A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Collagen Scaffolds
2.3. Environmental Assessment of Collagen Scaffolds
2.4. Characterization of Collagen Scaffolds
2.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
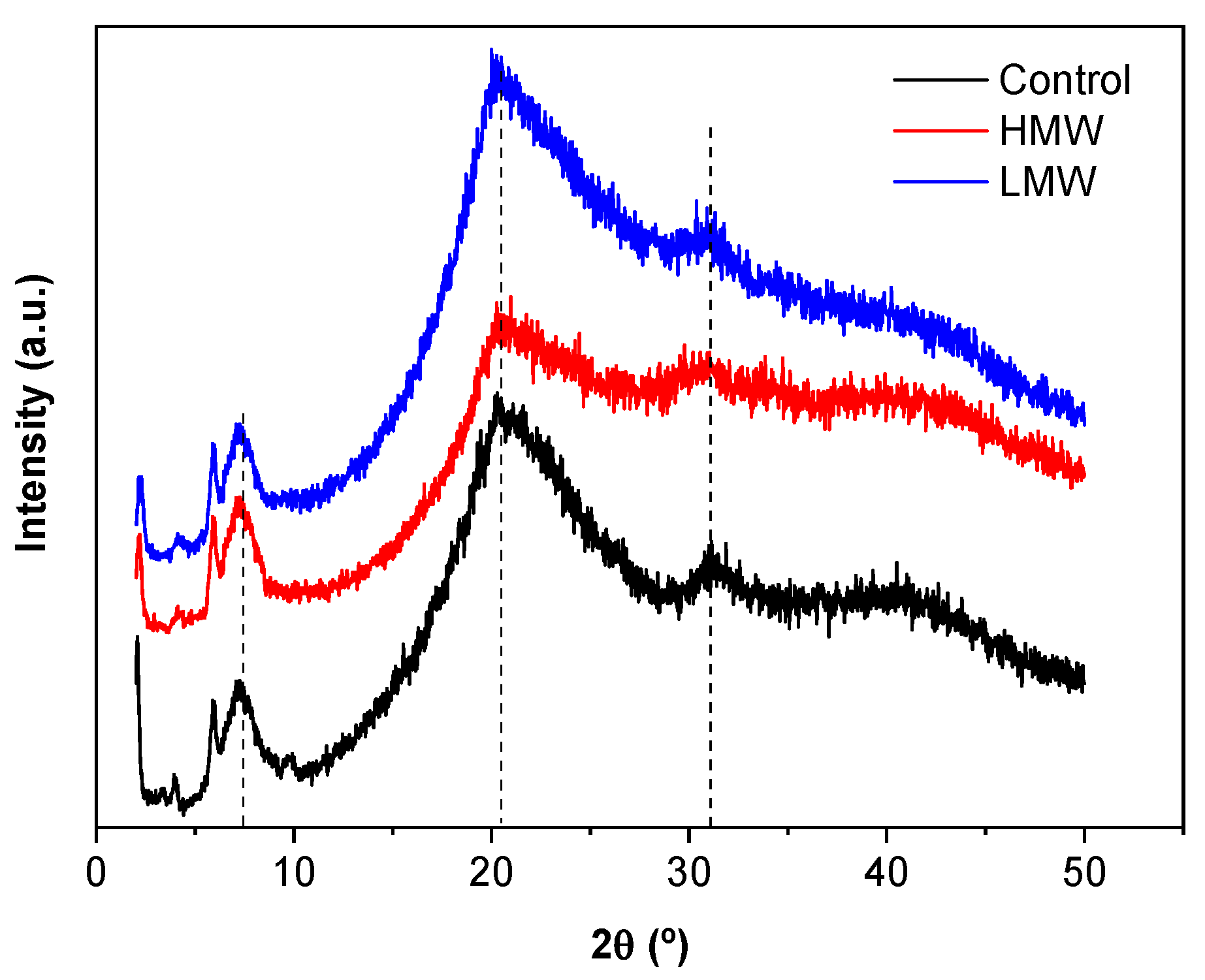
2.4.2. X-ray Diffraction (XRD)
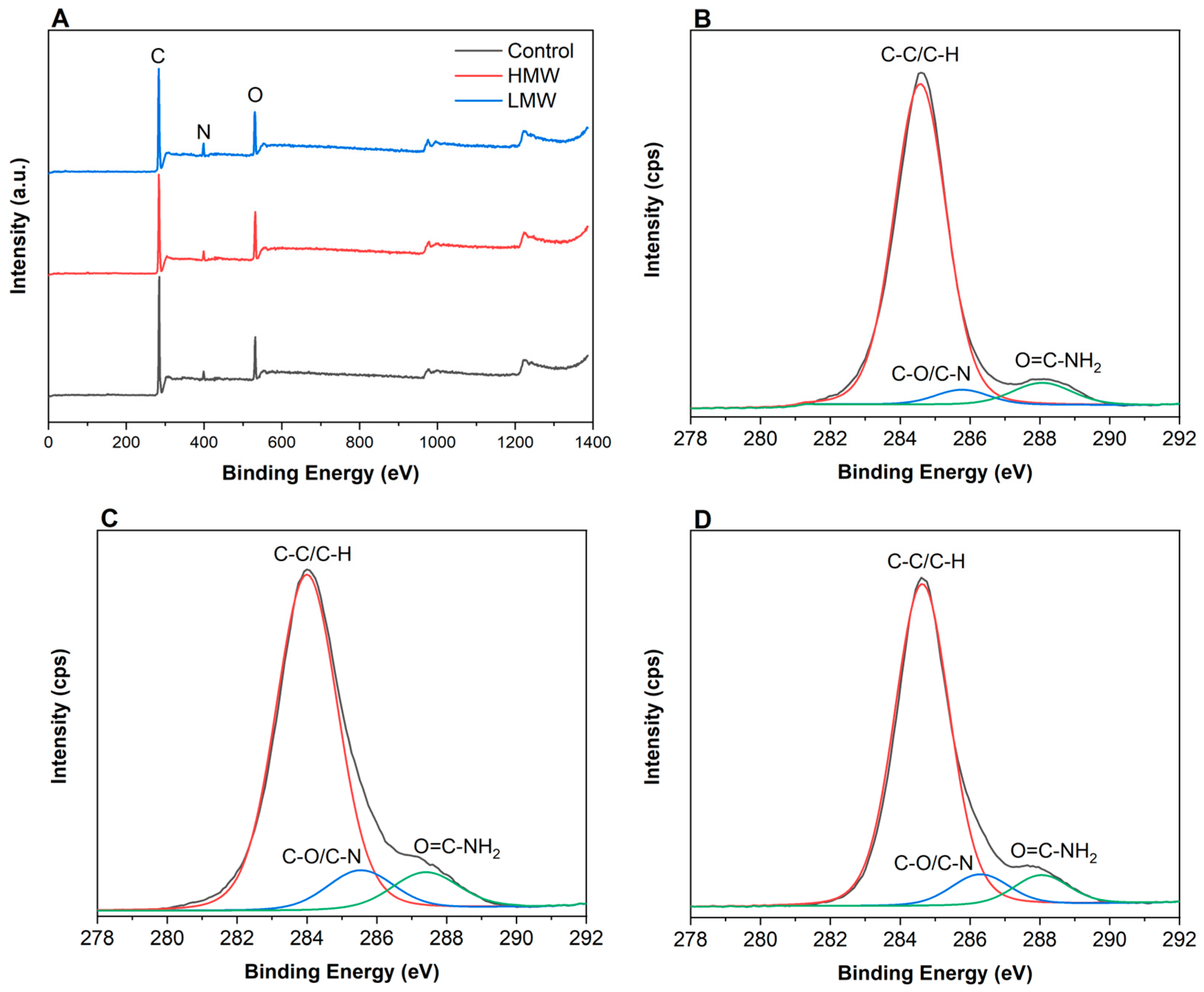
2.4.3. X-ray Photoelectron Spectroscopy (XPS)
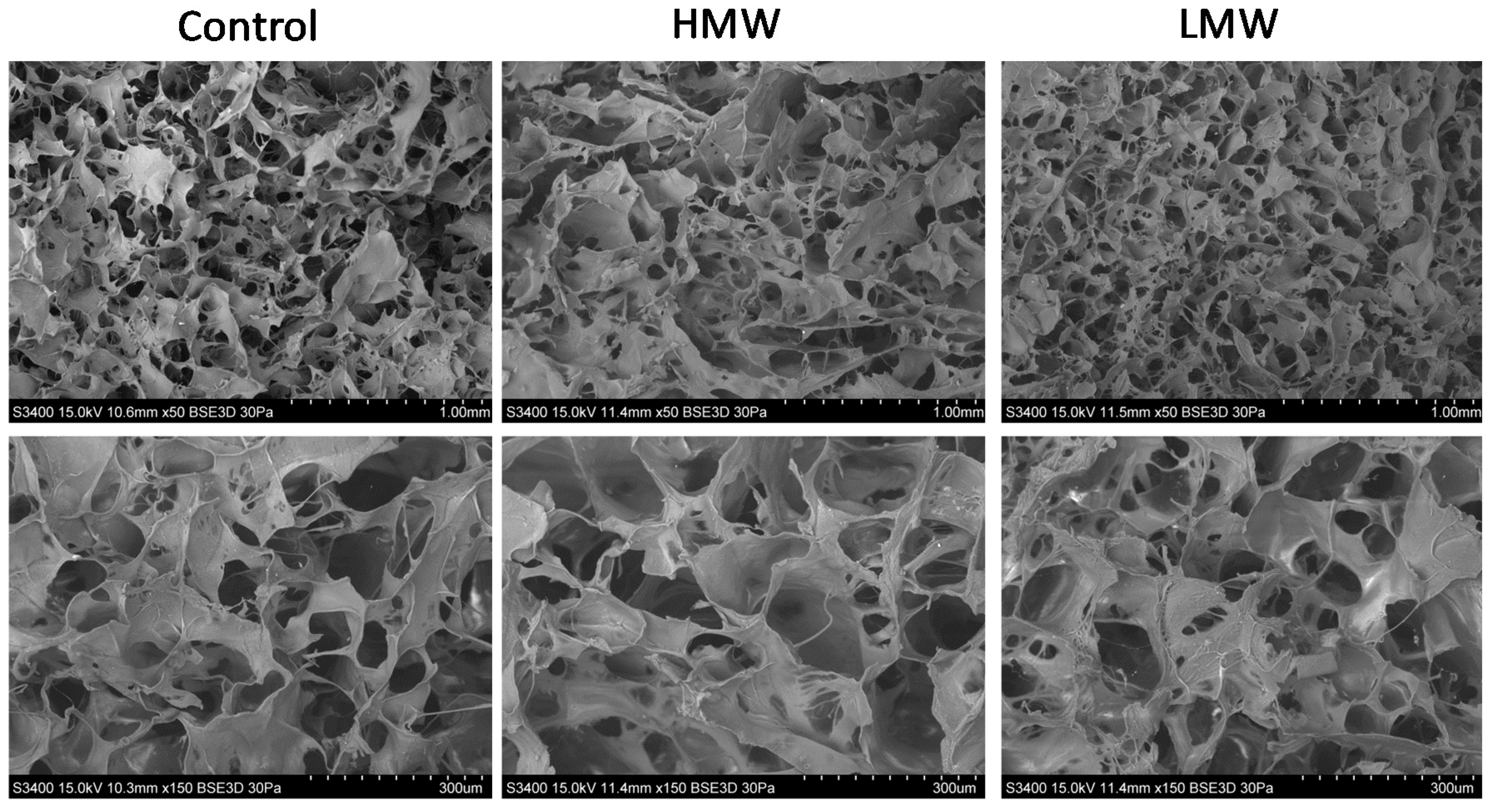
2.4.4. Scanning Electron Microscopy (SEM)
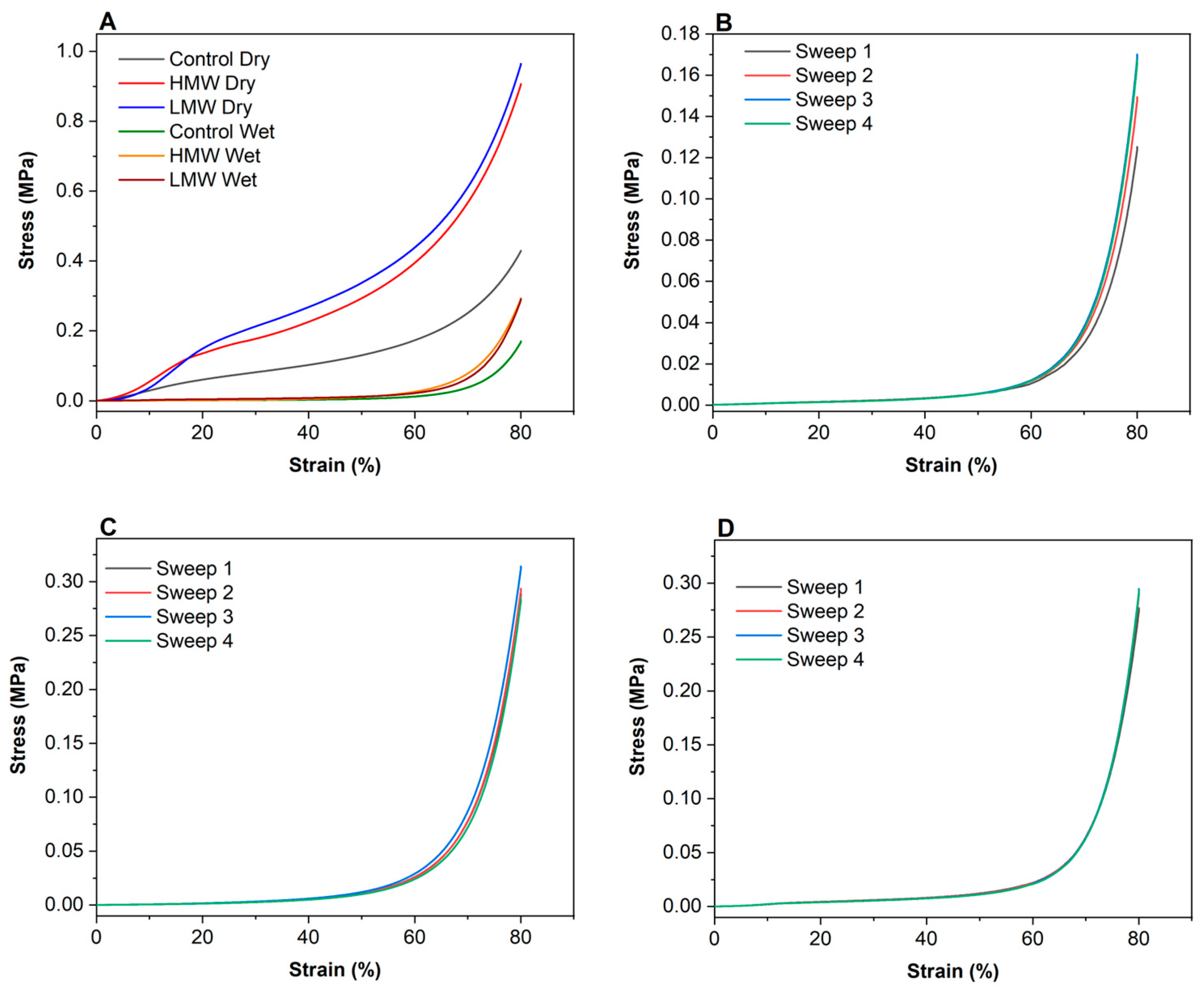
2.4.5. Compression Tests
2.4.6. Degradation Studies
3. Results and Discussion
3.1. Environmental Assessment of Collagen Scaffolds
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. X-ray Diffraction (XRD)
3.4. X-ray Photoelectron Spectroscopy (XPS)
3.5. Scanning Electron Microscopy (SEM)
3.6. Compression Tests
3.7. Degradation Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M. Integral valorization of pineapple (ananas comosus L.) by-products through a green chemistry approach towards added value ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council, the Economic and Social Committee and the Committee of the Regions. 2015. Available online: https://arquivo.pt/noFrame/patching/record/20170823204336/http://www.umic.pt/images/stories/publicacoes4/Single%20Market%20Act.pdf (accessed on 17 June 2020).
- Las Heras, K.; Santos-Vizcaíno, E.; Garrido, T.; Gutierrez, F.B.; Aguirre, J.J.; de la Caba, K.; Guerrero, P.; Igartua, M.; Hernandez, R.M. Soy protein and chitin sponge-like scaffolds: From natural by-products to cell delivery systems for biomedical applications. Green Chem. 2020, 22, 3445. [Google Scholar] [CrossRef]
- Andonegi, M.; de la Caba, K.; Guerrero, P. Effect of citric acid on collagen sheets processed by compression. Food Hydrocoll. 2020, 100, 105427. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Bio/Technol. 2010, 10, 151–163. [Google Scholar] [CrossRef]
- Martínez-Ortiz, M.A.; Hernandez-Fuentes, A.D.; Pimentel-Gonzalez, D.J.; Campos-Montiel, R.G.; Vargas-Torres, A.; Aguirre-Alvarez, G. Extraction and characterization of collagen from rabbit skin: Partial characterization. CyTA J. Food 2015, 13, 253–258. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valorization 2020, in press. [Google Scholar] [CrossRef]
- Joseph, K.; Nithya, N. Material flows in the life cycle of leather. J. Clean. Prod. 2009, 17, 676–682. [Google Scholar] [CrossRef]
- Bacardit, A.; Baquero, G.; Sorolla, S.; Ollé, L. Evaluation of a new sustainable continuous system for processing bovine leather. J. Clean. Prod. 2015, 101, 197–204. [Google Scholar] [CrossRef][Green Version]
- Shi, J.; Puig, R.; Sang, J.; Lin, W. A comprehensive evaluation of physical and environmental performances for wet-white leather manufacture. J. Clean. Prod. 2016, 139, 1512–1519. [Google Scholar] [CrossRef]
- Rosu, L.; Dragos Varganici, C.; Crudu, A.M.; Rosu, D.; Bele, A. Ecofriendly wet-white leather vs. conventional tanned wet—Blue leather. A photochemical approach. J. Clean. Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Cooper, J.A. Fibrous scaffolds for tissue engineering. In Biomaterials for Tissue Engineering Applications; Burdick, J.A., Mauck, R.L., Eds.; Springer: Berlin, Germany, 2011; pp. 47–73. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F. Biomaterials and scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Norris, E.G.; Dalecki, D.; Hocking, D.C. Acoustic modification of collagen hydrogels facilitates cellular remodeling. Mater. Today Bio 2019, 3, 100018. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, N. Collagen based biomaterials for tissue engineering applications: A review. In Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature; Springer: Berlin, Germany, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mat. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef]
- Raftery, R.M.; Woods, B.; Marques, A.L.; Moreira-Silva, J.; Silva, T.H.; Cryan, S.A.; Reis, R.L.; O’Brien, F.J. Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 2016, 43, 160–169. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Martínez, A.; Blanco, M.; Davidenko, N.; Cameron, R. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Cheng, X.R.; Wang, J.W.; Xu, D.X.; Wang, G. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J. Bioact. Compat. Pol. 2006, 21, 207–220. [Google Scholar] [CrossRef]
- Andonegi, M.; Peñalba, M.; de la Caba, K.; Guerrero, P. ZnO nanoparticle-incorporated native collagen films with electro—Conductive properties. Mater. Sci. Eng. C 2020, 108, 110394. [Google Scholar] [CrossRef] [PubMed]
- ISO 14040. Environmental Management—Life Cycle Assessment—Principals and Framework. 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 17 June 2020).
- Abdollahi, M.; Rezai, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; Mello, R.O.; Demiate, I.M.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manag. 2020, 102, 838–846. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Dan, W.H.; Dan, N.H.; Gu, Z.P. Evaluation of 1-ethyl-3-methylimidazolium acetate based ionic liquid systems as a suitable solvent for collagen. J. Appl. Polym. Sci. 2013, 130, 2245–2256. [Google Scholar] [CrossRef]
- Gao, D.; Cheng, Y.; Wang, P.; Li, F.; Wu, Y.; Lyu, B.; Ma, J.; Qin, J. An eco-friendly approach for leather manufacture based on P(POSS-MAA)-aluminum tanning agent combination tannage. J. Clean. Prod. 2020, 257, 120546. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Gao, M.M.; Liao, X.P.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Wang, B.L.; Han, Y.M.; Lin, Q.K.; Liu, H.H.; Shen, C.H.; Nan, K.H.; Chen, H. In vitro and in vivo evaluation of xanthan gum-succinic anhydride hydrogels for the ionic strength-sensitive release of antibacterial agents. J. Mater. Chem. B 2016, 4, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, X.; Ma, X.; Yang, R.; Zhao, W. A simple and eco—Friendly method of gelatin production from bone: One-step biocatalysis. J. Clean. Prod. 2019, 209, 916–926. [Google Scholar] [CrossRef]


| Impact Category | Unit | Total |
|---|---|---|
| Global warming | kg CO2 eq | 0.7730 |
| Stratospheric ozone depletion | kg CFC11 eq | 4.09·10−7 |
| Ionizing radiation | kBq Co-60 eq | 0.4080 |
| Ozone formation, human health | kg NOx eq | 0.0026 |
| Fine particulate matter formation | kg PM2.5 eq | 0.0019 |
| Ozone formation, terrestrial ecosystems | kg NOx eq | 0.0027 |
| Terrestrial acidification | kg SO2 eq | 0.0048 |
| Freshwater eutrophication | kg P eq | 0.0003 |
| Marine eutrophication | kg N eq | 3.32·10−5 |
| Terrestrial ecotoxicity | kg 1,4-DCB | 0.8840 |
| Freshwater ecotoxicity | kg 1,4-DCB | 0.0105 |
| Marine ecotoxicity | kg 1,4-DCB | 0.0146 |
| Human carcinogenic toxicity | kg 1,4-DCB | 0.0233 |
| Human non-carcinogenic toxicity | kg 1,4-DCB | 0.3050 |
| Land use | m2a crop eq | 0.0211 |
| Mineral resource scarcity | kg Cu eq | 0.0007 |
| Fossil resource scarcity | kg oil eq | 0.2350 |
| Water consumption | m3 | 0.0086 |
| Samples | Amide I (VI) | Amide I (VII) | VI/VII |
|---|---|---|---|
| Control | 1631 | 1544 | 87 |
| HMW | 1636 | 1555 | 81 |
| LMW | 1635 | 1552 | 83 |
| Samples | C–C/C–H 284.6 (eV) | C–O/C–N 285.9 (eV) | O=C–NH2 288.1 (eV) | C=N/C–N 399.7 (eV) | O–C=O/O=C–N 531.9 (eV) |
|---|---|---|---|---|---|
| Control | 75.98 | 3.59 | 5.89 | 1.81 | 12.73 |
| HMW | 69.11 | 6.93 | 6.21 | 3.96 | 13.79 |
| LMW | 64.93 | 8.18 | 7.51 | 3.54 | 15.84 |
| Samples | E (MPa) | σ (MPa) | ||
|---|---|---|---|---|
| Dry | Hydrated | Dry | Hydrated | |
| Control | 0.122 ± 0.002 | 0.006 ± 0.001 | 0.430 ± 0.033 | 0.170 ± 0.021 |
| HMW | 0.563 ± 0.011 | 0.013 ± 0.003 | 0.951 ± 0.041 | 0.294 ± 0.012 |
| LMW | 0.423 ± 0.013 | 0.018 ± 0.004 | 0.965 ± 0.049 | 0.291 ± 0.008 |
| Samples | HDD (%) | EDD (%) |
|---|---|---|
| Control | 0 | 100 |
| HMW | 10.6 ± 1.8 | 55.8 ± 1.6 |
| LMW | 15.1 ± 0.4 | 44.4 ± 4.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andonegi, M.; Irastorza, A.; Izeta, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment. Polymers 2020, 12, 1597. https://doi.org/10.3390/polym12071597
Andonegi M, Irastorza A, Izeta A, Cabezudo S, de la Caba K, Guerrero P. A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment. Polymers. 2020; 12(7):1597. https://doi.org/10.3390/polym12071597
Chicago/Turabian StyleAndonegi, Mireia, Ainhoa Irastorza, Ander Izeta, Sara Cabezudo, Koro de la Caba, and Pedro Guerrero. 2020. "A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment" Polymers 12, no. 7: 1597. https://doi.org/10.3390/polym12071597
APA StyleAndonegi, M., Irastorza, A., Izeta, A., Cabezudo, S., de la Caba, K., & Guerrero, P. (2020). A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment. Polymers, 12(7), 1597. https://doi.org/10.3390/polym12071597







