | 76.8 | 75.6 | 75.2 | 66.8 |
| Diiodomethane | 36.7 | 36.8 | 53.7 | 27.6 | 47.3 | 38.7 | 42.2 |
| Ethylene glycol | 44.8 | 55.7 | 51.1 | 47.4 | 51.0 | 45.3 | 51.5 |
| PLA/B-Carotene | |||||||
| Water | 69.9 | 80.5 | 73.4 | 76.7 | 77.9 | 81.2 | 78.5 |
| Diiodomethane | 35.3 | 39.8 | 38.2 | 38.9 | 32.9 | 45.4 | 38.2 |
| Ethylene glycol | 44.3 | 50.4 | 45.6 | 41.0 | 51.2 | 56.7 | 59.2 |
| PLA/Juglone | |||||||
| Water | 74.5 | 73.7 | 68.7 | 68.9 | 76.8 | 85.2 | 77.9 |
| Diiodomethane | 37.2 | 48.0 | 46.9 | 39.9 | 39.8 | 45.1 | 40.2 |
| Ethylene glycol | 42.3 | 50.9 | 46.7 | 40.4 | 50.9 | 58.2 | 53.7 |
| PLA/Morin | |||||||
| Water | 83.2 | 83.0 | 74.0 | 75.5 | 72.3 | 84.5 | 85.8 |
| Diiodomethane | 52.8 | 51.8 | 44.3 | 42.9 | 49.1 | 51.9 | 44.4 |
| Ethylene glycol | 58.8 | 44.1 | 57.7 | 43.6 | 48.2 | 56.4 | 55.8 |
| PLA/Curcumin | |||||||
| Water | 77.6 | 84.4 | 70.0 | 75.1 | 78.8 | 74.2 | 76.7 |
| Diiodomethane | 32.0 | 51.2 | 46.3 | 34.9 | 34.6 | 38.1 | 35.1 |
| Ethylene glycol | 51.5 | 46.5 | 49.0 | 52.2 | 53.0 | 51.5 | 54.9 |
| Liquid | Contact Angle during 6 Months of Composting [°] | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| PHA | |||||||
| Water | 70.0 | 67.2 | 89.2 | 82.8 | 74.7 | 74.5 | 80.2 |
| Diiodomethane | 50.1 | 53.9 | 57.7 | 55.4 | 52.2 | 37.2 | 47.7 |
| Ethylene glycol | 41.4 | 48.1 | 70.6 | 55.8 | 58.0 | 49.9 | 51.1 |
| PHA/B-Carotene | |||||||
| Water | 81.8 | 78.7 | 87.9 | 78.3 | 78.3 | 79.1 | 90.4 |
| Diiodomethane | 35.1 | 65.0 | 56.7 | 62.0 | 62.0 | 52.9 | 57.6 |
| Ethylene glycol | 76.6 | 53.5 | 53.4 | 45.2 | 45.2 | 49.3 | 50.6 |
| PHA/Juglone | |||||||
| Water | 83.0 | 60.1 | 84.8 | 73.4 | 83.5 | 76.0 | 75.4 |
| Diiodomethane | 57.1 | 56.6 | 49.8 | 49.3 | 50.0 | 52.7 | 53.3 |
| Ethylene glycol | 64.9 | 44.3 | 62.8 | 57.4 | 52.5 | 44.3 | 49.8 |
| PHA/Morin | |||||||
| Water | 70.7 | 83.1 | 71.8 | 77.9 | 80.1 | 87.6 | 88.4 |
| Diiodomethane | 32.2 | 40.3 | 43.8 | 51.4 | 60.5 | 60.8 | 52.3 |
| Ethylene glycol | 40.0 | 52.4 | 62.8 | 54.3 | 53.5 | 71.9 | 54.5 |
| PHA/Curcumin | |||||||
| Water | 65.9 | 70.3 | 76.6 | 60.8 | 80.1 | 81.6 | 80.4 |
| Diiodomethane | 34.3 | 57.1 | 58.2 | 44.5 | 56.0 | 54.4 | 38.1 |
| Ethylene glycol | 45.5 | 44.3 | 42.1 | 44.0 | 57.1 | 60.3 | 45.2 |
| Sample | Time of Composting [Month] | TFmax [MPa] | EFmax [%] | σ [MPa] | ε [%] |
|---|---|---|---|---|---|
| PLA | 0 | 44.9 | 6.4 | 39.6 | 8.1 |
| 1 | 98.3 | 3.8 | 83.3 | 4.4 | |
| 2 | 40.5 | 3.6 | 37.6 | 3.9 | |
| 3 | 45.0 | 4.8 | 42.7 | 4.8 | |
| 4 | 38.1 | 4.0 | 37.9 | 4.0 | |
| 5 | 54.1 | 3.8 | 52.5 | 3.8 | |
| 6 | 37.0 | 2.9 | 36.2 | 2.9 | |
| PLA/Juglone | 0 | 49.9 | 4.9 | 48.1 | 5.3 |
| 1 | 46.7 | 3.7 | 56.3 | 3.8 | |
| 2 | 59.3 | 3.8 | 58.3 | 4.0 | |
| 3 | 52.1 | 4.8 | 51.3 | 4.9 | |
| 4 | 51.5 | 4.3 | 51.4 | 4.3 | |
| 5 | 52.9 | 4.8 | 51.2 | 4.8 | |
| 6 | 57.1 | 4.7 | 55.2 | 4.8 | |
| PLA/Curcumin | 0 | 54.1 | 5.1 | 47.1 | 5.9 |
| 1 | 97.2 | 4.3 | 19.3 | 18.1 | |
| 2 | 45.4 | 3.6 | 42.7 | 4.7 | |
| 3 | 53.0 | 4.8 | 51.2 | 4.9 | |
| 4 | 49.3 | 5.4 | 47.4 | 5.5 | |
| 5 | 58.5 | 4.9 | 57.9 | 5.3 | |
| 6 | 51.9 | 4.6 | 50.2 | 4.6 |
| Sample | Time of Composting [Month] | TFmax [MPa] | EFmax [%] | σ [MPa] | ε [%] |
|---|---|---|---|---|---|
| PHA | 0 | 29.7 | 4.2 | 19.3 | 6.9 |
| 1 | 21.1 | 2.3 | 21.1 | 2.3 | |
| 2 | 18.6 | 2.5 | 18.5 | 2.5 | |
| 3 | 10.9 | 2.3 | 10.5 | 2.3 | |
| 4 | * | * | * | * | |
| 5 | * | * | * | * | |
| 6 | * | * | * | * | |
| PHA/Juglone | 0 | 32.5 | 3.8 | 6.4 | 5.8 |
| 1 | 18.7 | 2.2 | 18.7 | 2.2 | |
| 2 | 11.9 | 1.6 | 11.9 | 1.6 | |
| 3 | 9.2 | 1.8 | 9.9 | 1.8 | |
| 4 | * | * | * | * | |
| 5 | * | * | * | * | |
| 6 | * | * | * | * | |
| PHA/Curcumin | 0 | 30.0 | 4.4 | 5.99 | 6.1 |
| 1 | 17.3 | 1.9 | 17.2 | 1.9 | |
| 2 | 4.09 | 1.4 | 3.87 | 1.5 | |
| 3 | 7.75 | 0.9 | 7.75 | 0.9 | |
| 4 | * | * | * | * | |
| 5 | * | * | * | * | |
| 6 | * | * | * | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latos-Brozio, M.; Masek, A. The Effect of Natural Additives on the Composting Properties of Aliphatic Polyesters. Polymers 2020, 12, 1856. https://doi.org/10.3390/polym12091856
Latos-Brozio M, Masek A. The Effect of Natural Additives on the Composting Properties of Aliphatic Polyesters. Polymers. 2020; 12(9):1856. https://doi.org/10.3390/polym12091856
Chicago/Turabian StyleLatos-Brozio, Malgorzata, and Anna Masek. 2020. "The Effect of Natural Additives on the Composting Properties of Aliphatic Polyesters" Polymers 12, no. 9: 1856. https://doi.org/10.3390/polym12091856
APA StyleLatos-Brozio, M., & Masek, A. (2020). The Effect of Natural Additives on the Composting Properties of Aliphatic Polyesters. Polymers, 12(9), 1856. https://doi.org/10.3390/polym12091856






