Mucoadhesive Bletilla striata Polysaccharide-Based Artificial Tears to Relieve Symptoms and Inflammation in Rabbit with Dry Eyes Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Bletilla Striata Polysaccharide
2.3. Cell viability of Human Corneal Epithelial Cells (HCECs)
2.4. Antioxidant Effect of BSP
2.5. Anti-Inflammatory Effect of BSP by Gene Expression of Inflammatory Cytokines in HCECs
2.6. Characterization of Artificial Tears with BPS Addition
2.7. Ocular Retention Test for BPS Contained AT
2.8. Animal Model of DES and Treated by BSP Eye Drops
2.8.1. Measurement of Tear Production
2.8.2. Central Corneal Thickness Measurement (CCT)
2.8.3. Appearance of the Ocular Surface
2.8.4. Histological Examination of the Cornea
3. Results
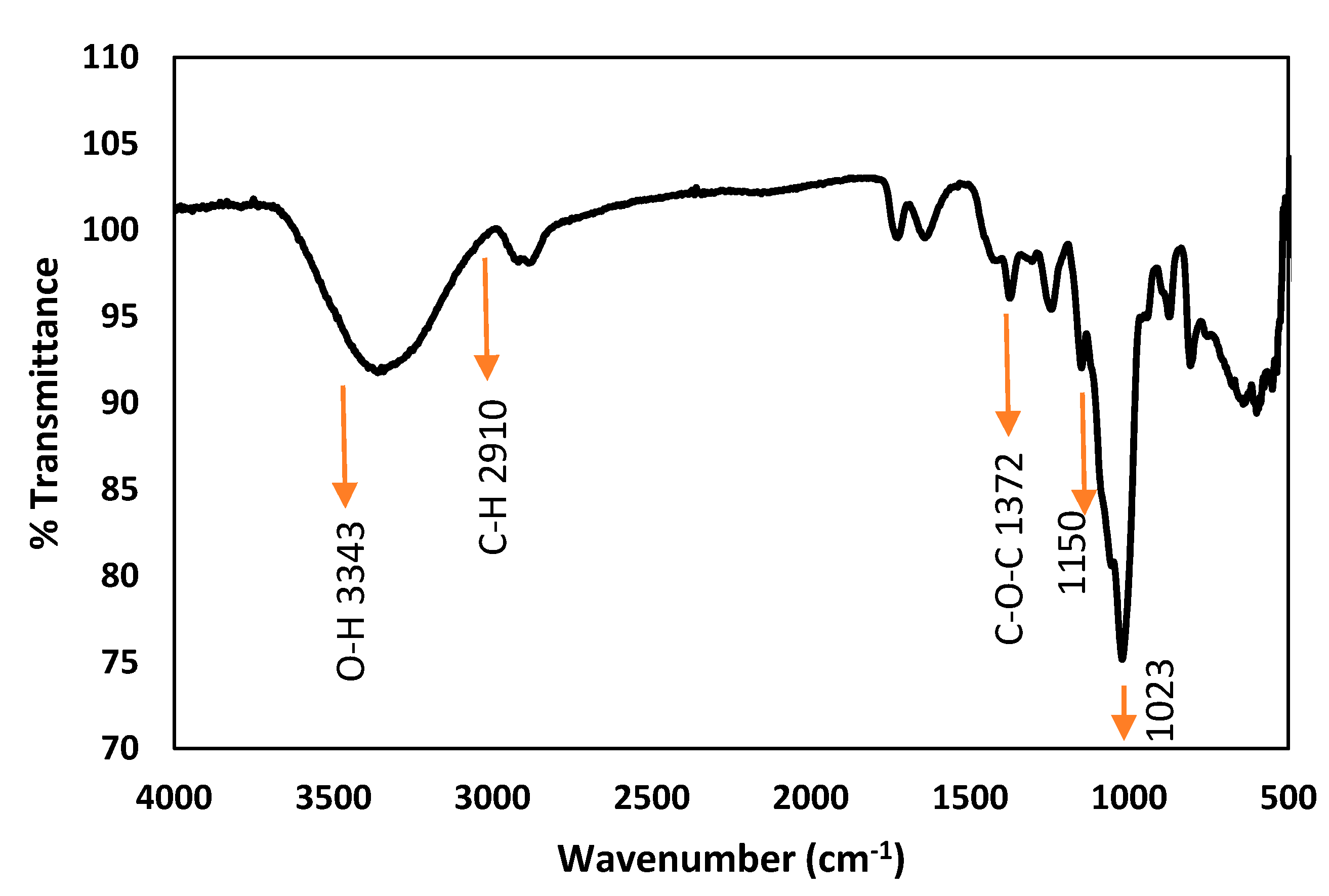
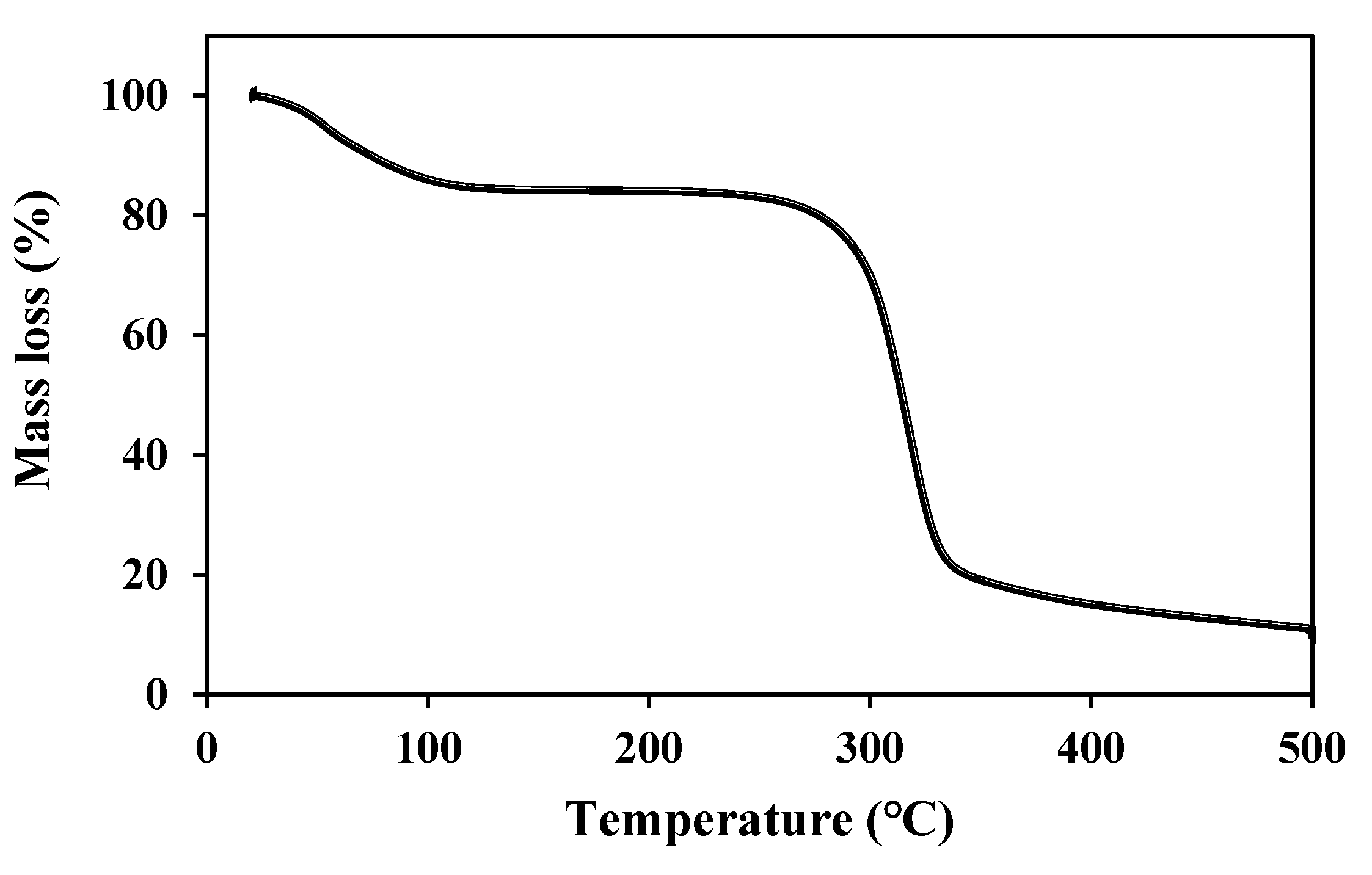
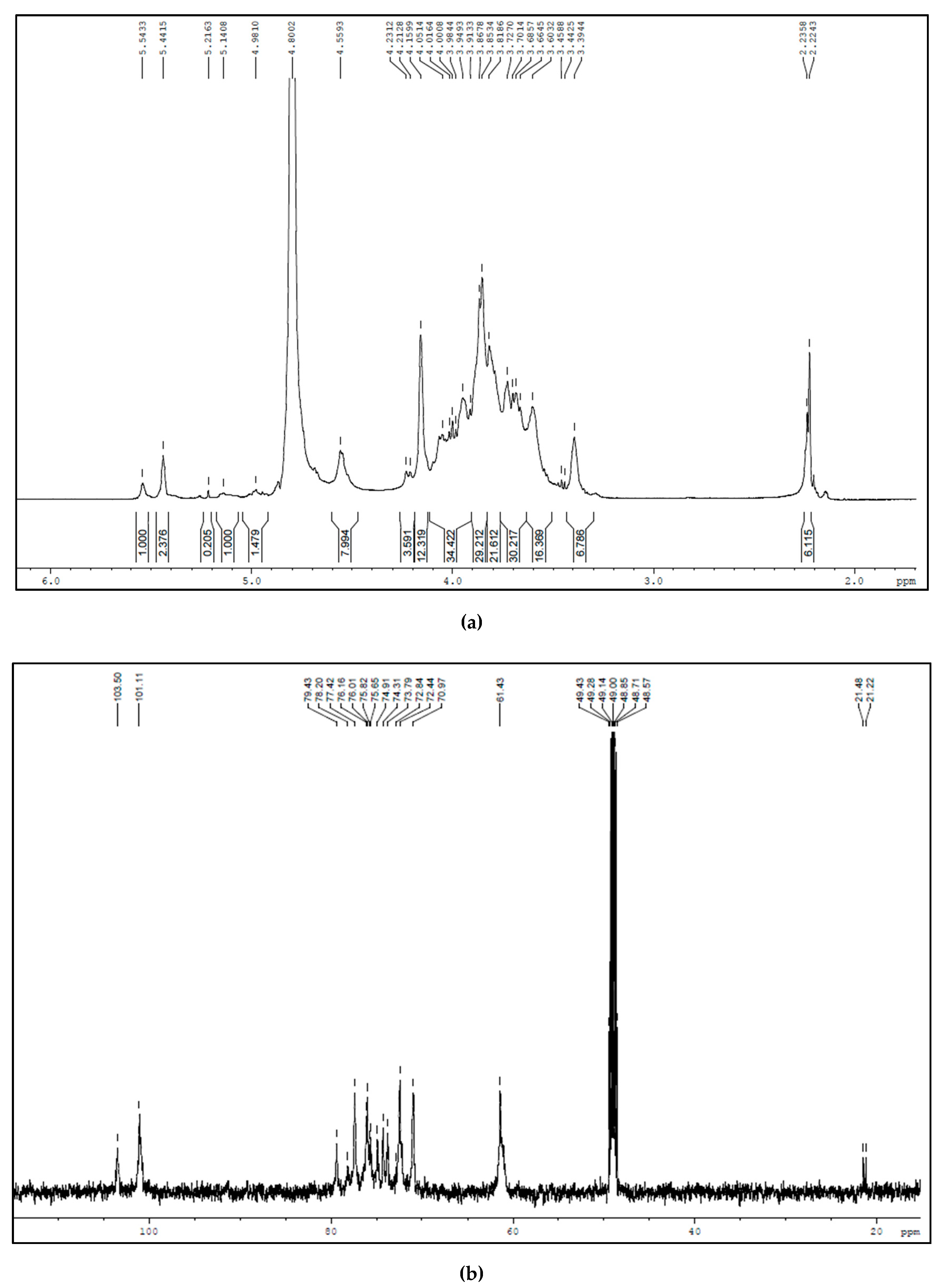
3.1. Characterization of BSP
3.2. Cell Viability of HCEC
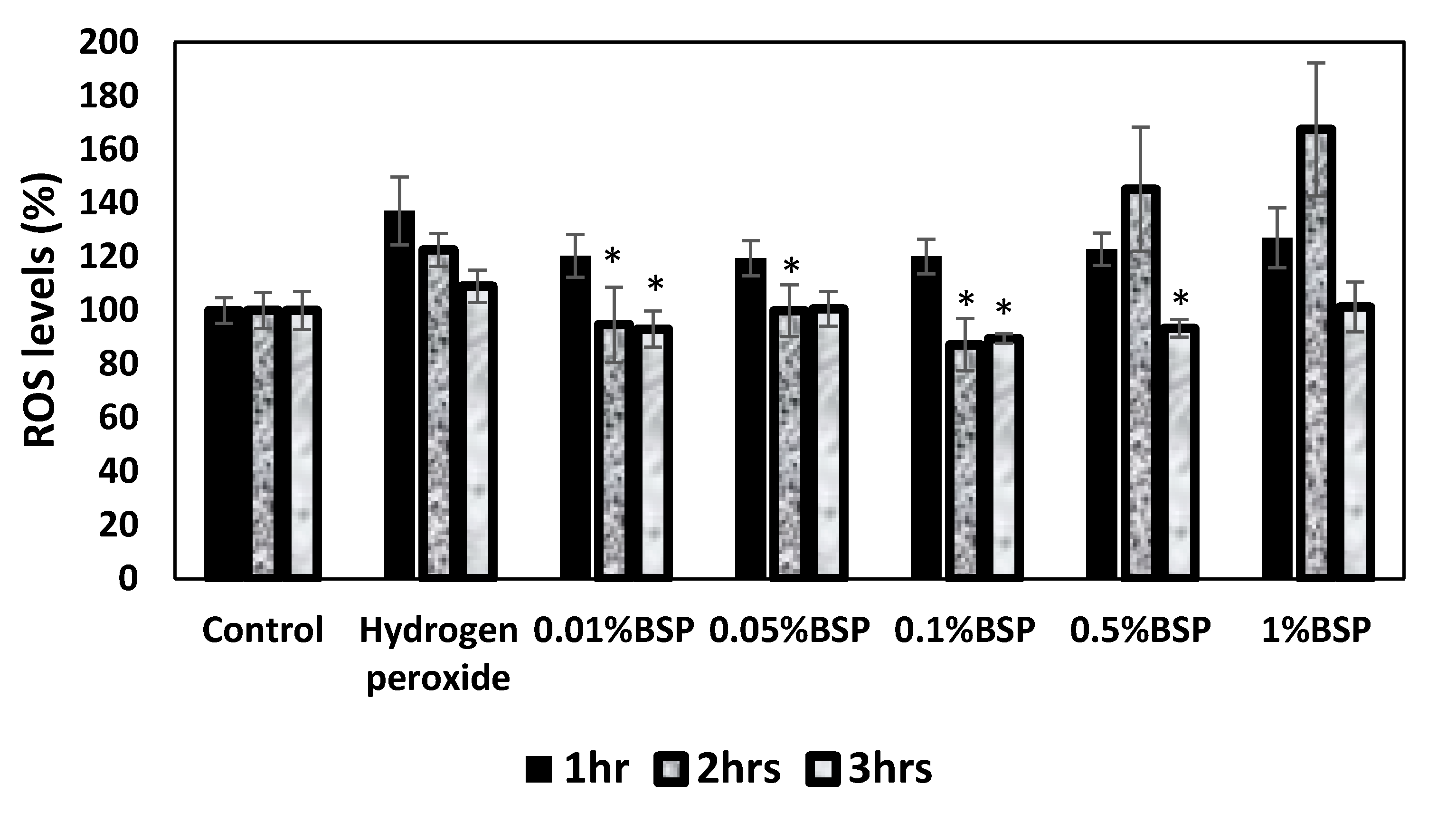
3.3. Antioxidant Effect of BSP by DCFDA Cellular ROS Detection Assay
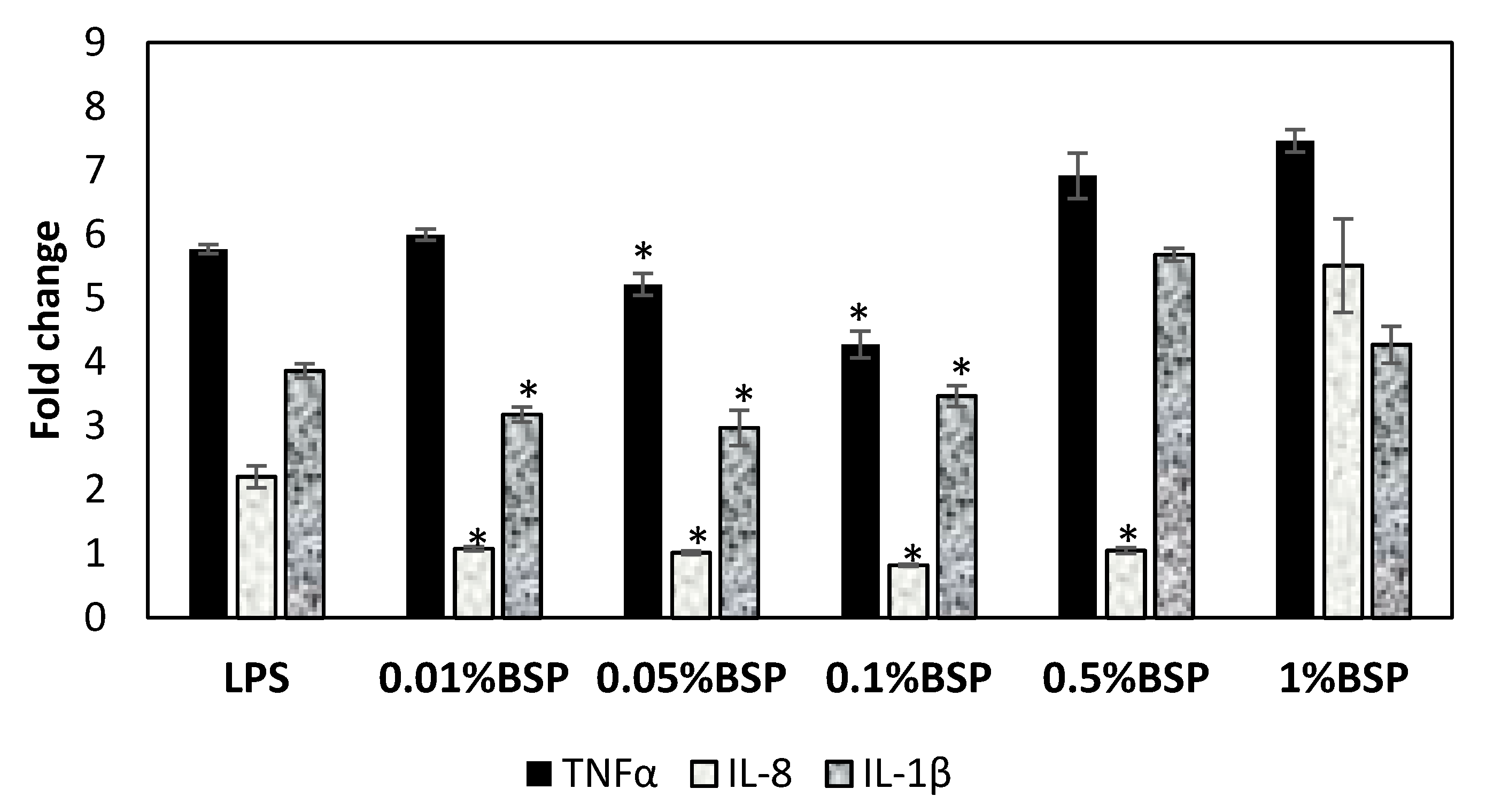
3.4. Anti-Inflammatory Effect of BSP by Gene Expression of Inflammatory Cytokines in HCECs
3.5. Physical Properties of Artificial Tears
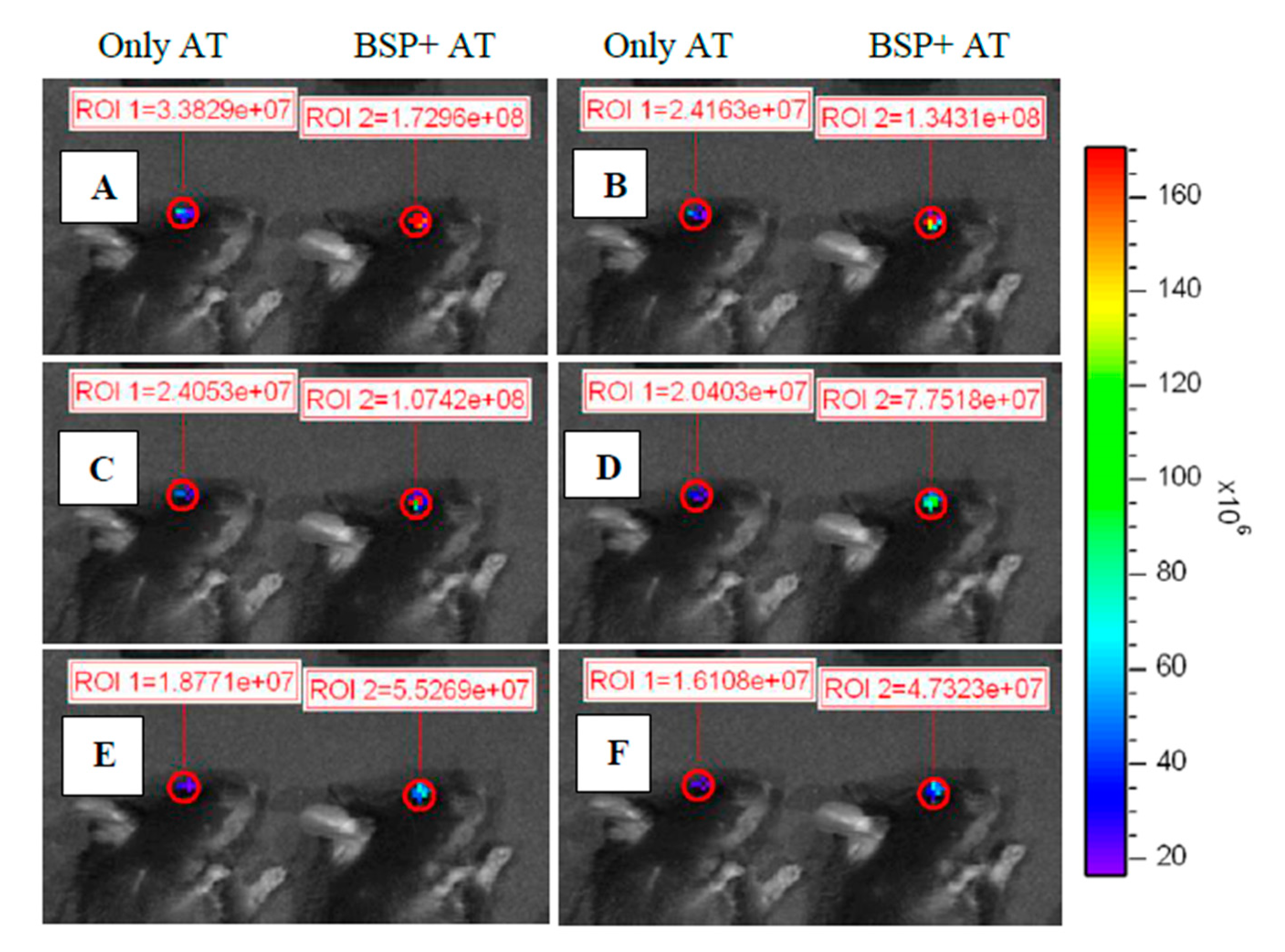
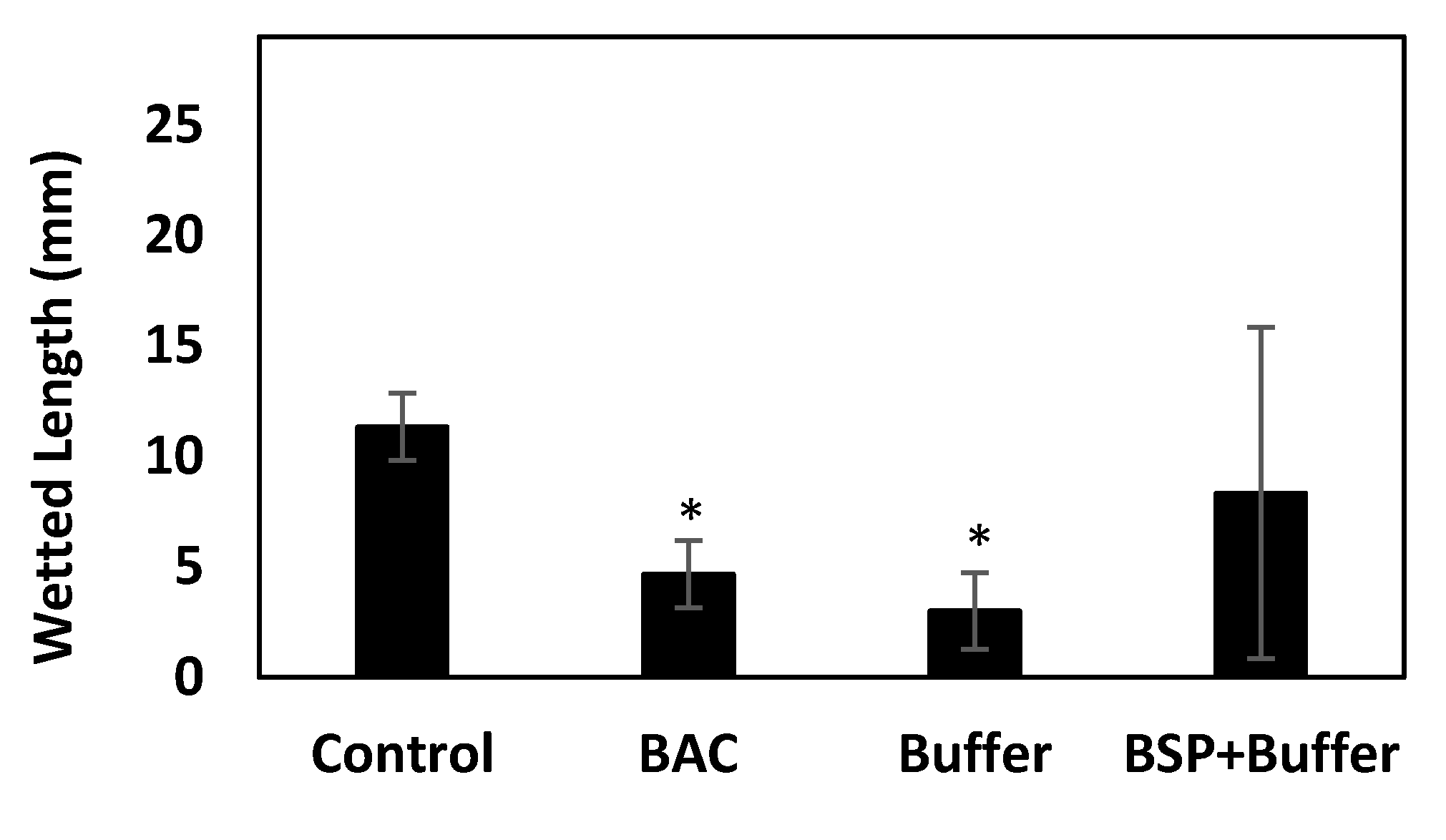
3.6. Effect of BSP to Enhance the Retention of AT on the Ocular Surface
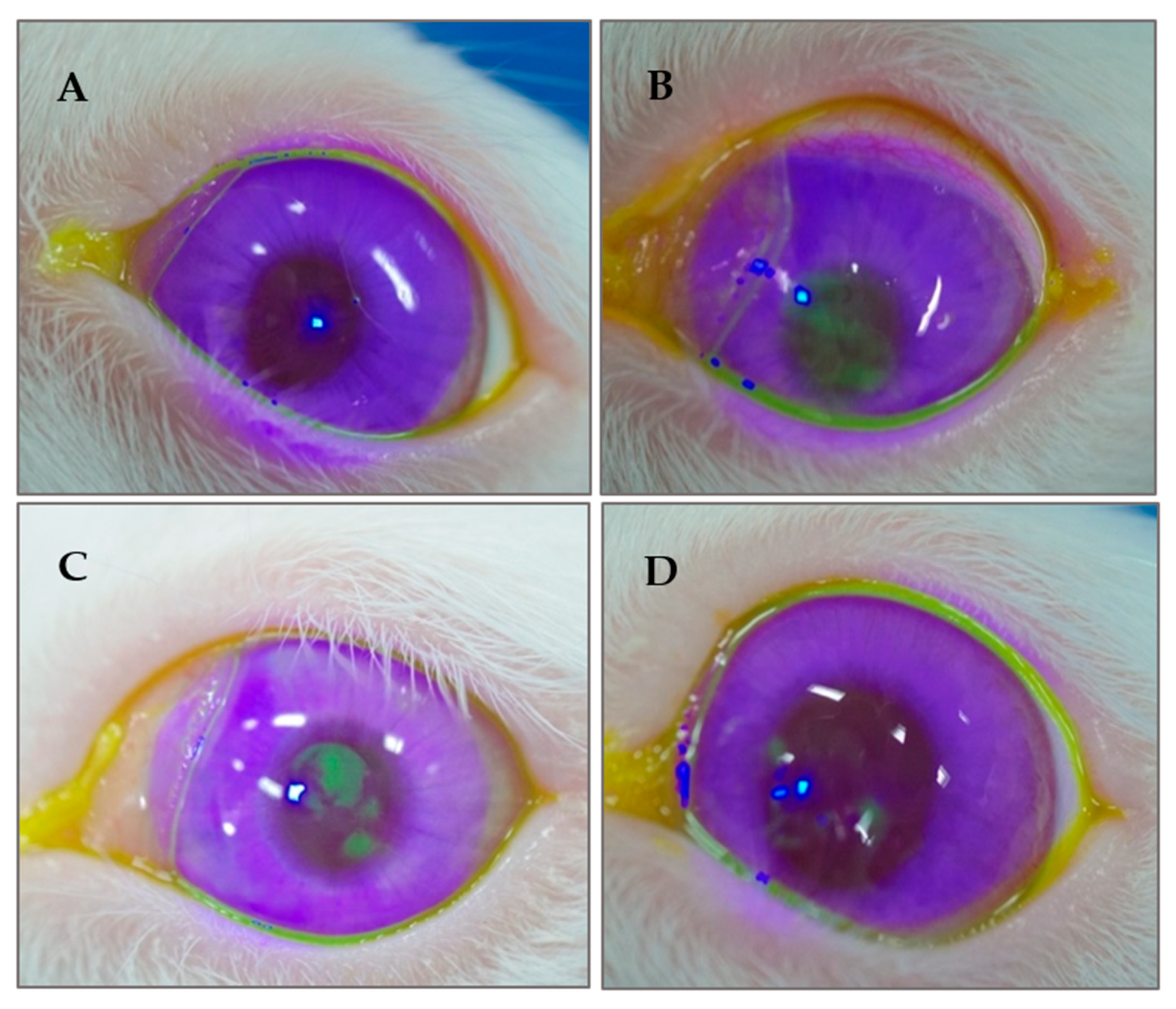
3.7. Appearance of the Ocular Surface
3.8. Changes in Tear Production
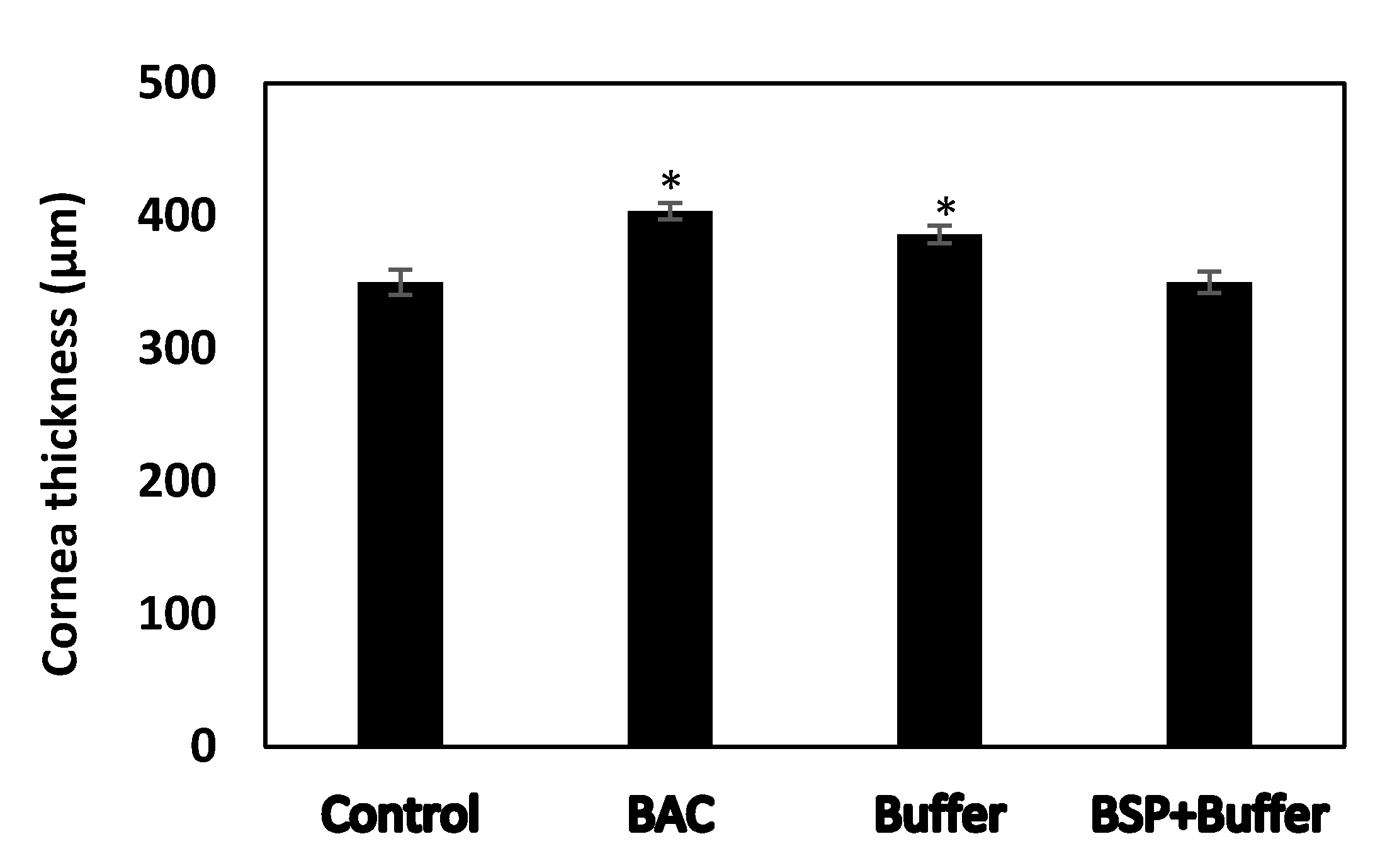
3.9. Changes in Cornea Thickness
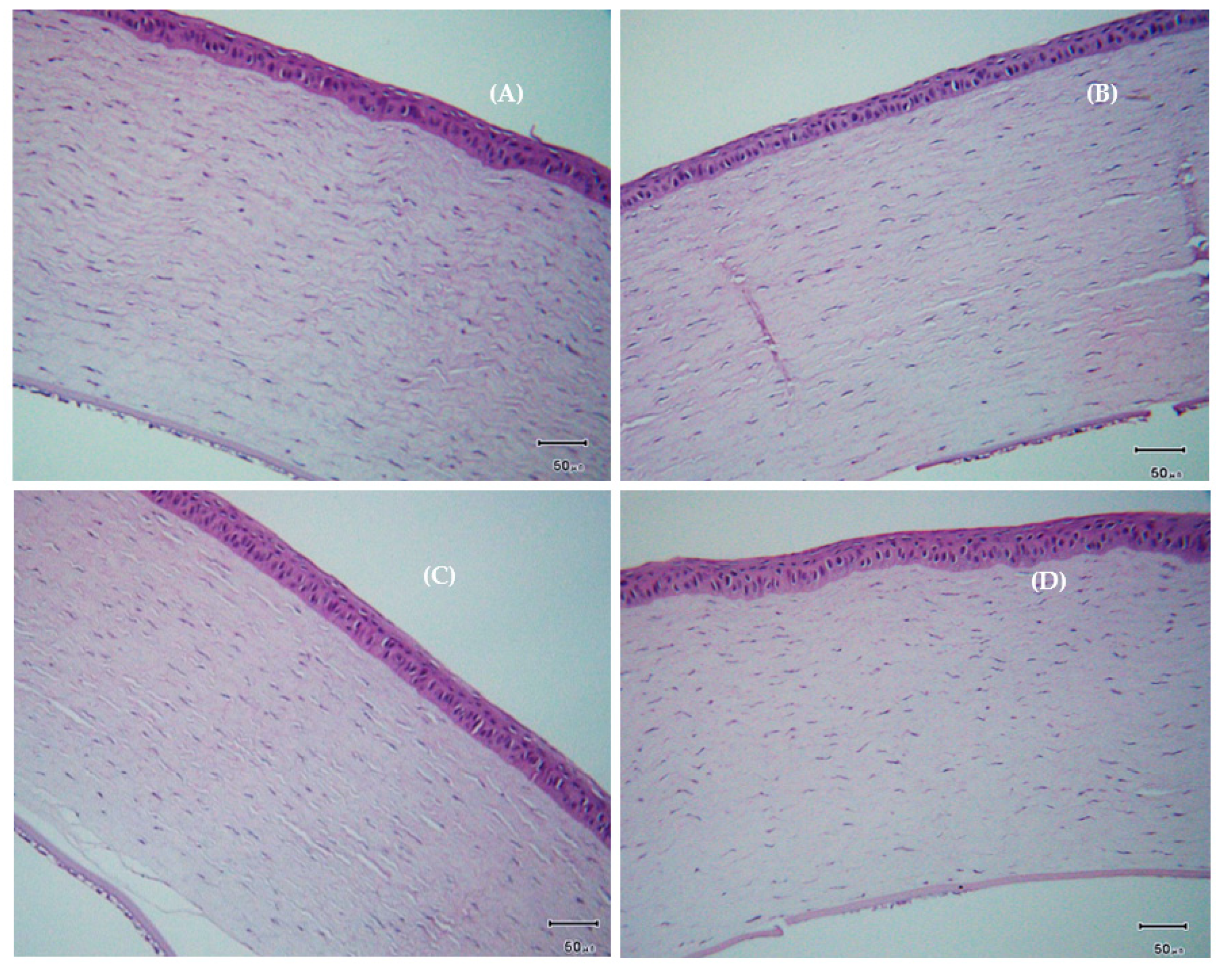
3.10. Histological Examination of the Cornea
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abengózar-Vela, A.; Schaumburg, C.S.; Stern, M.E.; Calonge, M.; Enríquez-de-Salamanca, A.; González-García, M.J. Topical Quercetin and Resveratrol Protect the Ocular Surface in Experimental Dry Eye Disease. Ocul. Immunol. Inflamm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Klein, R.; Klein, B.E.K. Prevalance of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000. [Google Scholar] [CrossRef] [PubMed]
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Miljanović, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of Dry Eye Syndrome on Vision-Related Quality of Life. Am. J. Ophthalmol. 2007. [Google Scholar] [CrossRef]
- Drew, V.J.; Tseng, C.L.; Seghatchian, J.; Burnouf, T. Reflections on dry eye syndrome treatment: Therapeutic role of blood products. Front. Med. 2018. [Google Scholar] [CrossRef]
- Sutu, C.; Fukuoka, H.; Afshari, N.A. Mechanisms and management of dry eye in cataract surgery patients. Curr. Opin. Ophthalmol. 2016, 27, 24–30. [Google Scholar] [CrossRef]
- Levitt, A.E.; Galor, A.; Weiss, J.S.; Felix, E.R.; Martin, E.R.; Patin, D.J.; Sarantopoulos, K.D.; Levitt, R.C. Chronic dry eye symptoms after LASIK: Parallels and lessons to be learned from other persistent post-operative pain disorders. Mol. Pain 2015. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Alcoholado, C.; Cifuentes, M.; Merayo-Lloves, J.; Andrades, J.A.; Becerra, J. Regenerative therapies in dry eye disease: From growth factors to cell therapy. Int. J. Mol. Sci. 2017, 18, 2264. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. Pathophysiology, diagnosis and treatment of dry eye. Dtsch. Arztebl. Int. 2015. [Google Scholar] [CrossRef]
- Wilson, S.E.; Perry, H.D. Long-term Resolution of Chronic Dry Eye Symptoms and Signs after Topical Cyclosporine Treatment. Ophthalmology 2007. [Google Scholar] [CrossRef]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014, 9, 240–250. [Google Scholar]
- Clayton, J.A. Dry eye. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Hung, Y.J.; Chen, Z.Y.; Fang, H.W.; Chen, K.H. Synergistic effect of artificial tears containing epigallocatechin gallate and hyaluronic acid for the treatment of rabbits with dry eye syndrome. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Galarreta, D.J.; Mrukwa-Kominek, E.; Böhringer, D.; Maurino, V.; Guillon, M.; Rossi, G.C.M.; Van der Meulen, I.J.; Ogundele, A.; Labetoulle, M. Clinical evaluation of an oil-based lubricant eyedrop in dry eye patients with lipid deficiency. Eur. J. Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yagci, A.; Gurdal, C. The role and treatment of inflammation in dry eye disease. Int. Ophthalmol. 2014, 34, 1291–1301. [Google Scholar] [CrossRef]
- Mateo Orobia, A.J.; Saa, J.; Lorenzo, A.O.; Herreras, J.M. Combination of hyaluronic acid, carmellose, and osmoprotectants for the treatment of dry eye disease. Clin. Ophthalmol. 2018, 12, 456–461. [Google Scholar] [CrossRef]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef]
- Kunert, K.S.; Tisdale, A.S.; Stern, M.E.; Smith, J.A.; Gipson, I.K. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: Effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Chen, S.; Wang, Y.; Jiang, H.; Yin, H. A new glucomannan from Bletilla striata: Structural and anti-fibrosis effects. Fitoterapia 2014. [Google Scholar] [CrossRef]
- Rjeibi, I.; Hentati, F.; Feriani, A.; Hfaiedh, N.; Delattre, C.; Michaud, P.; Pierre, G. Novel antioxidant, anti-α-amylase, anti-inflammatory and antinociceptivewater-soluble polysaccharides from the aerial part of nitraria retusa. Foods 2020, 9, 28. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Z.; Xue, J.; Wang, Y.; Zhang, J.; Cai, X.; Liu, Y.; Yang, F. Bletilla striata polysaccharide has a protective effect on intestinal epithelial barrier disruption in TAA-induced cirrhotic rats. Exp. Ther. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. Review Article A Review of Isolation, Chemical Properties, and Bioactivities of Polysaccharides from Bletilla striata. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Yue, L.; Wang, W.; Wang, Y.; Du, T.; Shen, W.; Tang, H.; Wang, Y.; Yin, H. Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways. Int. J. Biol. Macromol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Li, J.; Qiao, H.; Di, L. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. Int. J. Biol. Macromol. 2018. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Luo, Y.; Xue, W.; Diao, H.; Dong, L.; Chen, J.; Zhang, J. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol. Lett. 2006. [Google Scholar] [CrossRef]
- Xiong, C.; Chen, D.; Liu, J.; Liu, B.; Li, N.; Zhou, Y.; Liang, X.; Ping, M.; Ye, C.; Ge, J.; et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2008. [Google Scholar] [CrossRef]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992. [Google Scholar] [CrossRef]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human tear ph by direct measurement. Arch. Ophthalmol. 1981. [Google Scholar] [CrossRef]
- Benelli, U.; Nardi, M.; Posarelli, C.; Albert, T.G. Tear osmolarity measurement using the TearLabTM Osmolarity System in the assessment of dry eye treatment effectiveness. Contact Lens Anterior Eye 2010. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Xu, D.; Pan, Y.; Chen, J. Chemical constituents, pharmacologic properties, and clinical applications of bletilla striata. Front. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009. [Google Scholar] [CrossRef]
- Ohashi, Y.; Dogru, M.; Tsubota, K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta 2006, 369, 17–28. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubotav, K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Investig. Ophthalmol. Vis. Sci. 2018. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016. [Google Scholar] [CrossRef]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful in vitro techniques to evaluate the mucoadhesive properties of hyaluronic acid-based ocular delivery systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Zhou, T.; Wang, Y.; Bai, L.; He, H.; Liu, Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol. Vis. 2011, 17, 257–264. [Google Scholar]
- Wilson, W.S.; Duncan, A.J.; Jay, J.L. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br. J. Ophthalmol. 1975. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thacker, M.; Tseng, C.-L.; Chang, C.-Y.; Jakfar, S.; Chen, H.Y.; Lin, F.-H. Mucoadhesive Bletilla striata Polysaccharide-Based Artificial Tears to Relieve Symptoms and Inflammation in Rabbit with Dry Eyes Syndrome. Polymers 2020, 12, 1465. https://doi.org/10.3390/polym12071465
Thacker M, Tseng C-L, Chang C-Y, Jakfar S, Chen HY, Lin F-H. Mucoadhesive Bletilla striata Polysaccharide-Based Artificial Tears to Relieve Symptoms and Inflammation in Rabbit with Dry Eyes Syndrome. Polymers. 2020; 12(7):1465. https://doi.org/10.3390/polym12071465
Chicago/Turabian StyleThacker, Minal, Ching-Li Tseng, Chih-Yen Chang, Subhaini Jakfar, Hsuan Yu Chen, and Feng-Huei Lin. 2020. "Mucoadhesive Bletilla striata Polysaccharide-Based Artificial Tears to Relieve Symptoms and Inflammation in Rabbit with Dry Eyes Syndrome" Polymers 12, no. 7: 1465. https://doi.org/10.3390/polym12071465
APA StyleThacker, M., Tseng, C.-L., Chang, C.-Y., Jakfar, S., Chen, H. Y., & Lin, F.-H. (2020). Mucoadhesive Bletilla striata Polysaccharide-Based Artificial Tears to Relieve Symptoms and Inflammation in Rabbit with Dry Eyes Syndrome. Polymers, 12(7), 1465. https://doi.org/10.3390/polym12071465






