Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of a Chitosan Copper (II) Binary Composite: CCu
2.1.2. Synthesis of Chitosan, Alginate and Copper-Based Ternary Composites: CACu
2.2. Characterization
2.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.2. X-ray diffraction (XRD)
2.2.3. Thermal Gravimetric Analysis (TGA)
2.2.4. Solids C-13 NMR Spectroscopy
2.2.5. Raman Spectroscopy
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Point-of-Zero-Charge (PZC)
2.2.8. Solvent Swelling Test
2.2.9. X-ray Photoelectron Spectroscopy (XPS)
2.2.10. Leaching of Cu(II) Species
2.3. Adsorption Studies
2.3.1. Equilibrium Adsorption Experiments
2.3.2. Sorption Isotherms and Modeling
3. Results and Discussion
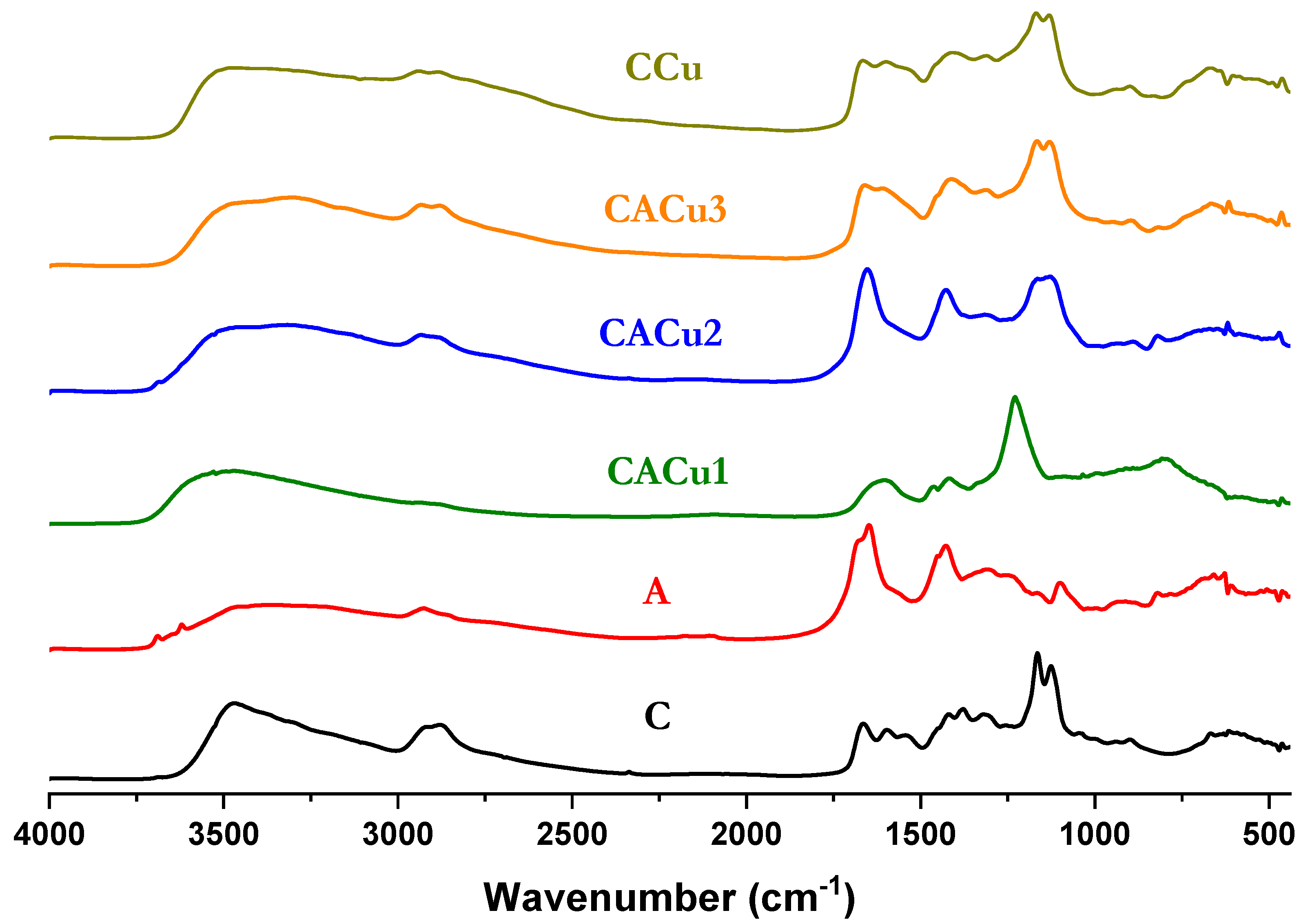
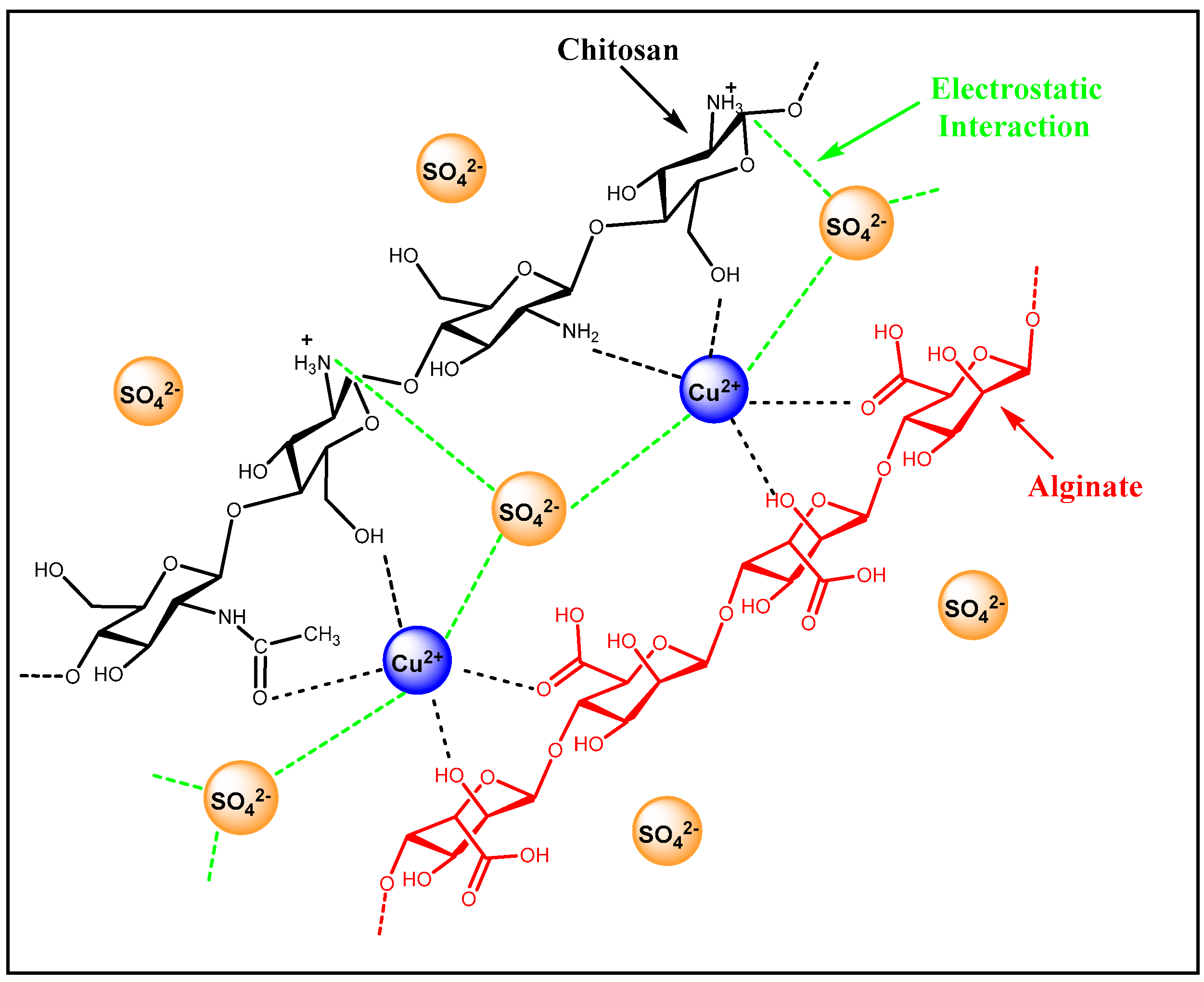
3.1. Fourier Transform Infrared (FTIR) Spectral Analysis
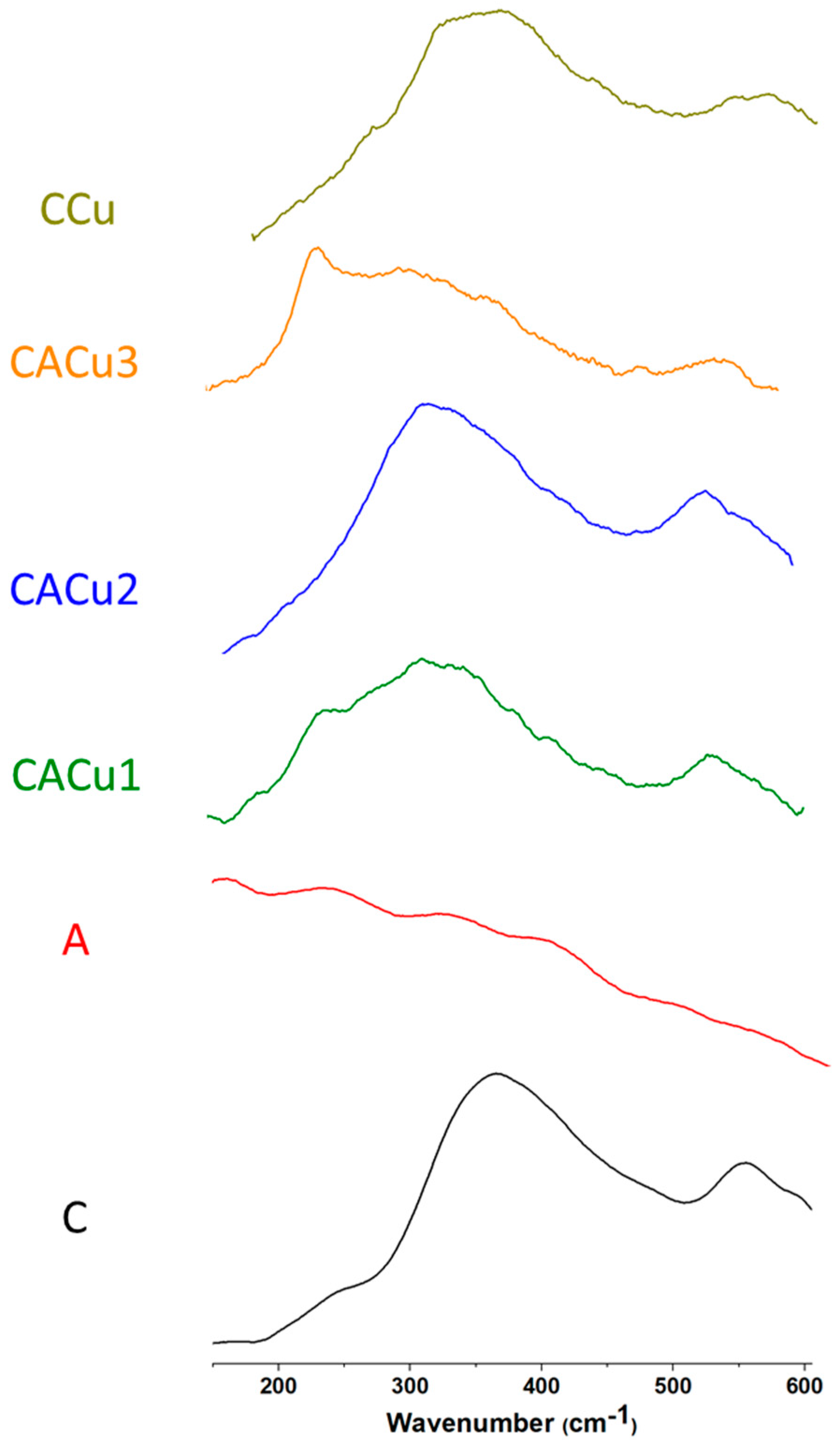
3.2. Raman Spectroscopy
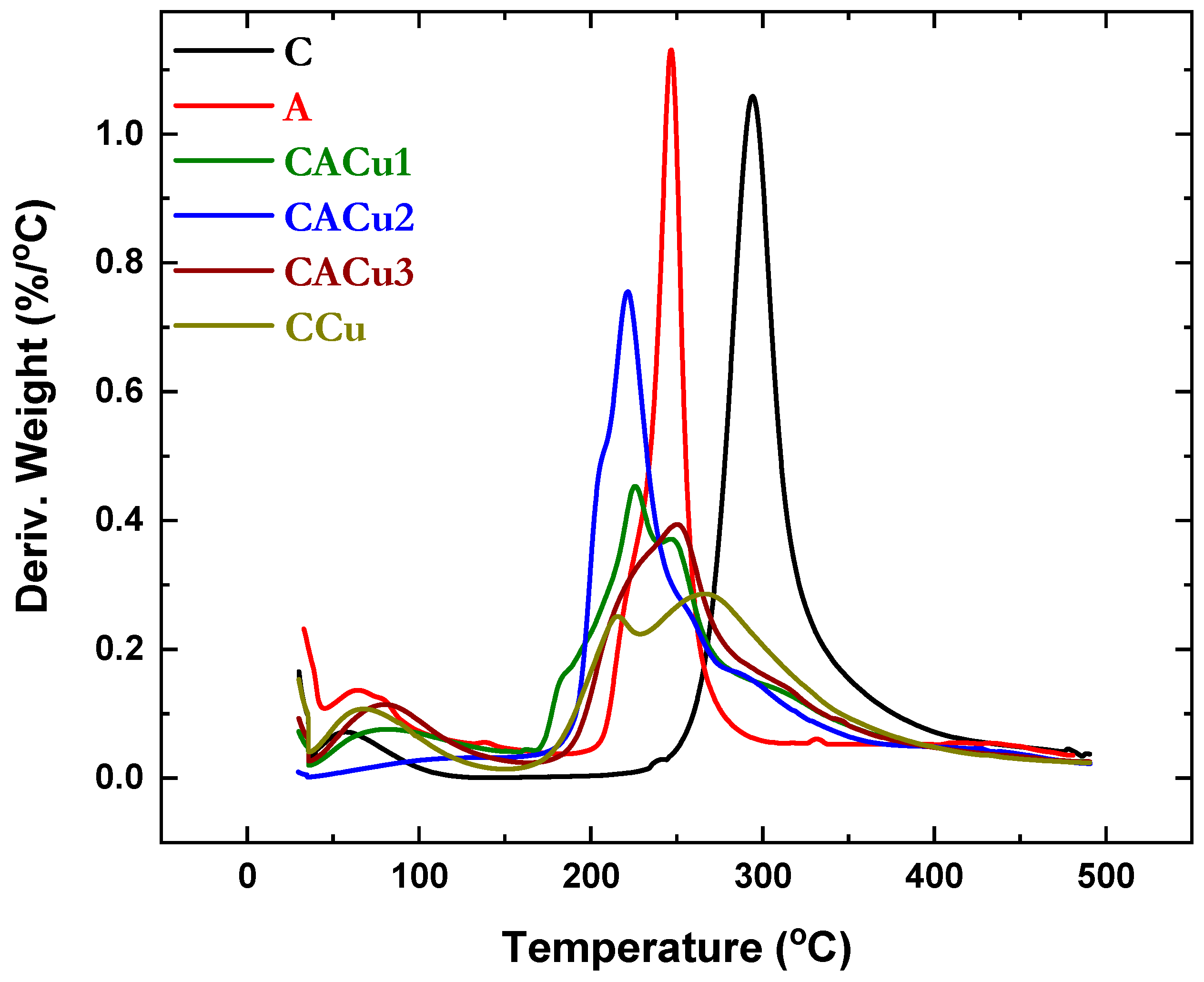
3.3. Thermogravimetric Analysis (TGA)
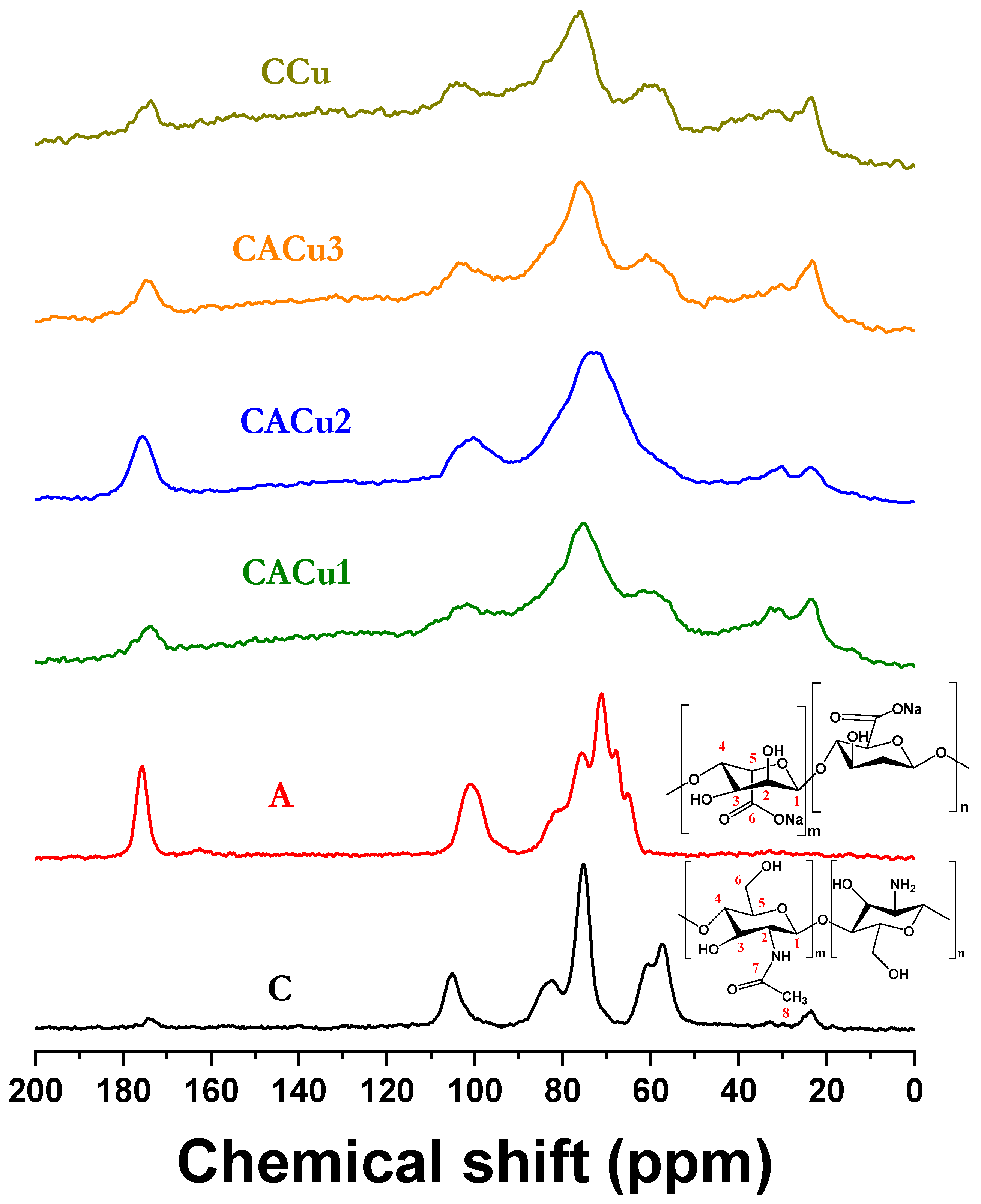
3.4. Solid State 13C NMR Spectroscopy
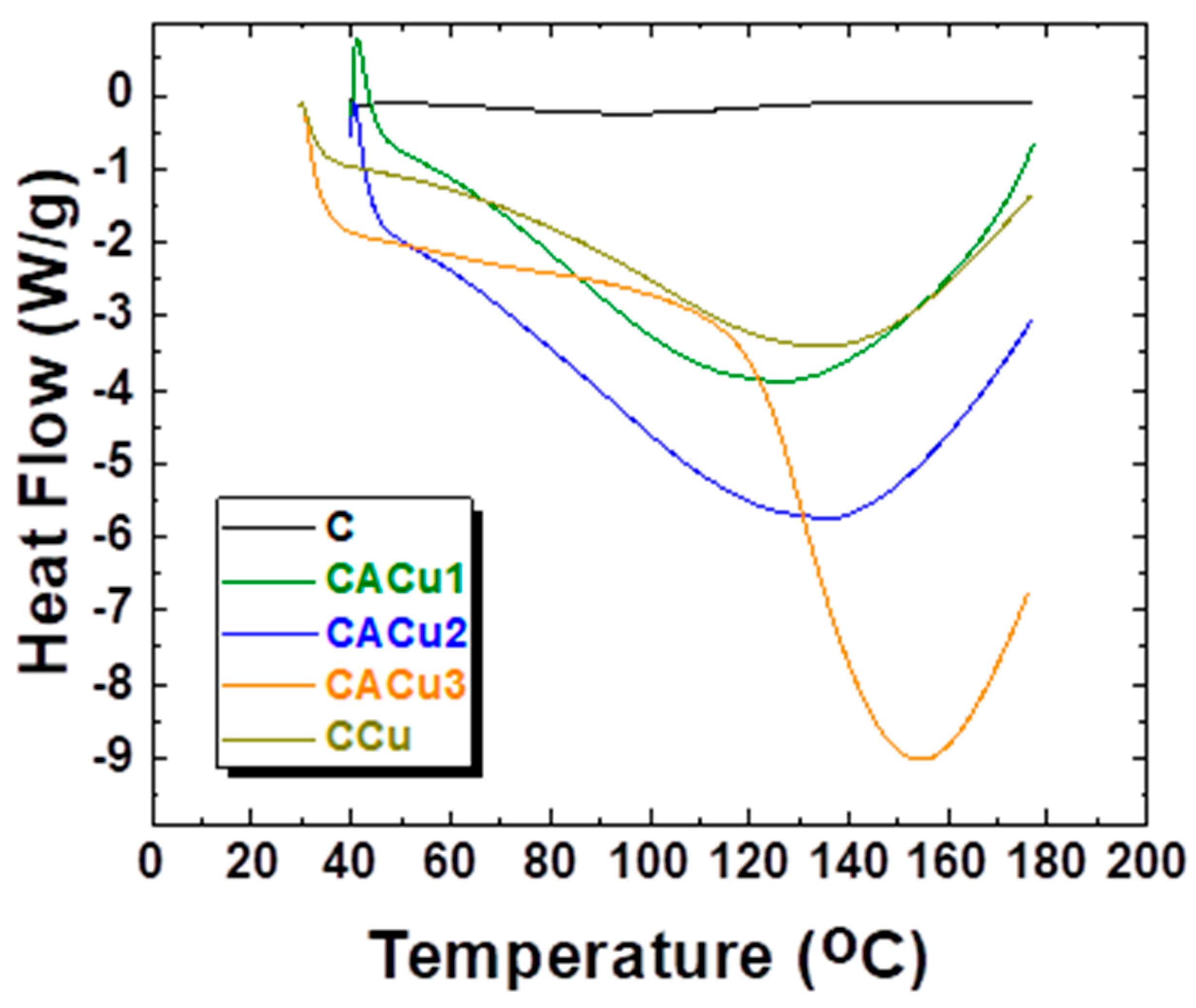
3.5. Differential Scanning Calorimetry (DSC)
3.6. Swelling Tests
3.7. X-ray Photoelectron Spectroscopy (XPS)
3.8. X-ray Diffraction (XRD) Results
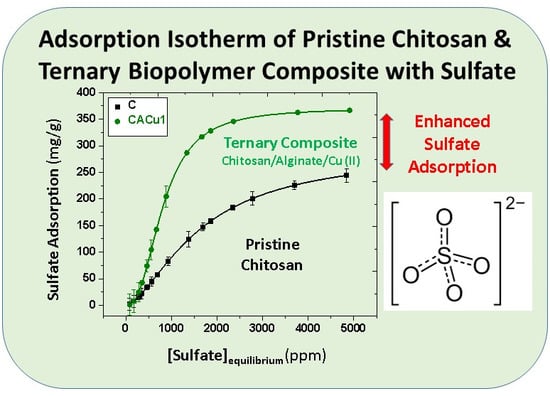
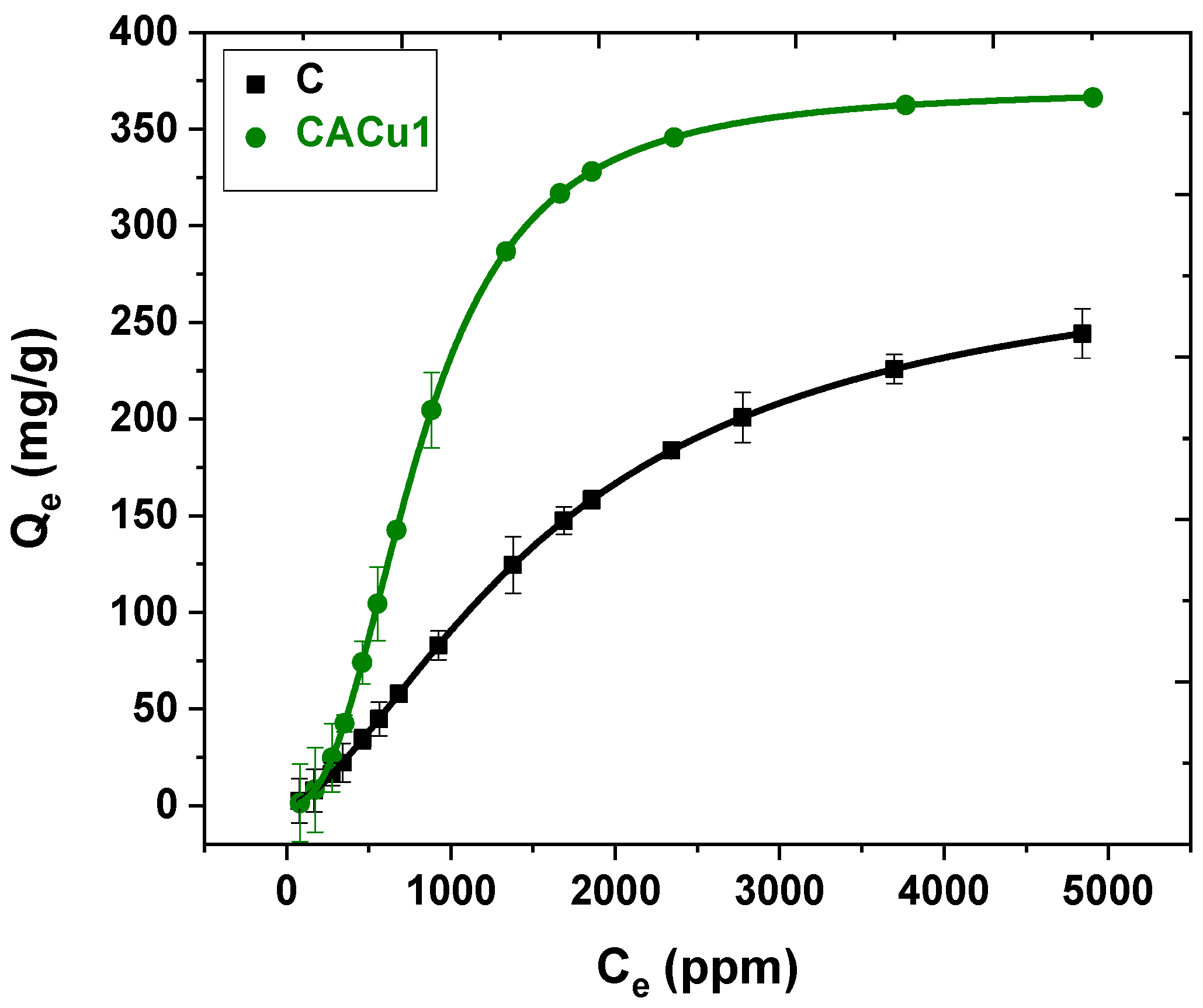
3.9. Sorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Runtti, H.; Tolonen, E.T.; Tuomikoski, S.; Luukkonen, T.; Lassi, U. How to tackle the stringent sulfate removal requirements in mine water treatment—A review of potential methods. Environ. Res. 2018, 167, 207–222. [Google Scholar] [CrossRef]
- Miao, Z.; Brusseau, M.L.; Carroll, K.C.; Carreón-Diazconti, C.; Johnson, B. Sulfate reduction in groundwater: Characterization and applications for remediation. Environ. Geochem. Health 2012, 34, 539–550. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef]
- Hao, T.; Xiang, P.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.; van Loosdrecht, M.C.M.; Chen, G.H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; De Ingeniería, F.; Portales, U.D. Environmental Impact and Toxicology of Sulphate. In Proceedings of the EnviroMine 2009, Santiago, Chile, 30 September–2 October 2009; pp. 1–10. [Google Scholar]
- Mbakop, S.; Mthombeni, N.H.; Leswifi, T.Y.; Onyango, M.S. Evaluation of Chitosan-Bentonite Composite Performance towards Remediation of Sulphate Containing Effluent. In Proceedings of the 2016 Annual Conference on Sustainable Research and Innovation, Nairobi, Kenya, 4–6 May 2016; pp. 65–70. [Google Scholar]
- Soucek, D.J.; Kennedy, A.J. Effects of Hardness, Chloride, and Acclimation on the Acute Toxicity of Sulfate to Freshwater Invertebrates. Environ. Toxicol. Chem. 2005, 24, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Soucek, D.J.; Linton, T.K.; Tarr, C.D.; Dickinson, A.; Wickramanayake, N.; Delos, C.G.; Cruz, L.A. Influence of Water Hardness and Sulfate on the Acute Toxicity of Chloride to Sensitive Freshwater Invertebrates. Environ. Toxicol. Chem. 2011, 30, 930–938. [Google Scholar] [CrossRef]
- Bernardez, L.A.; de Andrade Lima, L.R.P.; de Jesus, E.B.; Ramos, C.L.S.; Almeida, P.F. A kinetic study on bacterial sulfate reduction. Bioprocess Biosyst. Eng. 2013, 36, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Jiang, J.; Chan, A.; Ali, S.; Saha, A.; Haushalter, K.J.; Lam, W.L.M.; Glasheen, M.; Parker, J.; Brenner, M.; Mahon, S.B.; et al. Hydrogen Sulfide--Mechanisms of Toxicity and Development of an Antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of Iron by Sulfate-Reducing Bacteria: New Views of an Old Problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of Hydrogen Sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Cocchetto, D.M.; Levy, G. Absorption of Orally Administered Sodium Sulfate in Humans. J. Pharm. Sci. 1981. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.G.; Sandler, R.S.; Seal, E. High Levels of Inorganic Sulfate Cause Diarrhea in Neonatal Piglets. J. Nutr. 1995. [Google Scholar] [CrossRef]
- Silva, A.M.; Lima, R.M.F.; Leão, V.A. Mine water treatment with limestone for sulfate removal. J. Hazard. Mater. 2012, 221, 45–55. [Google Scholar] [CrossRef]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Benatti, C.; Tavares, C.; Lenzi, E. Sulfate Removal From Waste Chemicals By Precipitation. J. Environ. Manag. 2008, 90, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Maree, J.P.; Strydom, W.F. Biological sulphate removal from industrial effluent in an upflow packed bed reactor. Water Res. 1987, 21, 141–146. [Google Scholar] [CrossRef]
- Maree, J.P.; Strydom, W.F. Biological sulphate removal in an upflow packed bed reactor. Water Res. 1985, 19, 1101–1106. [Google Scholar] [CrossRef]
- Bowell, R.; Dill, S.; Cowan, J.; Wood, A. A review of sulfate removal options for mine waters. In Proceedings of the International Mine Water Association Symposium, Johannesburg, South Africa, 7–13 September 1998; pp. 329–342. [Google Scholar]
- Maddah, H.A.; Alzhrani, A.S.; Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.; Almalki, A.M. Evaluation of various membrane filtration modules for the treatment of seawater. Appl. Water Sci. 2018, 8, 150. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Lubis, M.R.; Takagi, R.; Matsuyama, H. Removal profile of sulfate ion from mix ion solution with different type and configuration of anion exchange membrane in elctrodialysis. J. Water Process Eng. 2017, 20, 173–179. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Rance, D.P.M.; Bellotindos, L.M.; Lu, M.C. Removal of sulfate by fluidized bed crystallization process. J. Environ. Chem. Eng. 2017, 5, 2431–2439. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Wan, J.; Wang, Y.; Guan, Z. Removal of Sulfate Ions from Aqueous Solution by Adsorption with Hydrotalcite- like Composite. Huan Jing Ke Xue 2016, 37, 1000–1007. [Google Scholar]
- Pavlikova, P.; Balintova, M.; Holub, M. Study of sulphate ions removal from acidic waters using ion exchange resin. Sel. Sci. Pap. J. Civ. Eng. 2018, 13, 51–58. [Google Scholar] [CrossRef]
- Moshoeshoe, M.; Silas Nadiye-Tabbiruka, M.; Obuseng, V. A Review of the Chemistry, Structure, Properties and Applications of Zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar] [CrossRef]
- Dong, R.; Liu, Y.; Wang, X.; Huang, J. Adsorption of Sulfate Ions from Aqueous Solution by Surfactant-Modified Palygorskite. J. Chem. Eng. Data 2011, 56, 3890–3896. [Google Scholar] [CrossRef]
- Ramos, B.G.Z.; Kundu, P.P.; Sarkar, K. Biopolymers Employed in Drug Delivery. In Biopolymers; Wiley Online Library, Scrivener Publishing: Beverly, MA, USA, 2011; pp. 559–573. [Google Scholar] [CrossRef]
- Erõs, I.; Ramos, B.G.Z.; Kundu, P.P.; Sarkar, K. Polymers and Biopolymers in Pharmaceutical Technology. In Biopolymers; Scrivener Publishing: Beverly, MA, USA, 2011; pp. 525–558. ISBN 9780470639238. [Google Scholar]
- Raj, S.N.; Lavanya, S.N.; Sudisha, J.; Shetty, H.S.; Boufi, S.; Alila, S.; Erõs, I.; Ramos, B.G.Z.; Kundu, P.P.; Sarkar, K. Applications of Biopolymers in Agriculture with Special Reference to Role of Plant Derived Biopolymers in Crop Protection. In Biopolymers; Scrivener Publishing: Beverly, MA, USA, 2011; pp. 459–481. [Google Scholar]
- Fahnestock, K.J.; Austero, M.S.; Schauer, C.L. Natural Polysaccharides: From Membranes to Active Food Packaging. In Biopolymers: Biomedical and Environmental Applications; Wiley Online Books; Scrivener Publishing: Beverly, MA, USA, 2011; pp. 59–80. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Optimisation of Orthophosphate and Turbidity Removal Using an Amphoteric Chitosan-Based Flocculant-Ferric Chloride Coagulant System. Environ. Chem. 2019, 16, 599–612. [Google Scholar] [CrossRef]
- Wilson, L.; Tewari, B.T. Chitosan-Based Adsorbents: Environmental Applications for the Removal of Arsenicals; Nasar, A., Ed.; MRF: Millersville, PA, USA, 2018; pp. 133–160. ISBN 9781945291753. [Google Scholar]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on Recent Progress in Chitosan-Based Hydrogels for Wastewater Treatment Application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous Catalysis on Chitosan-Based Materials: A Review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Mekahlia, S.; Bouzid, B. Chitosan-Copper (II) complex as antibacterial agent: Synthesis, characterization and coordinating bond- activity correlation study. Phys. Procedia 2009. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Tao, H.C.; Li, S.; Zhang, L.J.; Chen, Y.Z.; Deng, L.P. Magnetic chitosan/sodium alginate gel bead as a novel composite adsorbent for Cu(II) removal from aqueous solution. Environ. Geochem. Health 2018. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef]
- Richau, K.; Schwarz, H.H.; Apostel, R.; Paul, D. Dehydration of organics by pervaporation with polyelectrolyte complex membranes: Some considerations concerning the separation mechanism. J. Membrane Sci. 1996, 113, 31–41. [Google Scholar] [CrossRef]
- Liao, I.C.; Wan, A.C.A.; Yim, E.K.F.; Leong, K.W. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release 2005, 104, 347–358. [Google Scholar] [CrossRef]
- Ayoub, A.; Venditti, R.A.; Pawlak, J.J.; Salam, A.; Hubbe, M.A. Novel Hemicellulose–Chitosan Biosorbent for Water Desalination and Heavy Metal Removal. ACS Sustain. Chem. Eng. 2013, 1, 1102–1109. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 214, 175–191. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Moret, A.; Rubio, J. Sulphate and molybdate ions uptake by chitin-based shrimp shells. Miner. Eng. 2003, 16, 715–722. [Google Scholar] [CrossRef]
- Chmielewská, E.; Sabova, L.; Sitek, J.; Gaplovska, K.; Morvova, M. Removal of nitrates, sulfate and Zn(II) ions from aqueous solutions by using biopolymeric alginate/ clinoptilolite rich tuff pellets. Fresenius Environ. Bull. 2010, 19, 884–891. [Google Scholar]
- Guo, Y.J.; Xue, J.Q.; Zhang, J.; Ma, J.; Quan, X.T.; Liu, Y. Adsorption behaviors of protonation modified chitosan and the analysis of spectra. Spectrosc. Spectr. Anal. 2014, 34, 78–81. [Google Scholar]
- Fosso-kankeu, E.; Waanders, F.; Steyn, B. Chitosan-graft-Polyacrylamide adsorbent for Sulphate removal from water. In Proceedings of the 7th International Conference on Latest Trends in Engineering and Technology (ICLTET’2015), Pretoria, South Africa, 26–27 November 2015; International Institute of Engineers: Desumajra, Kharar, India, 2015; pp. 7–10. [Google Scholar]
- Schwarz, S.; Schwarz, D.; Ohmann, W.; Neuber, S. Adsorption and desorption studies on reusing chitosan as an efficient adsorbent. In Proceedings of the 3rd World Congress on Civil, Structural, and Environmental Engineering (CSEE’18), Budapest, Hungary, 8–10 April 2018. [Google Scholar]
- Tian, Z.; Feng, T.; Yang, G.; Zhao, T.; Wang, L. Removal of sulfate from aqueous solution by magnetic chitosan microspheres. Desalin. Water Treat. 2019, 161, 293–303. [Google Scholar] [CrossRef]
- Singh, J.; Mishra, N.S.; Banerjee, S.; Sharma, Y.C. Comparative Studies of Physical Characteristics of Raw and Modified Sawdust for Their Use as Adsorbents for Removal of Acid Dye. Bioresources 2011, 6, 2732–2743. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. II. J. Chem. Phys. 1950, 18, 1024–1026. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent Using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Yu, H.; Guo, Z.; Wang, P.; Li, C.; Li, Z.; Li, P. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005, 340, 2150–2153. [Google Scholar] [CrossRef]
- Sadiq, A.; Choubey, A.; Bajpai, A.K. Biosorption of chromium ions by calcium alginate nanoparticles. J. Chil. Chem. Soc. 2018, 63, 4077–4081. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. Charact. 2011. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and Characterization of Sodium Alginate from Moroccan Laminaria Digitata Brown Seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Rashid, S.; Shen, C.; Chen, X.; Li, S.; Chen, Y.; Wen, Y.; Liu, J. Enhanced catalytic ability of chitosan-Cu-Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv. 2015. [Google Scholar] [CrossRef]
- Chan, H.Y.H.; Takoudis, C.G.; Weaver, M.J. Oxide Film Formation and Oxygen Adsorption on Copper in Aqueous Media as Probed by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. B 1999, 103, 357–365. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of Cu III Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper(I) Oxide Nanocrystals—One Step Synthesis, Characterization, Formation Mechanism, and Photocatalytic Properties. Eur. J. Inorg. Chem. 2013, 2013, 2640–2651. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and Characterization of Novel β-Cyclodextrin Based Copolymer Materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Iakovleva, E.; Mäkilä, E.; Salonen, J.; Sitarz, M.; Sillanpää, M. Industrial Products and Wastes as Adsorbents for Sulphate and Chloride Removal from Synthetic Alkaline Solution and Mine Process Water. Chem. Eng. J. 2015, 259, 364–371. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Faye, O.; Wilson, L.D. Adsorption of Phosphate Dianions by Hybrid Inorganic–Biopolymer Polyelectrolyte Complexes: Experimental and Computational Studies. ACS Appl. Polym. Mater. 2020, 2, 899–910. [Google Scholar] [CrossRef]
- Gopalakannan, V.; Viswanathan, N. One Pot Synthesis of Metal Ion Anchored Alginate–Gelatin Binary Biocomposite for Efficient Cr(VI) Removal. Int. J. Biol. Macromol. 2016, 83, 450–459. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.M.; Sudha, P.N.; Venkatesan, J.; Anil, S. Synthesis, Characterization and Applications of Nanochitosan/Sodium Alginate/Microcrystalline Cellulose Film. J. Nanomed. Nanotechnol. 2016, 7, 419. [Google Scholar] [CrossRef]
- Saito, H.; Tabeta, R.; Ogawa, K. High-Resolution Solid-State Carbon-13 NMR Study of Chitosan and Its Salts with Acids: Conformational Characterization of Polymorphs and Helical Structures as Viewed from the Conformation-Dependent Carbon-13 Chemical Shifts. Macromolecules 1987, 20, 2424–2430. [Google Scholar] [CrossRef]
- Vueva, Y.; Connell, L.S.; Chayanun, S.; Wang, D.; McPhail, D.S.; Romer, F.; Hanna, J.V.; Jones, J.R. Silica/alginate hybrid biomaterials and assessment of their covalent coupling. Appl. Mater. Today 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Vold, I.; Vårum, K.M.; Guibal, E.; Smidsrød, O. Binding of ions to chitosan—Selectivity studies. Carbohydr. Polym. 2003, 54, 471–477. [Google Scholar] [CrossRef]
- Geethamani, C.K.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Alkali-Treated Fly Ash for the Removal of Fluoride from Aqueous Solutions. Desalin. Water Treat. 2014, 52, 3466–3476. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.C. Adsorption of sulfate in aqueous solutions by organo-nano-clay: Adsorption equilibrium and kinetic studies. J. Cent. South Univ. 2014, 21, 1974–1981. [Google Scholar] [CrossRef]
- Runtti, H.; Luukkonen, T.; Niskanen, M.; Tuomikoski, S.; Kangas, T.; Tynjälä, P.; Tolonen, E.T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; et al. Sulphate Removal over Barium-Modified Blast-Furnace-Slag Geopolymer. J. Hazard. Mater. 2016, 317, 373–384. [Google Scholar] [CrossRef] [PubMed]

| Adsorbent | Year | References |
|---|---|---|
| Chitin-based shrimp shells | 2003 | Moret and Rubio [53] |
| Biopolymeric alginate/clinoptilolite pellets | 2009 | Chmielewská et al. [54] |
| Protonation modified chitosan | 2014 | Guo et al. [55] |
| Chitosan-grafted-polyacrylamide | 2015 | Fosso-Kankeu et al. [56] |
| Chitosan-bentonite composite | 2016 | Mbakop et al. [6] |
| Chitosan flakes | 2018 | Schwarz et al. [57] |
| Magnetic chitosan microspheres | 2019 | Tian et al. [58] |
| Reaction Conditions | CCu | CACu1 | CACu2 | CACu3 |
|---|---|---|---|---|
| Mass of Alginate (g) | 0 | 1.3 | 2.6 | 1 |
| Mass of Chitosan (g) | 1 | 1 | 1 | 2 |
| Mass of CuCl2 (g) | 0.83 | 0.83 | 0.83 | 0.83 |
| Material | Tmax (°C) | T0 (°C) | DSC Peak Area (J g−1) |
|---|---|---|---|
| C | 97.5 | 59.1 | 45.9 |
| CCu | 135.5 | 70.3 | 811.4 |
| CACu1 | 130.8 | 52.7 | 2016 |
| CACu2 | 125.3 | 51.9 | 1491 |
| CACu3 | 146.6 | 120.8 | 786.6 |
| Element | Well-1 (At. %) | Well-2 (At. %) | Well-3 (At. %) |
|---|---|---|---|
| C | 56.35 | 53.35 | 54.01 |
| N | 2.14 | 2.27 | 1.42 |
| O | 27.27 | 25.99 | 25.89 |
| Cu | 1.17 | 1.19 | 1.42 |
| S | - | - | - |
| Water Composition | Well-1 (mg/L) | Well-2 (mg/L) | Well-3 (mg/L) |
|---|---|---|---|
| Sulfate | 2062.6 | 2653.7 | 6030.0 |
| Nitrate | 6.9 | 27.6 | 1.6 |
| Chloride | 98.3 | 146.8 | 328.1 |
| Bicarbonate | 422 | 593 | 722 |
| Total Hardness | 1912 | 2198 | 4194 |
| Adsorbent | Qm (mg/g) | Reaction Conditions | References |
|---|---|---|---|
| Pulp and paper waste | 2.786 | Adsorbent: 40 g Mixture SO42−/Cl− 1000 mL pH: not reported Time: 480 h | Lakovleva [72] |
| Alkali-treated fly ash | 43.0 | Adsorbent: 2 g SO42− soln: 1000 mL pH: 7 Time: 2.5 h | Geethamani [79] |
| Organo-nano-clay | 63.5 | Adsorbent: 500 mg SO42− soln: 100 mL pH: 7 Time: 2 h | Wei and Hai-cheng [80] |
| Ba-modified blast-furnace-slag geopolymer | 119 | Adsorbent: 10 g SO42− soln: 1000 mL pH: 7–8 Time: 3 h | Runtti [81] |
| Chitin-based shrimp shells | 250.7 | Adsorbent: 750 mg SO42− soln: 200 mL pH: 7 Time: 1 h | Moret and Rubio [44] |
| Chitosan power (C) | 288.1 | Adsorbent: 20 mg SO42− soln: 15 mL pH: 6.8–7 Time: 24 h | This study |
| CACu1 | 371.4 | Adsorbent: 10 mg SO42− soln: 15 mL pH: 6.8 Time: 24 h | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.M.; Mohamed, M.H.; Udoetok, I.A.; Steiger, B.G.K.; Wilson, L.D. Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials. Polymers 2020, 12, 1502. https://doi.org/10.3390/polym12071502
Hassan MM, Mohamed MH, Udoetok IA, Steiger BGK, Wilson LD. Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials. Polymers. 2020; 12(7):1502. https://doi.org/10.3390/polym12071502
Chicago/Turabian StyleHassan, Md. Mehadi, Mohamed H. Mohamed, Inimfon A. Udoetok, Bernd G. K. Steiger, and Lee D. Wilson. 2020. "Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials" Polymers 12, no. 7: 1502. https://doi.org/10.3390/polym12071502
APA StyleHassan, M. M., Mohamed, M. H., Udoetok, I. A., Steiger, B. G. K., & Wilson, L. D. (2020). Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials. Polymers, 12(7), 1502. https://doi.org/10.3390/polym12071502